Thrombocytopenia and oxidative stress are the most frequent problems in patients with chronic liver diseases as viral cirrhosis and schistosomiasis. So, this study aimed to evaluate the role of thrombopoietin (TPO) on the occurrence of thrombocytopenia and in differentiation between these diseases. It also aimed to investigate the relation between TPO, oxidative stress and antioxidant status in these two types of chronic liver disease. So, We measured serum TPO level, lipid peroxide (MDA) and serum total antioxidant activity (TAO) in 40 patients with cirrhosis caused by hepatitis C virus and 37 patients with schistosomiasis from The Specialized Medical Hospital, Mansoura University. Results: Both serum TPO level and serum TAO activity were significantly lower (p < 0.05) in thrombocytopenic patients with viral cirrhosis when compared to both non thrombocytopenic and control groups. In contrast, TPO level was within the normal range in the patients with scistosomiasis either thrombocytopenic or not. while serum TAO activity was significantly lower (p < 0.05) in both thrombocytopenic and non thrombocytopenic patients with schistosomiasis in comparison to control subjects with no significant difference between these two subgroups. Serum MDA concentration was increased significantly (p < 0.05) in all diseased groups when compared to controls with significant increase in thrombocytopenic patients as compared to non thrombocytopenic. Conclusion: TPO hypoproduction played a role in the pathogenesis and treatment of viral cirrhosis associated with thrombocytopenia. Also, total antioxidant activity and MDA are useful markers for monitoring patients with these chronic liver diseases.
List of abbreviations:
Asn Asparagines
EDTA Ethylene diamine tetra acetic acid
ELISA Enzyme-linked immunosorbent assay
EPO Erythropoietin
HCV Hepatitis C virus
HNE 4-Hydroxynonenal
IL-1 Interleukin-1
kDa kilo-Dalton
LPO Lipid peroxidation
MDA Malondialdehyde
MK Megakaryocyte
mRNA Messenger Ribonucleic Acid
ROS Reactive oxygen species
TAO Total antioxidant activity
TBARS Thiobarbituric acid reactive substances
TNF-á
Tumor necrosis factor-?
TPO Thrombopoietin
PDF created with pdfFactory Pro trial version
www.pdffactory.com
IntroductionChronic liver diseases and their complications constitute a major health problem all over the world especially in our locality.
Major complications of chronic liver diseases included Varices and variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, portal hypertension, thrombocytopenia, and hepatorenal syndrome.1,2
One of the most frequent complications in patients with chronic liver disease was thrombocytopenia. Thrombocytopenia was defined as a subnormal number of platelets in the circulating blood (below the normal range of 150,000 to 400,000/μL). It was the most common cause of abnormal bleeding.3
The pathogenetic mechanisms leading to this disorder were incompletely understood.4 Several mechanisms had been suggested as a contributing cause to thrombocytopenia in liver diseases such as increased pooling in the enlarged spleen.5 but thrombocytopenia might even persist after splenectomy in some cases.6 Also it was seen in hepatic patients with normal splenic size.7 Also, inappropriate production of platelets in the bone marrow8 and reduced half life of platelets immunologically by increased platelet associated immunoglobulin IgG (autoantibodies) may participate in the induction or aggravation of thrombocytopenia.9,10 One of the recently postulated causes is reduced production of TPO.7
Thrombopoietin is the major hormone controlling platelet production which is primarily produced by the liver which is the predominant thrombopoietin producing organ.11 It is a polypeptide of 353 amino acids, including a 21-amino-acid secretory leader sequence with a predicted molecular mass of 35 kDa.12 It consists of two domains on the basis of its homology with erythropoietin (EPO).13 The 153-residue N-terminal region is homologous to human erythropoietin (EPO) with 4 conserved cysteine residues and is sufficient for receptor binding and signal transduction (It is also called the cytokine domain).14
In contrast, the 179-residue C-terminal region (or glycan domain), with no homology to any known proteins, has a large number of proline and glycine residues and six N-linked glycosylation sites.15 Among six Nglycosylation sites in the C-terminal region, two locations, Asn- 213 and Asn- 234, were found to be critical for secretion, and two other locations, Asn-319 and Asn-327, did not affect the secretion.16 In addition to its six N-glycosylation sites, the molecule possesses multiple O-glycosylation sites.13
TPO acts at both early and late stages of megakaryopoiesis, alone and in synergy with other cytokines (including interleukin-3, interleukin-11, steel factor (ST), and erythropoietin). The hormone acts in synergy with erythropoietin (EPO) to stimulate erythropoiesis17 and with interleukin-3 or steel factor to stimulate the proliferation and prolong the survival of hematopoietic stem cells and all types of blood-cell progenitors.18 TPO affects nearly every stage of megakaryocyte (MK) development from the committed progenitor to the mature platelet.19 It stimulates proliferation of progenitors and the development and complete maturation of polyploid megakaryocytes, which are fully capable of producing platelets.20 In the presence of TPO, MKs grow in size, develop several types of specific granules, and form demarcation membranes, a precursor of platelet formation.21 In the absence of TPO, immature megakaryocytes undergo apoptosis.11
Because TPO was the physiologic regulator of platelet production, circulating levels of TPO would be expected to vary inversely with changes in platelet demand.21 Measurement of serum TPO has been recently emerged as a new laboratory test for evaluation of patients with thrombocytopenia.22
Another complication in patients with chronic liver disease is related to Oxidative stress which was recognized in several forms of chronic liver disease.23 It was originally defined as the disequilibrium between prooxidants and antioxidants in biological systems. Once this imbalance appeared, cellular macromolecules might be damaged by the predominant free radicals. This leads to oxidative modifications of the genome, proteins, structural carbohydrates, and lipids; in the latter case, lipid peroxidation (LPO) occured.24
Lipid peroxidation was one of the most important expressions of oxidative stress25 because it amplified the free radical production process, and its products could lead to cellular and tissue damage. Direct determination of the primary products of oxidative attack had been shown to be the most accurate measure of lipid peroxidation.26 Lipid peroxidation was one of the main mechanisms of ROS-mediated liver injury.27 The deleterious consequences of this mechanism were related in part to the formation of aldehydic products such as malondialdehyde MDA and 4-hydroxynonenal (HNE).28 There was significant evidence that these aldehydic products might be involved in several chronic inflammatory diseases of the liver including alcoholic liver disease, hepatitis C, hepatic iron overload, primary biliary cirrhosis and schistosomiasis.29,30 Malondialdehyde (MDA) was the most abundant carbonyl compound and the major mutagenic and carcinogenic product generated by lipid peroxidation31 because it could be detected early in the course of some of these diseases, detection and prevention of lipid peroxidation by the aid of antioxidants could be of major interest in preventing evolution toward fibrosis and cirrhosis in human chronic liver diseases.28
Antioxidants are physiological substances that are derived from both endogenous and exogenous sources and act against oxidative stress. They modify ROS, resulting in the production of less reactive species and the decrease of their toxic effects. Antioxidants may delay or prevent direct oxidation of oxidizable substances. Cellular defense targeted against transient damaging species can be grouped under several mechanisms and collectively they operate to terminate free radical reaction or remove reactive species and their secondary products.29 From all of these, the present study aimed to evaluate the role of thrombopoietin (TPO) on the occurrence of thrombocytopenia and in differentiation between these diseases. It also aimed to investigate the relation between TPO, oxidative stress and antioxidant status in these two types of chronic liver disease.
Subjects and methodsPatientsThe present study was carried out on 77 patients with chronic liver diseases selected from the out-patient clinics of the Specialized Medical Hospital- Mansoura University-Mansoura- Egypt in the period between January 2004 and July 2004.
Patients with chronic liver diseases are treated by supportive treatment as combined diuretics (furosemide [Lasix 40 mg daily]), (oral spironolactone [Aldactone 100 mg daily]), Propranolol (Inderal 40 mg twice daily) and silymarin (legalon 140 mg three times daily).
Patients included in this study were classified into two groups:
Group I: 40 patients with cirrhosis caused by hepatitis C virus. The diagnosis was based on a positive test for HCV antibodies by third generation ELISA and quanitative PCR for HCV RNA beside clinical, radiological, biochemical and histopathological findings. It was subdivided according platelets count into:
Subgroup A: Thrombocytopenic patients (platelet count less than 100 x 103/μL):
Consisted of 27 patients (21 ♂ and 6 ♀) with age ranged between 36- 65 years with mean ± SE of 49.38 ± 2.12 years.
Subgroup B: Non-thrombocytopenic patients: Consisted of 13 patients (9 ♂and 4 ♀) with age ranged between 31- 59 years with mean ± SE of 41.15 ± 2.06 years.
Group II: 37 patients with chronic schistosomiasis. The diagnosis was based on the presence of viable bilharzial ova in fecal material as well as serologic testing and liver histopathology. It was subdivided into:
Subgroup C: Thrombocytopenic patients (platelet count less than 100 x 103/μL):
Consisted of 15 patients (10 ♂ and 5 ♀) with age ranged between 33- 65 years with mean ± SE of 51.93 ± 3.4 years.
Subgroup D: Non-thrombocytopenic patients: Consisted of 22 patients (19 ♂ and 3 ♀) with age ranged between 28- 54 years with mean ± SE of 40.77 ± 2.78 years.
Control group: This group Consisted of 12 normal healthy persons (8 ♂ and 4 ♀) with age ranged between 30- 45 years with mean ± SE of 36.56 ± 1.41 years.
Sample collectionBlood was taken from patients and control subjects by clean venipuncture using plastic disposable syringes.
Each blood sample was divided into two portions as follows:
- •
First portion was collected into EDTA-containing tube, and used for blood picture investigation within 5 hours.
- •
The second portion was collected into clean dry tube; allowed to coagulate and centrifuged for 10 minutes at 3,000 rpm. The clear nonhemolyzed serum is divided into different aliquots and used for estimation of: Liver function tests serum thrombopoietin concentration, Malondialdehyde concentration and Total antioxidant activity.
Serum TPO concentration was determined by a commercially available ELISA kit (Quantikine Human TPO Immunoassay, R&D Systems, Minneapolis, Minn., U.S.A.) according to the method used by Goulis et al., (1999).29
Serum MDA was measured according to the method of Draper and Hadley, (1990),30 in which serum proteins are precipitated by addition of trichloroacetic acid. Then thiobarbituric acid reacts with MDA to form thiobarbituric acid reactive product that is measured at 532 nm.
Serum total antioxidant activity was measured using the method of Koracevic et al., (2001).31 A standardized solution of Fe–EDTA complex reacts with hydrogen peroxide by a Fenton type reaction, leading to the formation of hydroxyl radicals (***entity***OH). These reactive oxygen species degrade benzoate, resulting in the release of thiobarbituric acid reactive substances (TBARS). Antioxidants from the added sample of human fluid cause suppression of the production of TBARS. This reaction can be measured spectrophotometrically and the inhibition of colour development is defined as the AOA.
Statistical analysisStatistical analysis of the present study was achieved by using GraphPad InStat program version 3.05 produced by GraphPad Software Inc. (2000). Significant differences in variables between two groups were tested by Student's t-test. Data were expressed as mean ± SE. Correlation coefficient (r) was used to measure the correlation between two numerical variables. P < 0.05 was considered statistically significant for comparison of the results in this study.
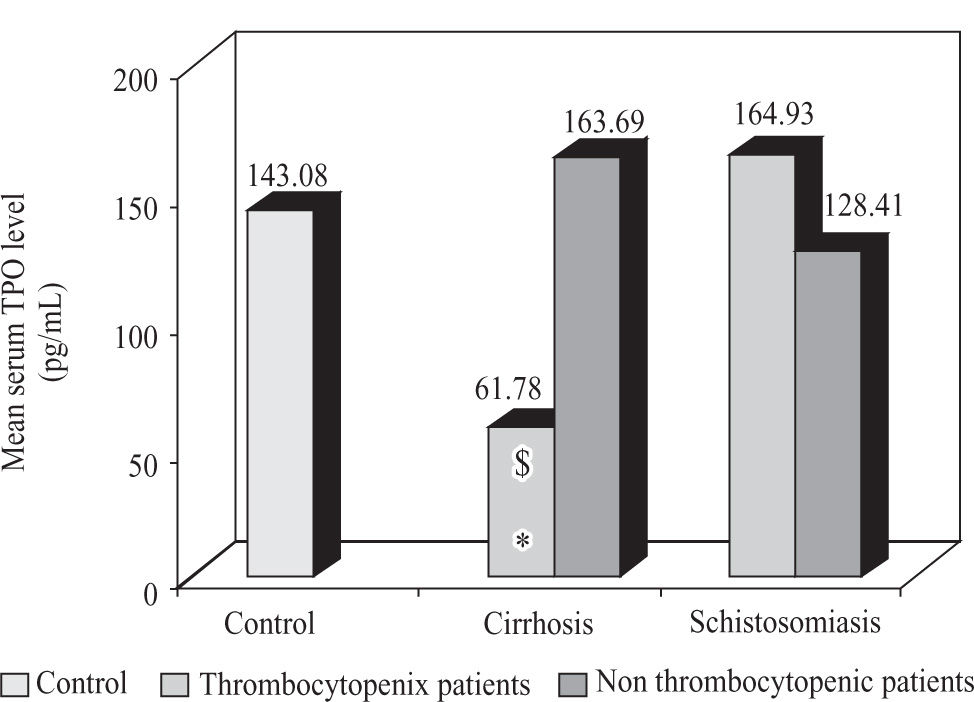
Results1)ThrombopoietinTables 1 and 2 shown the liver function test and the hematological parameters of patients included in this study. Thrombocytopenic patients with cirrhosis caused by hepatitis C virus showed a significant decrease (p < 0.05) in serum TPO level in comparison to both non thrombocytopenic patients and control subjects, while there was no significant difference in serum TPO level between thrombocytopenic, non thrombocytopenic patients with schistosomiasis and control subjects (Figure 1).
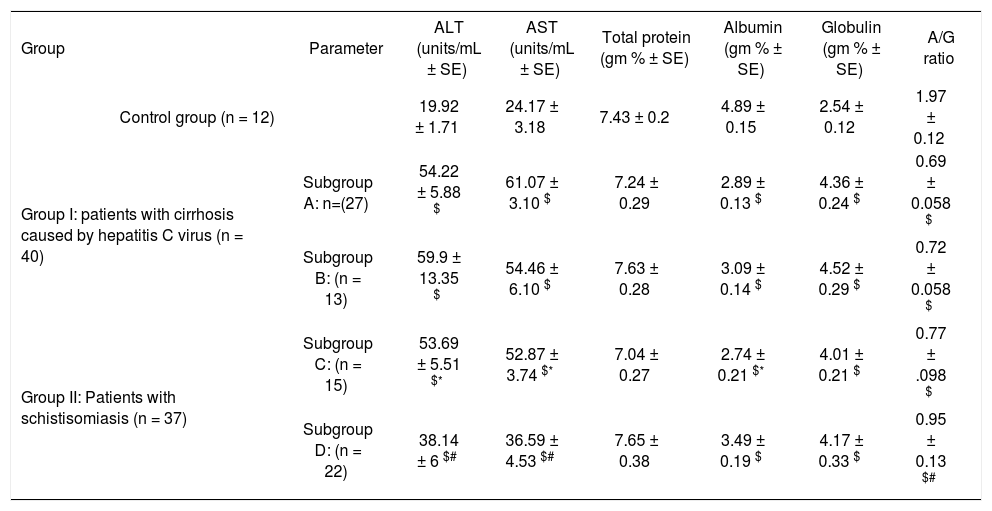
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities and serum levels of total protein (TP), albumin, globulin and A/G ratio in thrombocytopenic and non thrombocytopenic subgroups of patients with cirrhosis caused by hepatitis C virus and patients with schistosomiasis compared to control group (mean ± SE).
| Group | Parameter | ALT (units/mL ± SE) | AST (units/mL ± SE) | Total protein (gm % ± SE) | Albumin (gm % ± SE) | Globulin (gm % ± SE) | A/G ratio |
|---|---|---|---|---|---|---|---|
| Control group (n = 12) | 19.92 ± 1.71 | 24.17 ± 3.18 | 7.43 ± 0.2 | 4.89 ± 0.15 | 2.54 ± 0.12 | 1.97 ± 0.12 | |
| Group I: patients with cirrhosis caused by hepatitis C virus (n = 40) | Subgroup A: n=(27) | 54.22 ± 5.88 $ | 61.07 ± 3.10 $ | 7.24 ± 0.29 | 2.89 ± 0.13 $ | 4.36 ± 0.24 $ | 0.69 ± 0.058 $ |
| Subgroup B: (n = 13) | 59.9 ± 13.35 $ | 54.46 ± 6.10 $ | 7.63 ± 0.28 | 3.09 ± 0.14 $ | 4.52 ± 0.29 $ | 0.72 ± 0.058 $ | |
| Group II: Patients with schistisomiasis (n = 37) | Subgroup C: (n = 15) | 53.69 ± 5.51 $* | 52.87 ± 3.74 $* | 7.04 ± 0.27 | 2.74 ± 0.21 $* | 4.01 ± 0.21 $ | 0.77 ± .098 $ |
| Subgroup D: (n = 22) | 38.14 ± 6 $# | 36.59 ± 4.53 $# | 7.65 ± 0.38 | 3.49 ± 0.19 $ | 4.17 ± 0.33 $ | 0.95 ± 0.13 $# | |
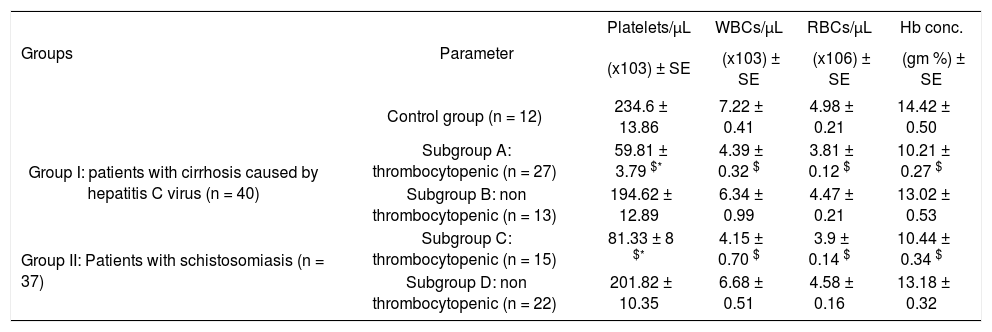
Platelets, white blood cells (WBCs) and red blood cells (RBCs) counts and hemoglobin (Hb) concentration in thrombocytopenic and non thrombocytopenic subgroups of patients with cirrhosis caused by hepatitis C virus and patients with schistosomiasis compared to control group (mean ± SE).
| Groups | Parameter | Platelets/μL | WBCs/μL | RBCs/μL | Hb conc. |
|---|---|---|---|---|---|
| (x103) ± SE | (x103) ± SE | (x106) ± SE | (gm %) ± SE | ||
| Control group (n = 12) | 234.6 ± 13.86 | 7.22 ± 0.41 | 4.98 ± 0.21 | 14.42 ± 0.50 | |
| Group I: patients with cirrhosis caused by hepatitis C virus (n = 40) | Subgroup A: thrombocytopenic (n = 27) | 59.81 ± 3.79 $* | 4.39 ± 0.32 $ | 3.81 ± 0.12 $ | 10.21 ± 0.27 $ |
| Subgroup B: non thrombocytopenic (n = 13) | 194.62 ± 12.89 | 6.34 ± 0.99 | 4.47 ± 0.21 | 13.02 ± 0.53 | |
| Group II: Patients with schistosomiasis (n = 37) | Subgroup C: thrombocytopenic (n = 15) | 81.33 ± 8 $* | 4.15 ± 0.70 $ | 3.9 ± 0.14 $ | 10.44 ± 0.34 $ |
| Subgroup D: non thrombocytopenic (n = 22) | 201.82 ± 10.35 | 6.68 ± 0.51 | 4.58 ± 0.16 | 13.18 ± 0.32 |
Mean serum TPO level in thrombocytopenic and non thrombocytopenic subgroups of patients with cirrhosis caused by hepatitis C virus and patients with schistosomiasis compared to control group (mean). $: significant against control group. *: significant against non thrombocytopenic subgroup (p < 0.05).
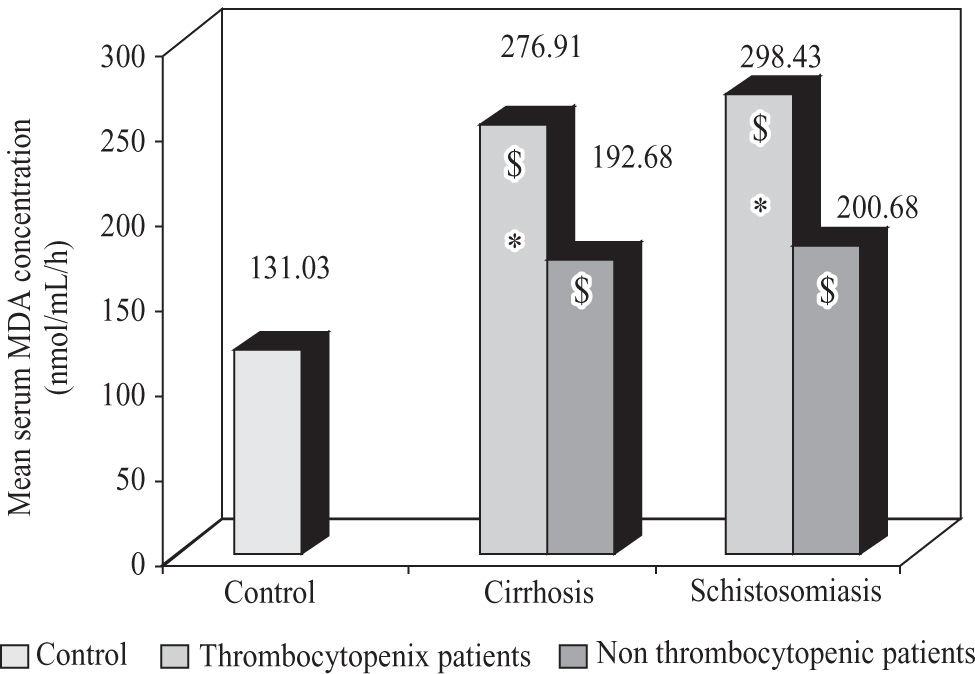
Significant increase (p < 0.05) in serum MDA concentration was observed in case of thrombocytopenic patients with viral cirrhosis and schistosomiasis when compared to corresponding non thrombocytopenic subgroups and control subjects. Also, non thrombocytopenic patients showed a significant increase (p < 0.05) when compared to control subjects (Figure 2).
Mean serum MDA concentration in thrombocytopenic and non thrombocytopenic subgroups of patients with cirrhosis caused by hepatitis C virus and patients with schistosomiasis compared to control group. $: significant against control group (p < 0.05). *: significant against non thrombocytopenic subgroup (p < 0.05).
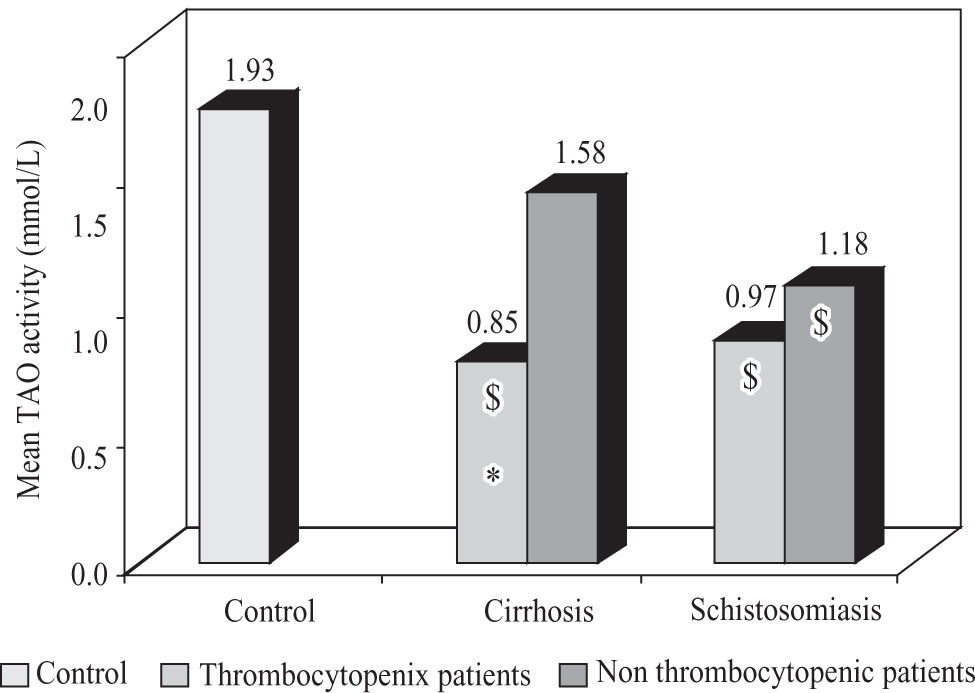
Thrombocytopenic patients with viral cirrhosis showed a significant decrease (p < 0.05) in TAO activity in comparison to non thrombocytopenic patients and control, while both thrombocytopenic and non thrombocytopenic patients with schistosomiasis showed a significant decrease (p < 0.05) in TAO activity in comparison to control subjects with no significant difference between the two subgroups (Figure 3).
Mean total antioxidant activity in thrombocytopenic and non thrombocytopenic subgroups of patients with cirrhosis caused by hepatitis C virus and patients with schistosomiasis compared to control group. $: significant against control group (p < 0.05). *: significant against non thrombocytopenic subgroup (p < 0.05).
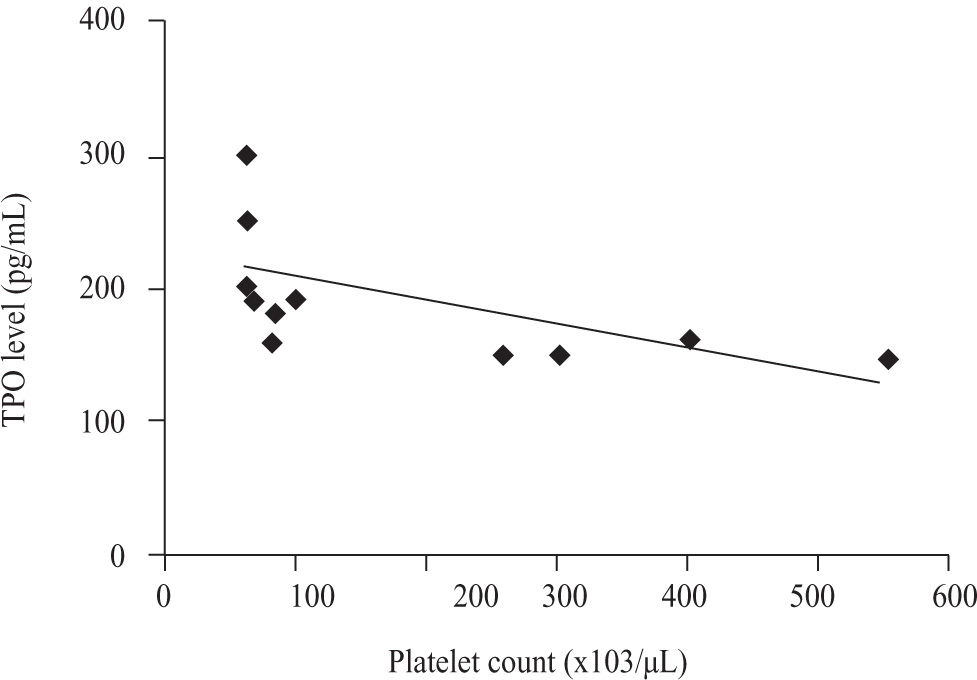
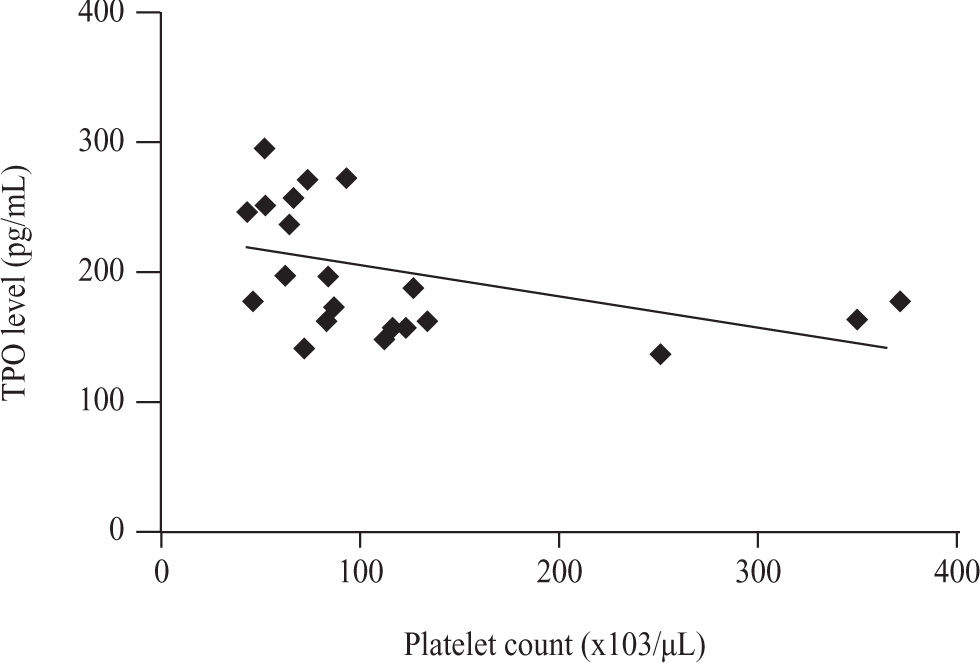
There was a significant negative correlation between serum TPO level and platelet count in non thrombocytopenic subgroup of both patients with cirrhosis caused by hepatitis C virus (r = -0.602, p = 0.030) and patients with schistosomiasis (r = -0.439, p = 0.041) (Figures 4and5).
No significant correlation was observed between serum TPO level and platelet count in thrombocytopenic subgroup of both Patients with cirrhosis caused by hepatitis C virus and patient with schistosomiasis.
The correlation between serum TPO level and platelet count in control group was negatively significant (r = -0.691, p = 0.0128).
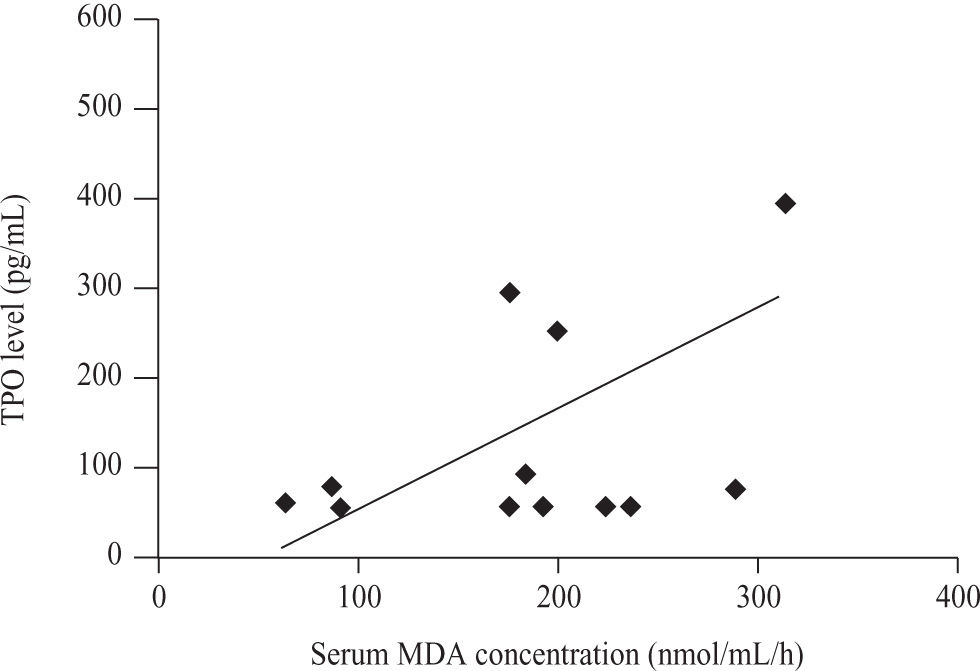
There was a Significant positive correlation (r = 0.555, p = 0.049)
between serum TPO level (pg/ml) and Serum MDA concentration (nmol/mL/h) only in non thrombocytopenic subgroup of patients with cirrhosis caused by hepatitis C virus (Figure 6).
DiscussionIn the present study, TPO level was significantly lower in the thrombocytopenic subgroup of cirrhotic patients with HCV (subgroup A) when compared to both non thrombocytopenic and control groups. This result is in agreement with previously published findings.32-36
Decreased TPO production in patients with cirrhosis related to HCV
infection was linked to decreased hepatocellular functioning mass and impaired protein synthesis in the cirrhotic liver (Giannini et al., 2003).37 The reasons for this belief include the progressive nature of TPO decline as cirrhosis evolves.38 In fact, TPO is produced by the liver at a constant rate and is cleared from circulation upon binding to its receptor on both megakaryocytes and platelets. Thus, circulating TPO levels depend upon hepatic synthesis and peripheral uptake. It is evident that impairment of the liver functioning mass may therefore cause a decrease in TPO production.19 This suggestion was based on the correction of both TPO level and platelet count after liver transplantation.7 The reduction in TPO mRNA levels was observed in the liver of cirrhotic patients.39 A 30-40% reduction in TPO mRNA levels was reported.40,41 Other cause of decreased TPO production is related to accumulation and destruction of platelets in the congested spleen, which enlarged due to portal hypertension. This could result in an increased TPO degradation and impair platelet production in the bone marrow.38,42
In contrast to our findings, Freni et al., (2002) stated that, serum TPO
level was significantly higher in the liver disease group associated with thrombocytopenia when compared to control group and this could be attributed to a feedback response to decreased platelet levels in those patients. Also, low TPO receptors expression, in addition to reduced platelet counts, might account for the normal or slightly elevated levels of serum TPO in these patients.43
In contrast to cirrhotic patient subgroups, the present study showed that TPO levels were within a normal range in the patients with scistosomiasis either thrombocytopenic or not.
Similar findings were reported by Souza et al., (2002) who stated that in patients with hepatosplenic schistosomiasis, no significant changes in serum TPO levels were found as compared to controls.44 This could be attributed to constant platelet mass since the splenic pool is in equilibrium with the circulatory pool and the hepatic production of TPO was not impaired in case of schistosomiasis which is fibrotic disease.7 The thrombocytopenia observed in patients with the hepatosplenic form of schistosomiasis is classically attributed to hypersplenism and portal hypertension. It was not due to platelet consumption or hypo-production of platelets.45
These result suggesting that hypersplenism and immune-mediated processes were predominant thrombocytopenic mechanisms in case of schistosomiasis.46
Platelets might act as defence mechanism against schistosome infection by aggregation on egg surfaces and destroying them47 and the thrombocytopaenia that occurs during schistosome infections might be a strategy that helps secondarily incoming parasites evade this type of host defensiveness.46
In the present study, MDA concentration showed a significance increase in all studied groups as compared to control subjects. This might be caused by direct interaction of HCV core protein with mitochondria is an important cause of the oxidative stress seen in chronic hepatitis C. This interaction caused an increase in mitochondrial ROS production, an oxidation of the mitochondrial glutathione pool and inhibition of electron transport.48 In viral hepatitis, ROS were produced in hepatocytes through the release of inflammatory cytokines such as TNF-α and IL-1 from inflammatory cells.49 The presence of the S. mansoni in the host hepatic mesenteries puts them under oxidative stress from immune-generated radicals as well as those potentially generated in the parasite during respiration and the breakdown and consumption of host hemoglobin with the concurrent release of toxic heme and ferrous ions.50
This result was in agreement with those observed by Romero et al., (1998); Ljubuncic et al., (2000); Cardin et al., (2002); Singh et al., (2005) and Levent et al, (2006). who reported that serum products of lipid peroxidation were increased in patients with liver diseases including cirrhosis when compared with healthy subjects and Lipid peroxidation in cell membranes and subcellular organelles had been proposed as a primary mechanism for cellular membrane dysfunction and tissue injury leading to hepatic damage.51-55
This also confirmed the results reported by (El-Sokkary et al, 2002) who stated that oxidative processes occured at the site of inflammation and were involved in the damaging effects of schistosomiasis and indicated that free radicals might be a major component of the disease.29
In this study, there was a significant difference in serum MDA level between thrombocytopenic and non thrombocytopenic sub groups of both viral cirrhosis and schistosomiasis. This confirm the results of Eboumbou et al., 2005 who stated that levels of MDA were associated with the severity of viral infection.56
In the present study, Thrombocytopenic patients with viral cirrhosis (subgroup A) showed a significant decrease in serum TAO activity in comparison to non thrombocytopenic patients (subgroup B) and control group, while the difference between non thrombocytopenic (subgroup B) and control subjects was not significant. This was due to the presence of an adequate antioxidant pool in the early stages of the disease.56 These results were in agreement with Yadav et al., (2002); Prakash and Joshi, (2004) and Saricam et al.,(2005).57-59
In contrast, Notas et al., (2005) reported that there was no significant difference in total antioxidant activity between patients with viral cirrhosis and healthy controls but he did not distinguish between thrombocytopenic and non thrombocytopenic patients.60
In the other hand, both thrombocytopenic and non thrombocytopenic patients with schistosomiasis (subgroup C and D) showed a significant decrease in serum TAO activity in comparison to control subjects, while there was no significant difference between these two subgroups.
Evidence of enhanced production of Lipid peroxidation products or significant decrease of antioxidant defense had been reported in all types of liver damage. So that lipid peroxidation can be prevented by administration of scavengers of free radicals or antioxidants. Because lipid peroxidation can be detected early in the course of some of these diseases, detection and prevention of lipid peroxidation could be of major interest in preventing evolution toward fibrosis and cirrhosis in human chronic liver diseases.28,60
The correlation between serum TPO level and platelet count was significantly negative in non thrombocytopenic subgroup of both Patients with cirrhosis caused by hepatitis C virus (r = -0.602, p = 0.030) and patient with schistosomiasis (r = -0.439, p = 0.041) and in control group (r = -0.691, p = 0.0128). This was because serum TPO levels were regulated normally by the platelet and megakaryocyte mass through binding to TPO receptors, internalization and degradation. Decreased platelets count lead to decreased number of TPO receptors and increased TPO serum level.56,61
But no significant correlation was observed in thrombocytopenic subgroups of both Patients with cirrhosis caused by hepatitis C virus and patient with schistosomiasis. This might attribute to involvement of more than one mechanism in production of thrombocytopenia such as portal hypertension and splenic sequestration of platelets. This was stressed by the observation that partial splenic embolization, a procedure that reduces spleen sequestration sharply and deeply, increases serum TPO levels among cirrhotic patients and restores the physiological relation between circulating platelet count and TPO.62,63 This not neglected the important role played by TPO in thrombocytopenia related to chronic liver disease. Even procedures that relieve portal hypertension, such as partial splenic embolization, produce an increase in platelet count that is mainly because of an improvement in liver function and consequently in TPO secretion.64 Also, it had been shown that successful interferon treatment of patients with chronic hepatitis C and thrombocytopenia is accompanied by an improvement in platelet count, which is mediated by an increase in TPO serum levels.65 This was in agreement with Okubo et al., (2000) and Ishikawa et al., (2002) who stated that no correlation between the platelet count and serum TPO level was shown in cirrhotic patients because of reduced TPO mRNA in the liver.61,66
Also, in this study, there was a Significant positive correlation (r = 0.555, p = 0.049) between serum TPO level (pg/mL) and Serum MDA concentration (nmol/ml/h) only in non thrombocytopenic subgroup of patients with cirrhosis caused by hepatitis C virus. In the same manner, Sattler et al., (1999) observed that normally, TPO induce increased formation of ROS, which act as second messengers to regulate activities of redox-sensitive enzymes, including protein kinases and protein phosphatases and potentiate signal transduction needed for TPO to exert its action.67
In the other hand, with Disease progression, these ROS levels elevated beyond that which could be neutralised by antioxidants and could propagate cellular damage by oxidative modification of cellular components and lipid peroxidation inducing apoptotic cell death.68,69
From all of these, it could be concluded that TPO serum levels played an important role in induction of thrombocytopenia in case of viral cirrhosis and human recombinant TPO could be used as treatment therapy for this thrombocytopenia. It also appears that the mechanism controlling serum TPO levels and thrombocytopenia might be different in viral cirrhotic patients compared to schistosomiasis patients.
Also, total antioxidant activity and lipid peroxidation products were considered as useful markers for monitoring patients with these chronic liver diseases. Early detection and prevention of lipid peroxidation by the aid of antioxidants could be of major interest in preventing evolution toward fibrosis and cirrhosis in human chronic liver diseases.
AcknowledgementDeep appreciation is for Prof. Dr. Ayman Naseem Menessy, Professor of Internal Medicine, Faculty of Medicine, Mansoura University for his medical assistance and generous help in following up the patients.