Introduction and aim. Transplant recipients are chronically ill patients who rely on medical treatment throughout life to achieve positive results. Despite that, medication nonadherence after liver transplantation is extremely common. The self-report, one of several methods for measuring adherence, is easy to apply and low cost. Thus, this study aims to translate and validate the Immunosuppressant Therapy Adherence Instrument (ITAS) in Brazilian Portuguese for liver transplant recipients.
Material and methods. A total of 139 liver transplant recipients were selected from a general hospital, who were assessed by using the Portuguese version of ITAS. The scale was translated based on the model proposed by Wild, et al. and its psychometric properties were assessed.
Results. The average Cronbach’s a coefficient was 0.830. ITAS and Basel Assessment of Adherence with Immunosuppressive Medications Scale (BAASIS) presented significant correlation, with a Spearman’s p coefficient = 0.300 (S = 309,580; p < 0.001). The area under the receiver operating characteristics (ROC) curve was 0.638 (95% CI: 0.557 – 0.715). Factor analysis results indicated that the carelessness factor model was the optimal model, and the factor “feeling worse” was the lowest.
Conclusion. The Portuguese version of ITAS has adequate psychometric properties to measure adherence to immunosuppressant therapy.
Adherence to a medical regimen is defined as the extent to which the patient’ behavior coincides with the clinical prescriptions.1 Among the greatest challenges to the success of transplants is to ensure regular adherence of immunosuppressive drugs. This is essential for the proper functioning of the graft.2
Immunosuppressant therapy nonadherence after liver transplantation is reported in 72.9% subjects who took less than 100% of the prescribed doses, tracked with electronic monitoring.3 Therefore, almost half of transplant recipients have some non-adherent behavior, such as not using the medication regularly, nor taking the correct dose, nor the required timescales.4,5 Despite the clinician’s efforts to inform patients about the importance of immunosuppression to the maintenance of the graft, to avoid its rejection and other clinical outcomes negative, such consequences often occur.6 In addition, nonadherence generates significant socioeconomic impacts on the health systems.7
There are several methods for measuring adherence. One of them, the self-report can measure adherence easily and with very low cost, that being the most employed method in the clinical setting and research of medication nonadherence.8,9 A validated self-report instrument is recommended for investigation of adherence behavior and can predict clinical outcomes. There is no gold standard to measuring adherence to immunosuppressive drugs,10 so other objective methods and clinical outcomes can be used for correlation.11 Adherence should be evaluated in the long term and strengthened through therapeutic strategies such as systematic education that may contribute to the adherence of medications.12
Brazil is the second country in the world in terms of numbers of liver transplantations.13 Despite this fact there is no validated specific instrument to measure immunosuppressant therapy adherence for liver transplantations in Brazil. The aim of this study was to translate and assess the validation of the Immuno- suppressant Therapy Adherence Instrument (ITAS) to Brazilian Portuguese for patients submitted to liver transplantations.
Material and MethodsDesign, sample and settingThis psychometric study was conducted in a general hospital (Hospital Português da Bahia) and in a Teaching Hospital (Universidade Federal da Bahia). Patients were recruited between September 01, 2014 and June 20, 2015. The assessments were applied to the participants who agreed to sign an informed consent form. The general sociodemographic survey was administered to all participants (n = 139). Patients were included in the study if they met the following criteria: received a liver transplant, able to understand the Portuguese language, and 18 years or older at the time of the study. Patients unable to read (illiteracy) and those who were submitted to retransplantation were excluded from the sample.
Demographic characteristicsAge, gender, marital status, and time post-transplant were assessed.
Variables and measurementThe ITAS is a self-report measure of immunosuppressant therapy adherence targeted to solid-organ transplant recipients, developed to be a reliable measure of adherence to immunosuppressant therapy in the three months prior to when research is conducted. The four items assess the behaviors of forgetfulness, carelessness, neglect and cessation due to feeling worse. Responses are designed for the patient to choose each behavior’s frequency, in order to minimize patients’ providing a positive adherence response of “yes”. Response option levels are: 0 % of the time, 1–20 %’, 21–50% and greater than 50%. Raw scores can range from 0 (greater than 50% for all items), indicating very poor adherence, to 12 (0% for all items), indicating perfect adherence. Scores below 80% indicate poor adhesion.14
A psychometric re-evaluation of the ITAS was performed and two theoretically linked psychosocial constructs were selected to design the construct validity analysis: social support and resilience. The results demon-strated the ITAS statistical relationships with these constructs and confirmation that the ITAS is a valid and reliable measure of IST adherence.15
The Brazilian Portuguese version of the Basel Assessment of Adherence with Immunosuppressive Medications Scale (BAASIS), validated in kidney transplant patients was used as a standard for comparison. The BAASIS is a self-report instrument for measuring nonadherence (NA) in transplantations, that measures: taking adherence, drug holidays, timing adherence, and dose reduction in a four-week period. Responses are given a six-point scale: Never (0), once per month (1), every second week (2), every week (3), more than once per week (4), and every day (5).16
TranslationThe ITAS was translated as according to the method proposed by Wild, et al.17 The original questionnaire was translated independently by two fluent English speakers. This process resulted in two preliminary versions. A consensus among both translators resulted in a reconciled version. Next, a reverse translation from Portuguese to English was conducted.
The final version (Table 1) was applied to 30 liver transplant patients, who were asked about their understanding of the instrument.
The final version of the ITAS scale in Brazilian Portuguese.
| ESCALA DE ADESÃO À TERAPIA IMUNOSSUPRESSORA (ITAS). | ||||
|---|---|---|---|---|
| Circule a letra da resposta que melhor estima a porcentagem de tempo descrita em cada uma das 4 questões. | ||||
| 0% (nenhuma) | 1–20% | 21–50% | Mais de 50% (Muito frequentemente) | |
| 1. Nos últimos 3 meses, com que frequência você esqueceu de tomar seu(s) medicamento(s) imunossupressor(es)? | A | B | C | D |
| 2. Nos últimos 3 meses, com que frequência você foi descuidado ao tomar seu(s) medicamento(s) imunossupressor(es)? | A | B | C | D |
| 3. Nos últimos 3 meses, com que frequência você parou de tomar seu(s) medicamento(s) imunossupressor(es) porque se sentiu pior? | A | B | C | D |
| 4. Nos últimos 3 meses, com que frequência você deixou de tomar seu(s) medicamento(s) imunossupressor(es) por qualquer razão? | A | B | C | D |
Legenda: 3para “0% (nenhuma frequência) do tempo”; 2 para “1%–20% do tempo”; 1 para “21–50% do tempo”; 0 para “mais de 50% do tempo”. Pontuação: Alta - baixa; sendo 0 baixa e 12 alta.
Items were coded as 0, 1, 2, and 3 according to the Likert scale responses of “greater than 50%”, “21-50%”, “1-20%”, and “0%”, respectively.14 Since ITAS and BAASIS present opposite punctuation directions, BAASIS raw score was inverted before analysis.
Cronbrach’s α based on a polychoric correlation matrix was calculated to assess internal validity. Polychoric based α is considered to be more reliable in ordinal structured data.18
Convergent validity was assessed with Spearman’s ρ correlation coefficient between ITAS and BAASIS (previously validated). ITAS accuracy considering BAASIS classification as a gold standard was evaluated by logistic regression. Individuals were labeled non-adherent if BAA-SIS items presented any answer different from “never”.19,20
The Area Under the Receiver Operating Characteristic (AUROC) curve was calculated with respective confidence intervals estimated using bootstrap resampling. An optimal cut-point was determined using Youden criterion and used to determine accuracy, sensitivity, specificity, positive and negative predictive values.
Maximum-likelihood exploratory factor analysis with Varimax rotation was performed to analyze the optimal number of latent factors and to investigate factor loadings related to each item.
Analysis was performed using R programming language and environment.21
EthicThis study was approved by the local Institutional Review Board (MCO-UFBA - process number 14/2002) and was carried out in accordance to Declaration of Helsinki (version dated 2013). The researchers ensured that the documents would be kept confidential.
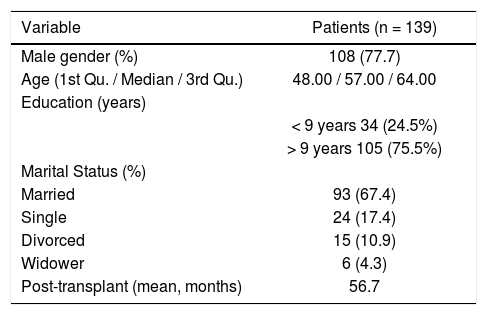
ResultsSample characteristicsVisual inspection (histogram) and normality tests (Shapiro-Wilk; p <0.001) suggested non-normality of the data. Descriptive analysis on the overall sample (n = 139) revealed that the majority of the participants were male (11%). The median age was 55.00 (Interquartile range [IQR]: 46.00–61.00). The participants were predominantly married (61.4%). The average time between transplant and collection was approximately 56.1 months IQR 30,00– 19,00) (Table 2).
Socio-demographic characteristics of liver transplant recipients who were assessed by using the Portuguese version of ITAS.
| Variable | Patients (n = 139) |
|---|---|
| Male gender (%) | 108 (77.7) |
| Age (1st Qu. / Median / 3rd Qu.) | 48.00 / 57.00 / 64.00 |
| Education (years) | |
| < 9 years 34 (24.5%) | |
| > 9 years 105 (75.5%) | |
| Marital Status (%) | |
| Married | 93 (67.4) |
| Single | 24 (17.4) |
| Divorced | 15 (10.9) |
| Widower | 6 (4.3) |
| Post-transplant (mean, months) | 56.7 |
ITAS: Immunosuppressant Therapy Adherence Instrument. SD: Standard deviation.
Internal consistency measured by polychoric Cronbach’s alpha coefficient value was high (a = 0.830; Standardized a = 0.800).
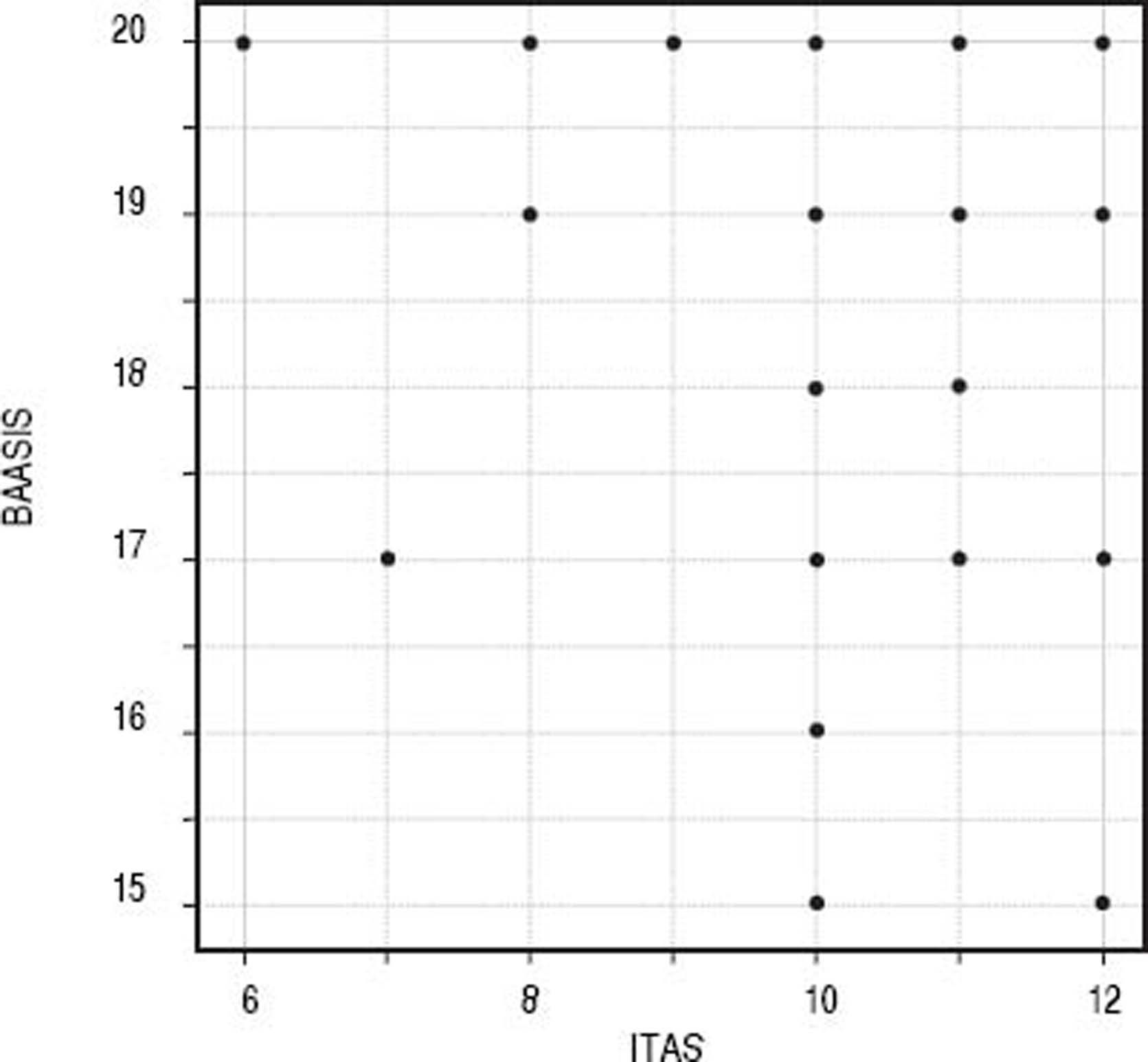
Convergent validityITAS and BAASIS (inverted) presented significant correlation, with a Spearman’s coefficient = 0.302 (S = 312.500; p < 0.001) (Figure 1).
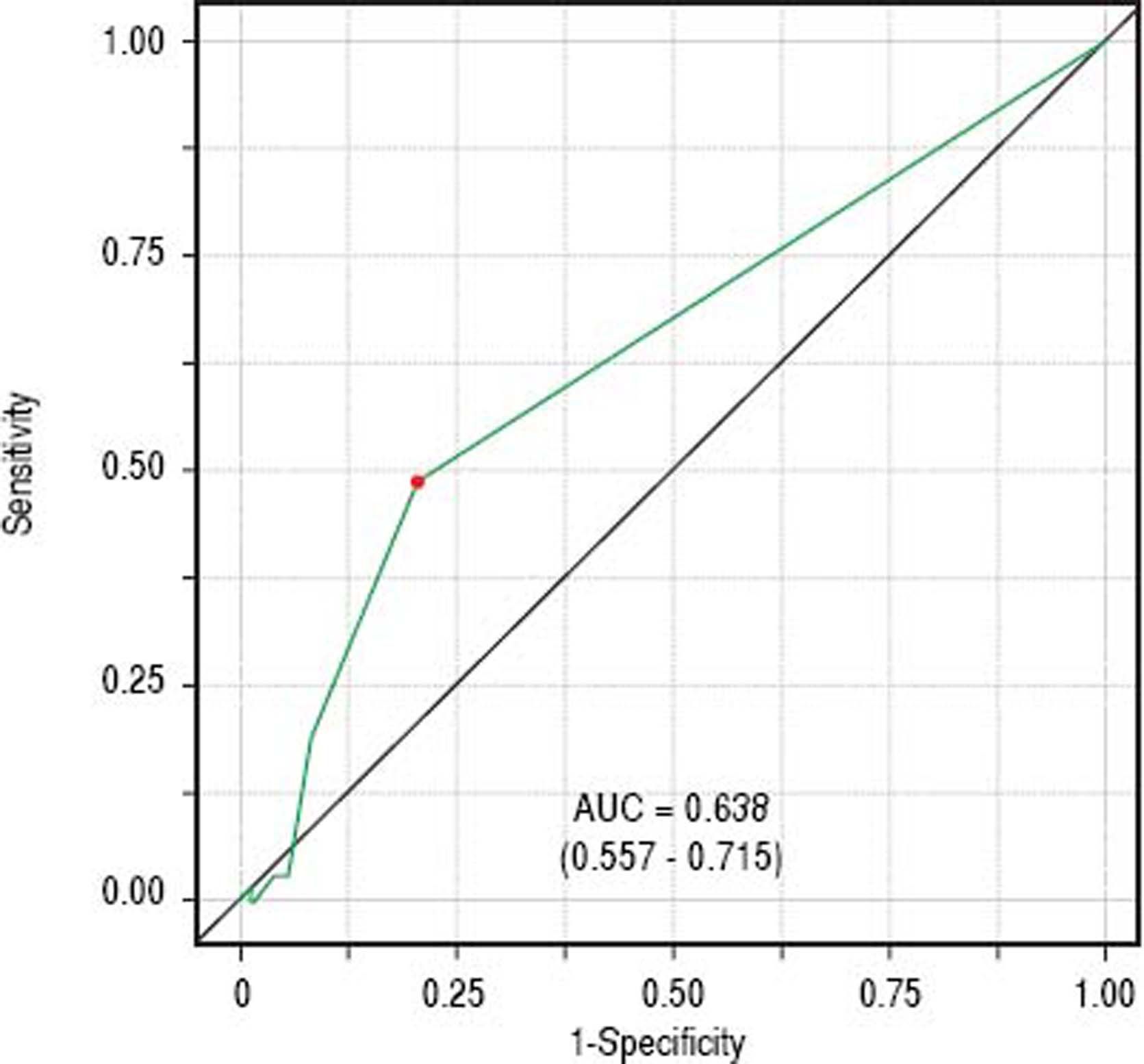
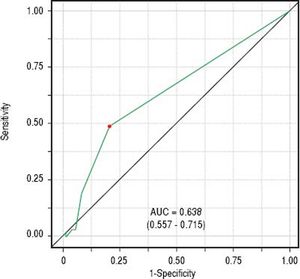
Classificatory performance and accuracy measuresITAS discriminatory performance considering BAASIS classification as the outcome can be seen in figure 2 (AU-ROC = 0.638; 95% CI: 0.557 – 0.715). Reporting at least one negative response was the optimal cut-point (accuracy = 0.647; sensitivity = 0.492; specificity = 0.792; positive predictive value = 0.688; negative predictive value = 0.626).
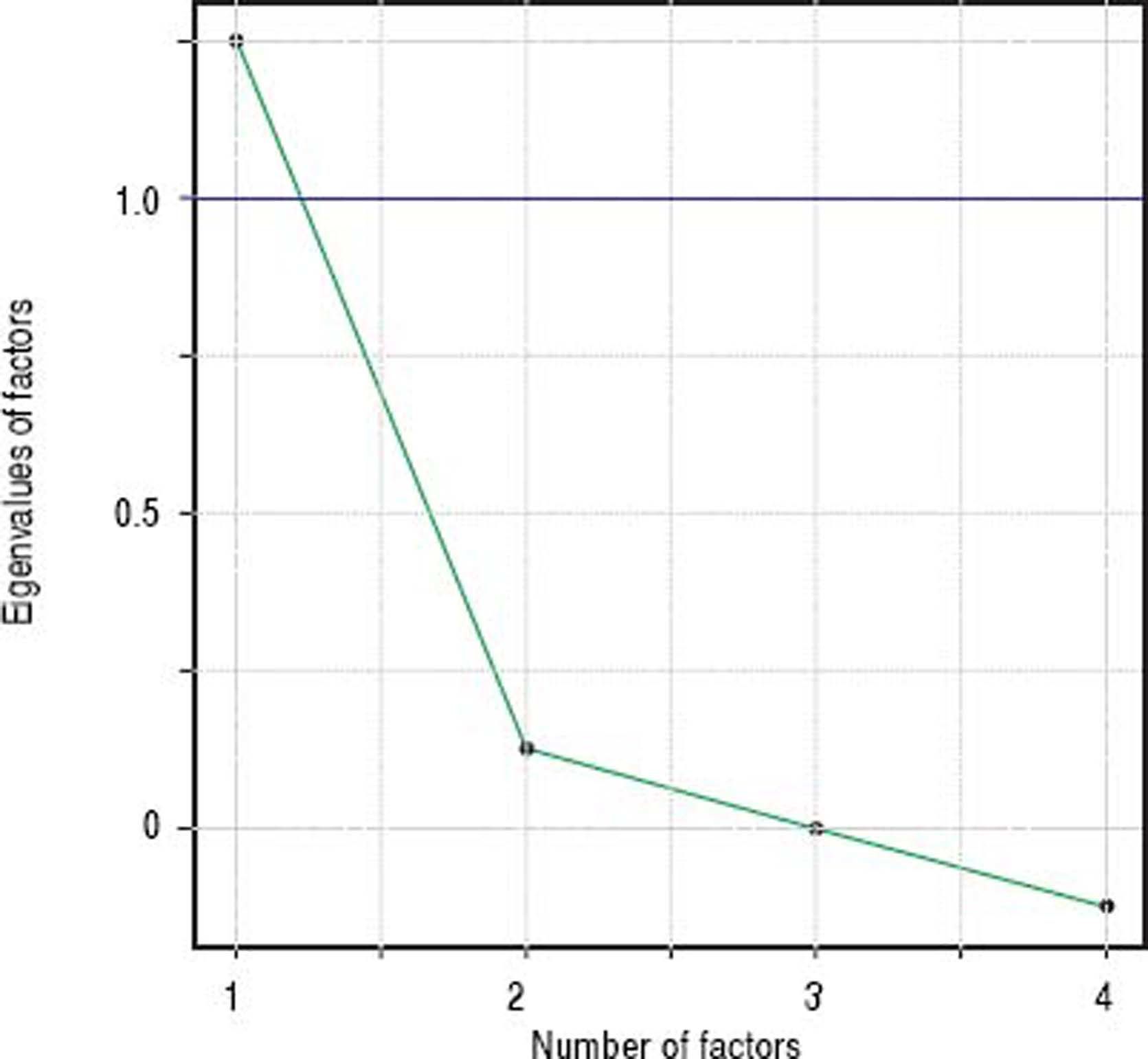
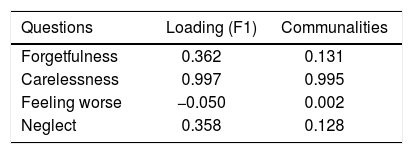
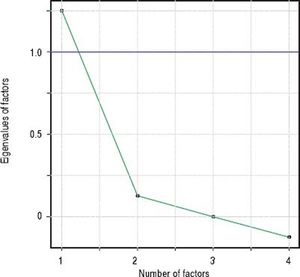
Factor analysisA single factor model was adequate to ITAS test data (χ2 = 2.77; df = 2; p = 0.250). Carelessness presented higher loading (0.997), followed by Forgetfulness (0.362) and Neglect (0.358), in table 3. Factor analysis data is shown in table 4 and the scree plot with eigenvalues for different number of factors is displayed in figure 3.
The ITAS contributes as a valid instrument for immunosuppressant medication adherence in solid organ transplants22 and several studies use the ITAS as an adherence measure.22–25 The aim of this study was to translate the ITAS to Brazilian Portuguese and to evaluate its psychometric properties in adult liver transplant recipients. The version of the ITAS - scale Brazilian Portuguese facilitates the measurement of immunosuppressant adherence in transplant patients, and reduces negative outcomes for example graft loss and death.
The ITAS is an instrument easy to apply that takes no longer than 5–10 min to complete. It is relatively inexpensive, simple, and can be conducted rapidly when compared with other methods of adherence assessment. In this study, we included patients from various parts of Brazil who had their transplants in the state of Bahia, therefore our study sample reflects a wider scale in Brazil. The answers (in percentage ranges) of this version were maintained to preserve continuality, but may present difficulties to patients presenting cognitive deficits, poor educational levels and low social support.22
Internal consistency provides an estimate of the equivalence of items from the same scale, and values between 0.70 and 0.95 are considered to be acceptable.26,27 Our Brazilian Portuguese version of the scale presented good internal consistency and was similar to previously published studies - Cronbach’s alpha = 0.81.15 Items within the scale were correlated as expected.
Factor analysis solution with a single factor was adequate, indicating higher loading values for Carelessness (0.997), Forgetfulness (0.362), and Neglect (0.358). Feeling Worse (item 3) factor loading was close to zero (− 0.050), since almost all patients included in the sample (98.6%) answered this item with option A: 0% (none). This behavior was not observed in the original ITAS validating studies14 and might be due to regional differences. This hypothesis can be verified in further studies replicating the experiments in other regions.
Concerning convergent validity, our findings indicate that the translated ITAS correlates well with the translation of the BAASIS scale, an instrument validated in Brazil.16 AUROC value of 0.5 should be considered a minimum.28 Therefore, our results (AUROC = 0.638) indicate satisfactory discrimination for adherence.
The result of the psychometric properties analysis suggest that the Portuguese translated version of ITAS in Brazil is a psychometric scale internally consistent, with good convergent validity with BAASISa. These findings need to be replicated in further studies. Altogether, these results require confirmation in larger samples with regional variance.
This study has some limitations. The sample may be subject to a bias recruitment due to convenience sampling, because the participants included in the study were those who attended routine consultations. Non-adherent patients may be more prone to miss consultations and, therefore, to not be included in the survey. Our sample covers only liver transplant patients and the results cannot be generalized to other types of transplants.
In conclusion, the ITAS instrument was successfully translated and an analysis of the data confirmed its consistency and convergent validity with a validated tool. The translation and validation of the ITAS instrument contributes to the applicability and relevance of the instrument for the Brazilian population.
Abbreviations- •
AUROC: Area Under the Receiver Operating Characteristic.
- •
BAASIS: Basel Assessment of Adherence with Immunosuppressive Medications Scale.
- •
ITAS: Immunosuppressant Therapy Adherence Instrument.
This project was partially supported by the National Council of Technological and Scientific Development (CNPq): 462014/2014-2 - Edital Universal MCT/CNPQ 2014. Another part of the funding (totaling 100% with the funding above) was supported by the scholarships from the Coordination for the Improvement of Higher Level Personnel (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of InterestThe authors declare no conflicts of interest.
AcknowledgementsThe authors thank all the patients who agreed to be included in this study for their cooperation. We are also grateful to Raquel Roberts for proofreading, José Edson Oliveira Filho, Carolina Arruda and Maria Isabel Teles.