Hepatocellular carcinoma (HCC) ranks third on the list of the leading cause for cancer death globally. The treatment of HCC patients is unsatisfactory. However, the traditional Chinese medicine Chebulae Fructus has potential efficacy in the treatment of HCC.
Materials and methodsWe mined the active ingredients of Chebulae Fructus and its main targets from the Traditional Chinese Medicine Systems Pharmacology database. HCC-related datasets were downloaded from The Cancer Genome Atlas database and differentially expressed genes (DEGs) in HCC were obtained by differential expression analysis. Top10 small molecule compounds capable of reversing HCC pathology were screened by the Connectivity Map database based on DEGs. Ellipticine, an extract of Chebulae Fructus, had the potential to reverse HCC pathology. Protein-Protein Interaction (PPI) networks of DEGs in HCC were constructed using STRING. Eighteen potential targets of Chebulae Fructus for the treatment of HCC were obtained by taking intersection of DEGs in HCC with targets corresponding to the active constituents of Chebulae Fructus. In addition, MTT assay was also employed to examine the effect of ellipticine on HCC cell viability.
ResultsIt has been shown that ellipticine and ellagic acid have antitumor activity. Random Walk with Restart analysis of PPI networks was performed using potential targets as seeds, and the genes with the top 50 affinity coefficients were selected to construct a drug-active constituent-gene interaction network. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses of key genes involved in the treatment of HCC with Chebulae Fructus demonstrated that these genes were mainly enriched in signaling pathways related to tumor metabolism such as cAMP signaling pathway and Ras signaling pathway. Finally, it was verified by MTT assay that proliferation of HCC cells could be remarkably hindered.
ConclusionsWe excavated ellipticine, a key active constituent of Chebulae Fructus, by network pharmacology, and elucidated the signaling pathways involved in Chebulae Fructus, providing a theoretical basis for the use of Chebulae Fructus for HCC clinical application.
World Health Organization global cancer statistics show that primary liver cancer is on the list of the most common cancer, ranking 6th in incidence and 3rd in mortality [1]. As the largest subtype of primary liver cancer, hepatocellular carcinoma (HCC) accounts for 75%-85% of primary liver cancers [1]. Currently, treatment strategies for advanced HCC mainly include surgical resection, radiation therapy, chemotherapeutics, and immunotherapy [2–5]. However, the clinical results showed unsatisfactory efficacy, with only a few patients benefiting. Currently, patients with liver dysfunction cannot withstand chemotherapy treatment, and HCC is recognized by medical practitioners as a chemorefractory tumor [6]. A large proportion of patients with advanced HCC adopt palliative therapy, which causes great physical and psychological pressure on patients, therefore, there is a great need to find effective chemicals with few side effects for HCC chemotherapy.
Network pharmacology is based on the theory of systems biology, network analysis of biological systems, and selection of specific signaling nodes, which is a new discipline for the molecular design of multi-target drugs. Network pharmacology effectively reveals interconnections among drugs, targets, and diseases [7]. At present, many studies have shown the use of network pharmacology to reveal the molecular mechanism between the active constituents of traditional Chinese medicine and its targets in the treatment of cancer or disease. For example, Tao and his colleagues used pharmacological and chemogenomic approaches to reveal potential therapeutic targets of Radix Curcumae formula in the treatment of cardiovascular diseases, and elaborate the mechanism of this formula regarding the prevention of cardiovascular and cerebrovascular diseases [8]. Guo et al.[9] elaborated on the mechanism of Zuojin pill in the treatment of HCC based on network pharmacology. Huang et al.[10] explored the key mechanism of HuanglianJiedu Decoction for the treatment of HCC using network pharmacology. Xu et al.[11] used network pharmacology to excavate the potential role of Huangqin-Baishao herb pair in the treatment of cancer. Thus, network pharmacology is more and more important in the development of new drugs for diseases and research of drug mechanism, and the use of network pharmacology in the development of new drugs has great advantages.
Chebulae Fructus, a traditional Chinese medicine known as the king of Tibetan medicine, is commonly used in traditional Chinese medicine to relieve diarrhea with astringents, astringe lung to stop cough, and reduce pathogenic fire to relieve sore-throat. One study has found that Chebulae Fructus extracts significantly inhibit growth of breast cancer cells and lung cancer cells [12]. Saleem and colleagues used the ethanol extract of Chebulae Fructus to treat liver cancer, breast cancer, osteosarcoma, prostate cancer cell lines, and the results showed that Chebulae Fructus extracts can significantly inhibit the growth of these cell lines [13]. However, there is no evidence to show whether the extract of Chebulae Fructus can be used in the clinical treatment of HCC, and its active constituents, targets and pathways remain to be studied.
Based on the network pharmacology, this study identified ellipticine, a key active constituent of Chebulae Fructus for the treatment of HCC, and potential targets of it were also obtained. Those key genes closely affected by targets were then mined, clarifying the key signaling pathways involved in the treatment of HCC with Chebulae Fructus. In this study, we preliminarily elucidated the molecular mechanism of Chebulae Fructus and provided a scientific basis for the use of Chebulae Fructus in HCC treatment.
2Materials and methods2.1Acquisition of active constituents and targets of Chebulae FructusIn the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://tcmspw.com/index.php), the active constituents of the traditional Chinese medicine Chebulae Fructus were searched. The main medicinal active constituents of Chebulae Fructus were downloaded with Oral bioavailability (OB) ≥30% and Drug-likeness (DL) ≥0.18, while the targets corresponding to the ingredients were collected.
2.2Dataset downloadFrom The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/), the mRNA expression data of HCC were downloaded (normal: 50, tumor: 374) in count format.
2.3Differential expression analysis and prediction of small molecule compoundsDifferentially expressed genes (DEGs) in HCC samples (|logFC |>2.0, FDR<0.05) were obtained by differential expression analysis using the R package edgeR, taking gene expression profiles of normal samples as controls [14]. The up-regulated and down-regulated DEGs were imported into the Connectivity Map (CMap) database (https://clue.io/), and HEPG2 and HUH7 were selected regarding the cell type. Pert_type was selected for the perturbation type and trt_cp was selected for the compounds, which were compared with the reference dataset. Based on the enrichment of DEGs in the reference expression profile, small molecule compounds that may have a role in reversing HCC pathology were predicted by correlation analysis.
2.4Protein-protein interaction (PPI) network construction and random walk with restart (RWR) analysisUsing the STRING database (https://string-db.org/), protein interaction analysis of DEGs in HCC was performed to select interaction relationships with confidence scores greater than or equal to 0.7 to construct PPI networks. Cytoscape was employed for the visualization of the PPI network. And ClueGO was employed to classify the above results into modules for the analysis between PPI modules. The drug target gene in the PPI network was seed gene, and RWR analysis of the PPI network was performed using the R package dnet with a set restart probability of 0.85 [15]. The laplacian method was used to normalize the adjacency matrix of the network graph, and the affinity coefficient between each gene and seed was obtained after RWR analysis. We considered the top 50 nodes of affinity coefficient as key genes of Chebulae Fructus acting on HCC and performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses on key genes using the R package clusterProfiler [16]. The interaction network of drug-active component-gene was mapped using cytoscape 3.8.0 software (https://cytoscape.org/release_notes_3_8_0.html).
2.5Cell cultureThe HCC HepG2 cell line (BNCC338070; bnbio, China) was employed in cell experiments of this study. And cells were cultured in Dulbecco's Modified Eagle Medium (Gibco, USA) containing 10% fetal bovine serum at 37 °C with 5% CO2 and 95% humidity.
2.6MTT assayCell viability was detected by employing the MTT assay (Beyotime, China). HCC cells in the logarithmic growth phase were seeded in 96-well plates, and 6 replicate wells were taken from each group. Cells were grown adherent and then replaced, and taken 1-16 μM as a concentration gradient, cells were treated with ellipticine. After 48 hours, 20 μl MTT solution (50 mg/ml) was added to each well. And additional 4 hours later, 150 μl dimethyl sulfoxide (DMSO) was added to each well, and the optical density values (light absorption values) of the cells in each group were measured at a wavelength of 490 nm using a microplate reader.
2.7Ethics approval and consent to participateNot applicable.
3Results3.1Main active constituents and their targets of Chebulae FructusIn order to search the chemical constituents of Chebulae Fructus, its active constituents were queried from TCMSP using Chebulae Fructus as the search term. We collected eight active constituents of Chebulae Fructus (OB≥30%, DL≥0.18) (Table 1). Further, 122 targets of 7 active constituents were obtained through the TCMSP database (Table S1), and only 72 targets remained after deletion of duplications, of which ellipticine and ellagic acid had antitumor activity.
Main active constituents of Chebulae Fructus.
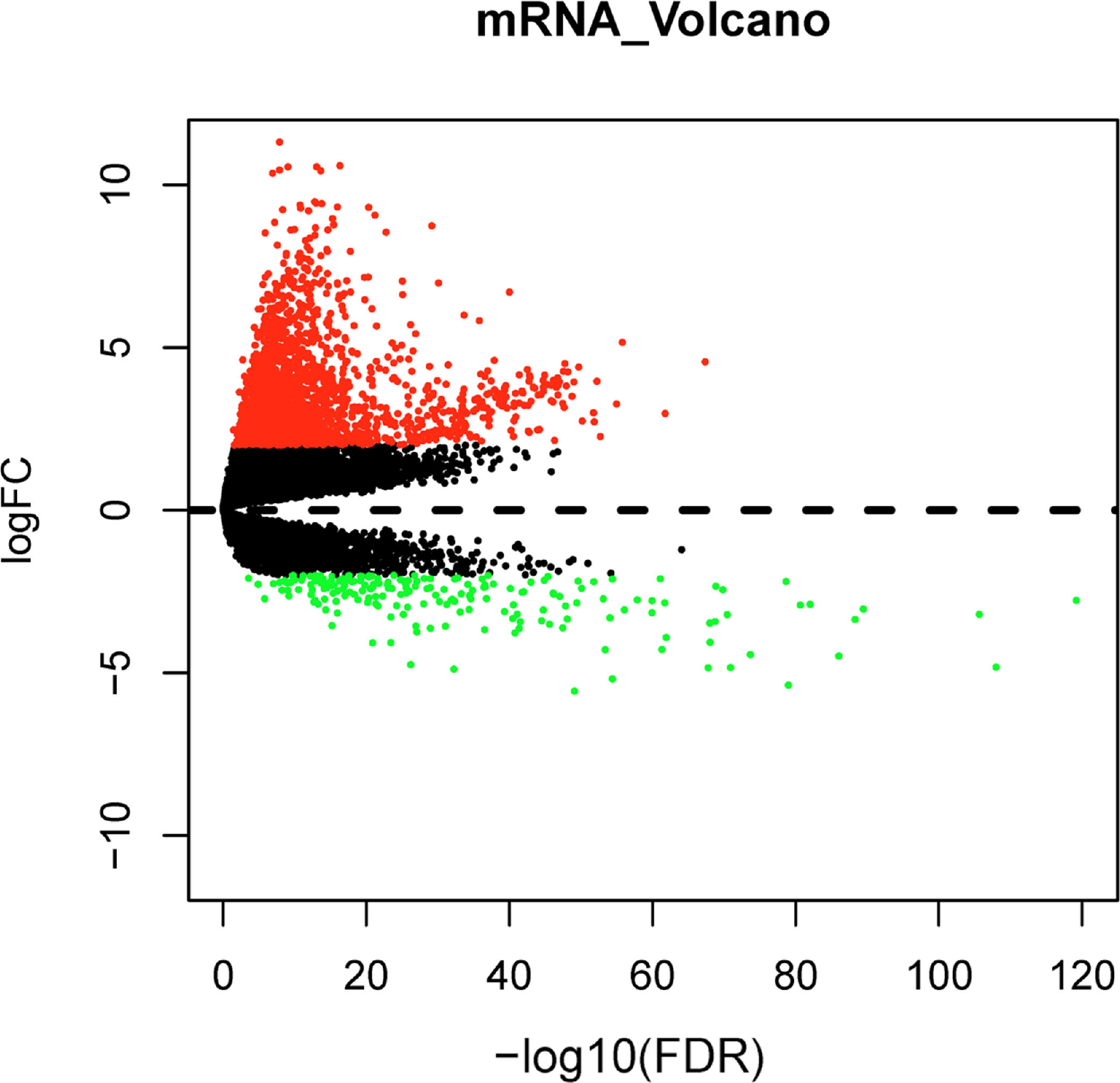
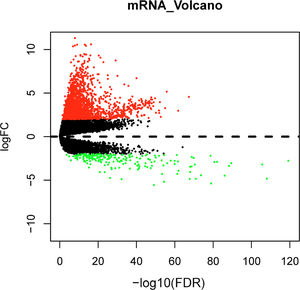
Differential expression analysis between normal and tumor samples in the TCGA-LIHC dataset yielded 1992 DEGs, including 1787 significantly up-regulated DEGs and 205 significantly down-regulated DEGs (Fig. 1). Top150 up-regulated DEGs and down-regulated DEGs were subsequently imported into the CMap database for comparison, and small molecule compounds that may reverse disease pathology were predicted by negative correlation. Among the top 10 small molecule compounds with negative correlations, ellipticine has the potential to reverse HCC pathology (Table 2). This also confirmed that the active constituents of Chebulae Fructus possessed the potential to treat HCC.
Small molecule compounds capable of reversing HCC pathology predicted by CMap.
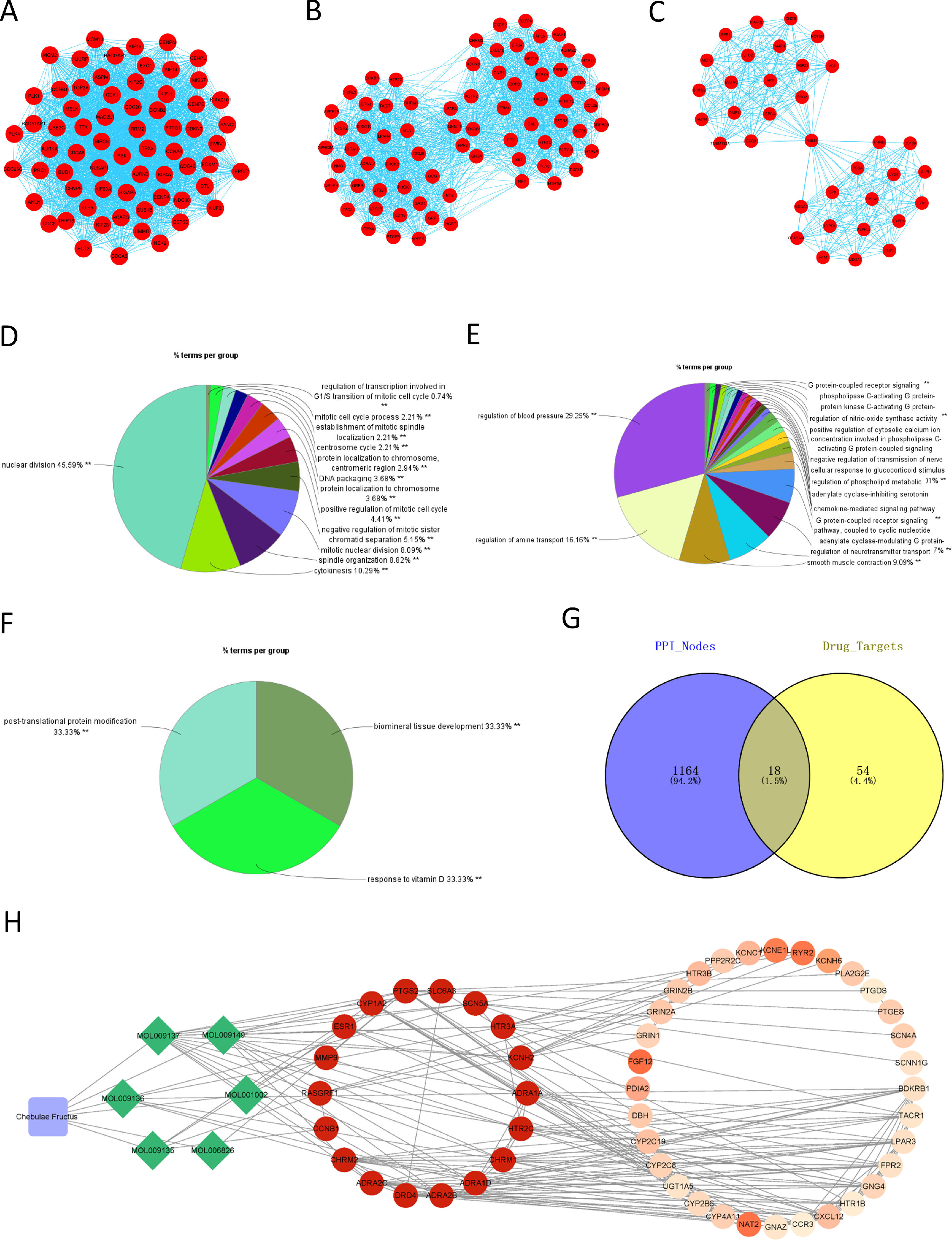
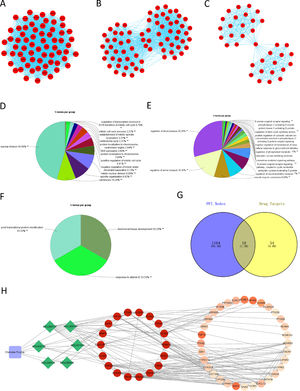
The PPI network of DEGs in HCC was constructed using STRING, with a total of 1182 nodes and 8163 interactions in the network. Subsequently, the PPI network was divided into modules using ClueGO, and the modules with correlation TOP3 were selected for analysis. The results demonstrated the connection of genes in module 1 with mitosis and cell cycle of cells (Fig. 2A, 2D), genes in module 2 with metabolism of phospholipase (Fig. 2B, 2E), and genes in module 3 with protein modification, mineral absorption (Fig. 2C, 2F). Combined with the above analysis, we surmised that Chebulae Fructus might hinder tumor growth with its effect on these biological functions.
Network of Chebulae Fructus and active constituents in the treatment of HCC A: PPI network of DEGs in HCC. B: Venn diagram showing potential targets of Chebulae Fructus for the treatment of HCC. C: Drug-active constituent-gene interaction network. Blue rounded square nodes represent Chebulae Fructus; Green diamond nodes represent the active constituents of Chebulae Fructus; Circular nodes represent genes that may respond to drugs; Gene nodes with direct interaction relationship with green diamonds are potential target genes of Chebulae Fructus for the treatment of HCC; Gene color from deep to light represents affinity coefficients from high to low.
RWR analysis was performed on this PPI network. Genes in the PPI network were intersected with the target genes of active constituents of Chebulae Fructus, and 18 potential target genes were obtained for HCC treatment (Fig. 2G), which were used as seed genes for RWR analysis to obtain the affinity coefficient of each gene node with seed gene. Finally, the genes with affinity coefficient ranking top 50 were selected as the key genes of Chebulae Fructus for HCC treatment, and then we constructed a drug-active constituent-gene interaction network (Table S2,Fig. 2H). Overall, the drug-active constituent-gene interaction network constructed by us revealed the interaction relationships among active constituents, targets o and key genes of Chebulae Fructus.
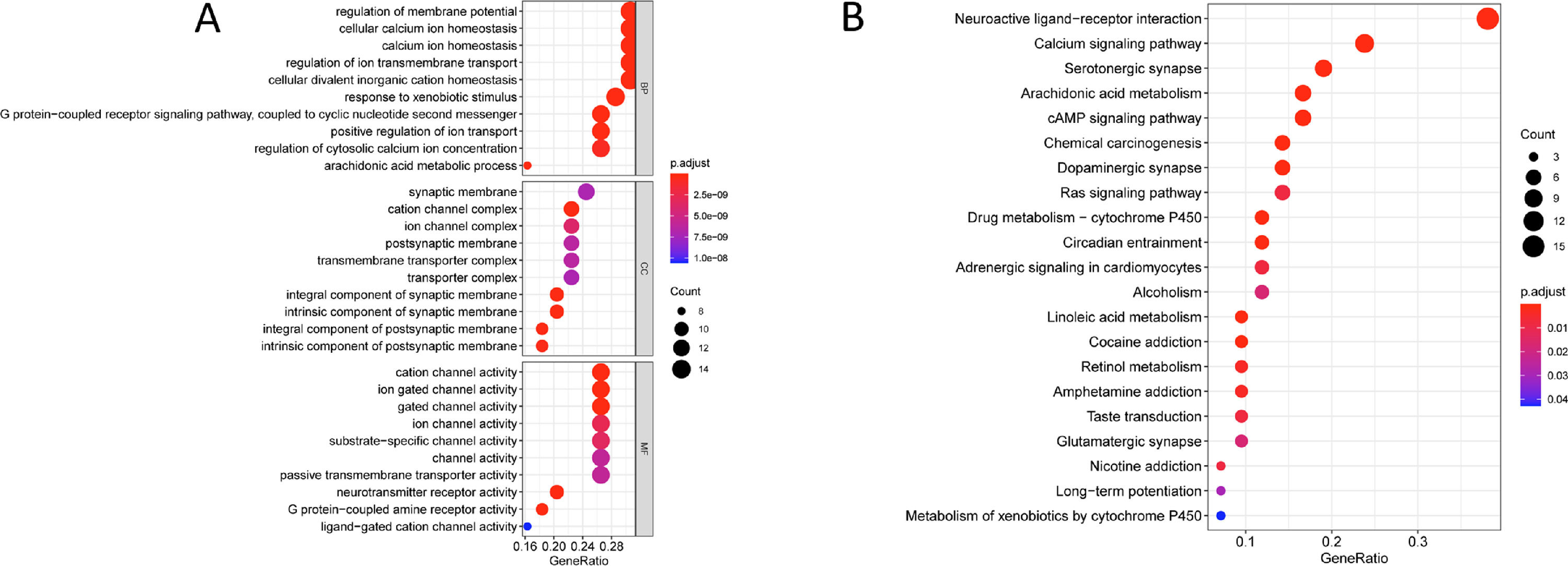
3.4Key signaling pathways of Chebulae Fructus in the treatment of HCCFinally, GO and KEGG enrichment analyses were performed for the key genes of Chebulae Fructus in the treatment of HCC. The results of GO enrichment analysis showed that these genes were mainly enriched in biological functions such as regulation of membrane potential, cellular calcium ion homeostasis, positive regulation of ion, response to xenobiotic stimulus and G protein-coupled receptor signaling pathway, coupled to nucleotide second messenger (Fig. 3A). The results of KEGG enrichment analysis showed that these genes were mainly enriched in signaling pathways such as Neuroactive ligand-receptor interaction, Calcium signaling pathway, Arachidonic acid metabolism, cAMP signaling pathway and Ras signaling pathway (Fig. 3B).
Key signaling pathway of Chebulae Fructus for HCC A: Bubble diagram of GO enrichment analysis of key genes of Chebulae Fructus acting on HCC. B: Bubble diagram of KEGG enrichment analysis of key genes of Chebulae Fructus acting on HCC. The bubble size is the number of enriched genes in the term, and the color indicates the p-value, the darker the color, the smaller the p-value.
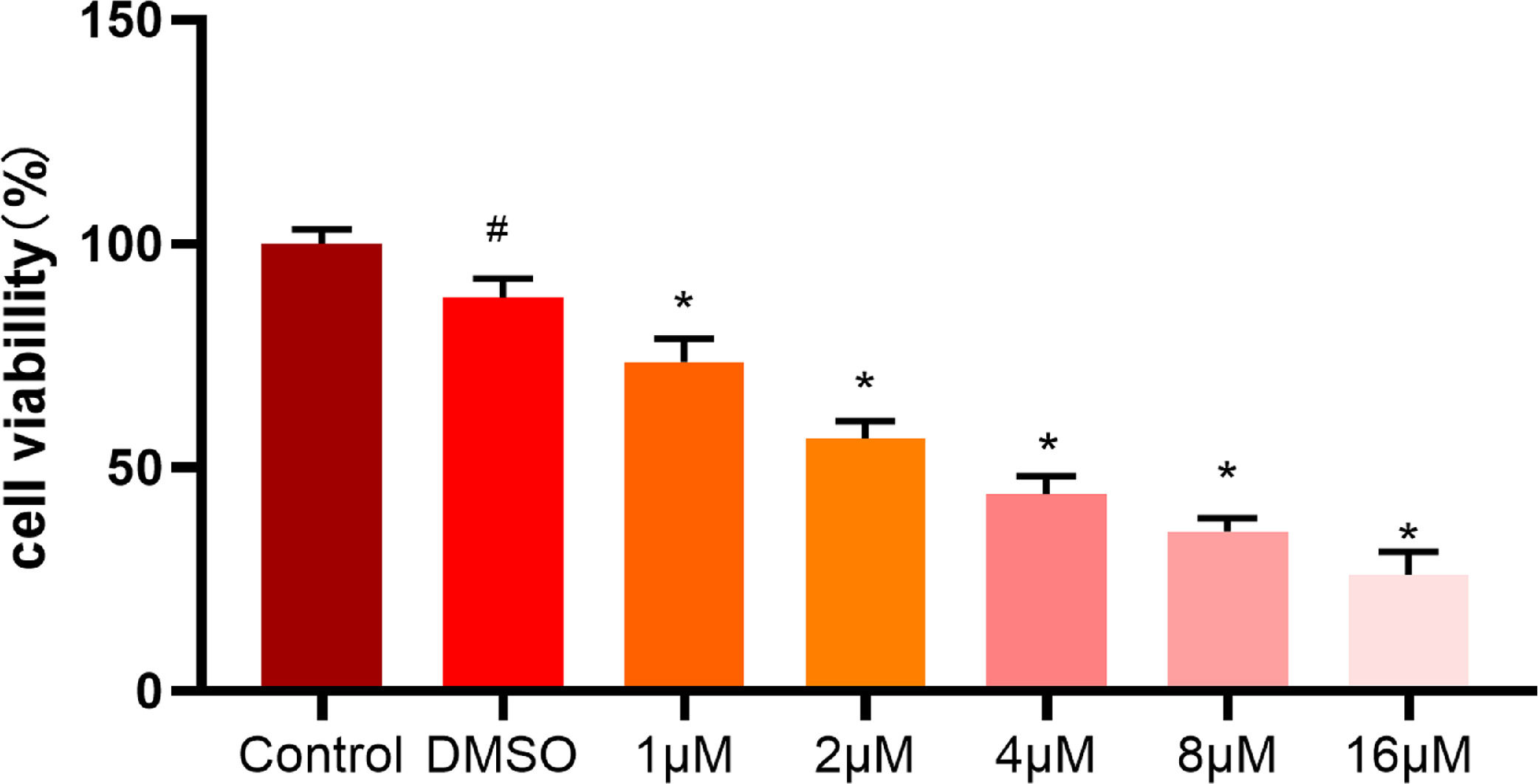
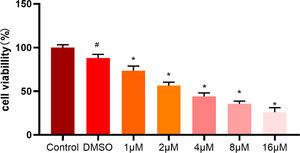
To further validate the effect of ellipticine on HCC cells, different concentrations (0-16 μM) of ellipticine were employed to act on HepG2 cells. After 48 hours, the effect of ellipticine on HepG2 cell viability was then observed. MTT results demonstrated (Fig. 4) that ellipticine at different concentrations had varying degrees of inhibitory effect, and with the increase of ellipticine concentration, the inhibitory effect on the cell viability of HCC cells was more significant.
MTT assay analyzes the viability of HCC cells MTT assay was employed to detect the viability of HCC cells treated with different concentrations of ellipticine, where # represents significant difference between control and DMSO groups, and * represents significant difference between ellipticine groups at different concentrations and DMSO groups (p<0.05).
In China, liver cancer is the third most common cancer. The incidence of liver cancer in males is about 2 to 3 times that in females. Before 60 years old, liver cancer is the most commonly diagnosed cancer type and the most important cause of cancer death in males [2]. In this study, we mined the active constituents of Chebulae Fructus based on TCMSP database and found that ellipticine and ellagic acid were respectively one of the eight effective chemical constituents. Ellipticine is favored by medical researchers because of its high anticancer efficiency, limited toxic side effects, and limited intrinsic toxicity [17]. Existing studies have found that ellipticine shows strong anti-cancer activity in the second stage of cancer such as breast cancer [18], HCC [19] and non-small cell lung cancer [20]. In addition, Guo and colleagues found that ellipticine initiates the mitochondrial apoptotic pathway by regulating Bcl-2 family protein expression (Bak, Bax, and Bcl-XS), altering mitochondrial membrane potential (DeltaPsim) and activating caspase-9 and caspase-3, which ultimately induces apoptosis in hepatoma cells [19]. Cyclin-Dependent Kinase 6 (CDK6) plays an important role in tumor progression, and Yousuf and colleagues found that ellagic acid has the highest binding affinity with CDK6, which is an effective inhibitor of CDK6 and is expected to be a new drug for cancer therapy [21]. In addition, we found that ellipticine could reverse the pathological conditions of HCC through the CMap database using the DEGs of HCC. We therefore reasoned that Chebulae Fructus may have great potential in the clinical treatment of HCC.
From TCMSP database, several potential therapeutic targets of Ellipticine for the treatment of HCC were obtained, namely, PTGS2, CYP1A2, CCNB1 and RASGRF1. Ellipticine Potential therapeutic targets. PTGS2 also known as COX-2, is found to be expressed in a variety of cancers, is closely related to tumor cell anti-apoptosis, proliferation, angiogenesis, inflammation, invasion and metastasis, and plays a multifaceted role in promoting tumorigenesis as well as cancer cell tolerance to chemotherapy and radiotherapy [22]. Chen et al.[23] also found that the inhibition of PTGS3 could regulate the HCC cell apoptosis mediated by PINK1-PRKN. CYP1A2 promoter region polymorphisms have been associated with cancer susceptibility in humans, and there has been evidence that they are associated with lung cancer and bladder cancer susceptibility in Caucasians [24]. While Yu and colleagues found that CYP1A2 inhibits HCC by antagonizing HGF/MET signaling [25]. CCNB1 high expression accelerates the progression of HCC. Gu and his colleagues discovered that knockdown of CCNB1 by RNA interference significantly inhibits the migration, proliferation and invasion of HCC cells [26]. RASGRF1 is closely related to glucose metabolism and has been found that it may be a new oncogenic factor in lung cancer, while in mouse models, RASGRF1 deletion delays aging process in mice [27, 28]. In addition, the RWR analysis yielded key genes closely affected by the target of Chebulae Fructus, which included the adrenaline α family gene ADRA2C, ADRA2B, ADRA1D, ADRA1A. It has been found that adrenal signaling in cellular pathways can promote cancer development [29]. We speculated that the epinephrine signaling pathway was inhibited by influence of effective chemical constituents of Chebulae Fructus, thus hindering the progression of HCC. In this study, the active constituent of Chebulae Fructus, ellipticine, mined by network pharmacology, is closely related to cancer cell proliferation, migration, and invasion. In addition, CYP1A2 was identified as the potential therapeutic target of HCC. These results indicate that the potential therapeutic target of Chebulae Fructus is of great significance to the treatment of HCC.
Finally, we performed enrichment analysis of the key genes involved in the treatment of HCC by Chebulae Fructus, and the results of KEGG enrichment analysis showed that these target genes were mainly involved in the cAMP signaling pathway and Ras signaling pathway. cAMP is one of the first identified and most versatile second messengers, and it is involved in different cellular responses to extracellular signals. cAMP signaling pathway exhibits anticancer effects through PKA independent pathway, which induces mesenchymal to epithelial cell transition, inhibits cancer cell growth and migration, and enhances body sensitivity to traditional anti-tumor drugs [30]. The RAS is the driver of the second largest mutated gene in human cancer, and its function is to switch the extracellular environment into an intracellular signal transduction cascade. RAS mutant proteins regulate tumor cell proliferation, apoptosis, metabolism, and angiogenesis through downstream MAPK, PI3K, and other signaling pathways [31]. These two signaling pathways play an important role in targeted cancer therapy. Overall, the targets we mined may inhibit HCC cell metastasis and invasion through cAMP signaling pathway and RAS signaling pathway under the induction of major components of Chebulae Fructus.
However, this study has two shortcomings. First, it is a bioinformatics-based study. Although wet experiments were introduced, contents related to it were inadequate. And other experiments are still needed to verify the effect of Chebulae Fructus on HCC later. Secondly, several active ingredients and potential targets of Chebulae Fructus were obtained by using network pharmacology screening, but only the efficacy of ellipticine was validated in this study. And the effects of other ingredients on HCC cells are not yet known. It is still of great necessity to verify the mechanism of Chebulae Fructus in the treatment of HCC in combination with cell experiments and animal experiments.
5ConclusionsIn this study, we employed network pharmacology to reveal the validation active constituents of Chebulae Fructus as well as the potential targets of the treatment of HCC were mined successfully. And the key genes for treating HCC were also obtained through PPI network and RWR analysis. Then a drug-active constituent-gene interaction network was constructed. Herein, the mechanism of Chebulae Fructus on HCC was only in the early phases of exploration, and there are few studies on the application of Chebulae Fructus in HCC treatment. Besides, the anticancer mechanism of the active ingredients in Chebulae Fructus remains to be further discovered. Our study found that ellipticine had a significant inhibitory effect on the viability of HCC cells. However, since ellipticine has certain toxic side effects, the construction of ellipticine derivatives will be better employed for cancer therapy if ellipticine is used as a lead compound. Based on the conclusions drawn from our study, Chebulae Fructus has the potential to be used as an adjuvant therapy for HCC.
Availability of data and materialsThe data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
FundingThis work was supported by Jiaxing Key Laboratory of Oncology Radiotherapy (2021-zlzdsys), 2019 Jiaxing Key Discipline of Medicine-Oncology (Supporting Subject) (2019-zc-11).
Authors' contributionsJLJ and ZPY contributed to the study design. GXH conducted the literature search. XMY acquired the data. JJ wrote the article. JLJ performed data analysis and drafted. ZPY revised the article. All the authors gave the final approval of the version to be submitted.
Declarations of interestNone