Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD) that can progress to liver cirrhosis, liver failure and hepatocellular carcinoma. It is the second leading cause of liver transplant in the US. We aim to investigate the prevalence, demographics and risk factors NASH patients in the US.
Patients and methodsWe used a large database (Explorys IBM) that aggregates electronic health records from 26 nationwide healthcare systems. We identified adults with NASH between 2010-2020. Demographics including age, gender and race were collected. NASH risk factors including Diabetes Millets (DM), Hyperlipidemia (HLD), Hypertension (HTN) and Obesity were also collected. Cochran-Armitage test was used to assess the statistical significance of year-by-year trend. Univariable and multivariable logistic regression were used to estimate the odds ratio (OR) of risk factors.
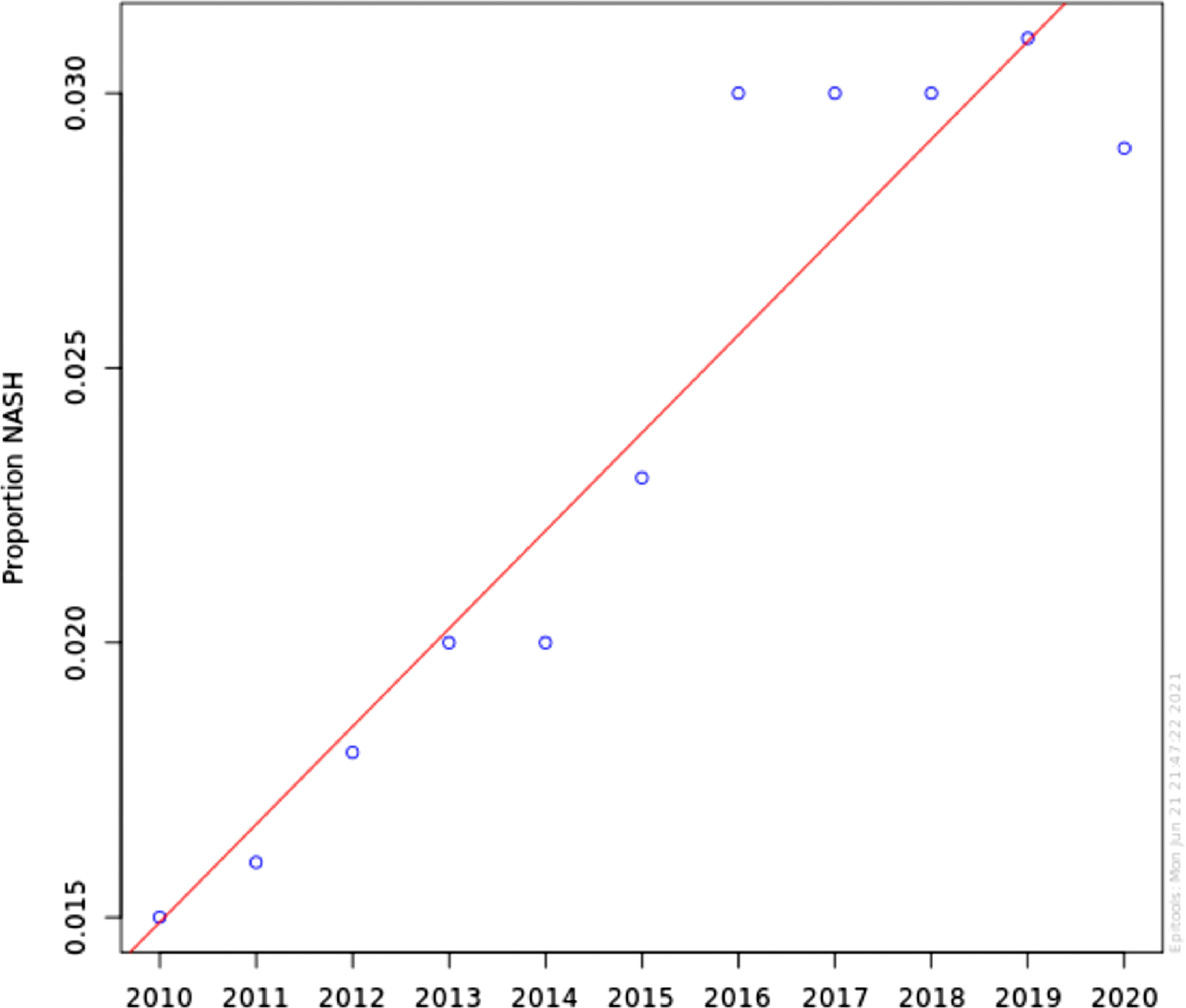
ResultsNASH annual prevalence rate increased from 1.51% in 2010 to 2.79% in 2020 (p < 0.0001). The proportion of patients with NASH by gender was 54.1% female vs 45.9% male (OR 1.04 [0.91-1.11]). Caucasian had higher odds of NASH than non-Caucasian (OR 1.42 [1.31-1.54]). NASH is strongly associated with DM and obesity (OR 18.61 [17.35-19.94]) and (OR 20.97 [17.87-23.21]), respectively. Other components of metabolic syndrome were associated with NASH to a lesser degree; HTN (OR 3.24 [3.20-3.28]) and HLD (OR 4.93 [4.85-4.01]).
ConclusionThe prevalence of NASH has significantly increased in the US in the last decade. This is likely related to the increased prevalence of risk factors as well as increased awareness of the disease.
Non-Alcoholic Fatty Liver Disease (NAFLD) is characterized histologically into Non-Alcoholic Fatty Liver (NAFL) and Non-Alcoholic Steatohepatitis (NASH). NASH patients are at an increased risk of advanced fibrosis and cirrhosis [1]. It is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States [2,3]. Moreover, NASH imposes a significant economic burden in the US. In 2017, the lifetime costs of NASH patients in the US were estimated at $222.6 billion, and the cost of the advanced NASH population is estimated at $95.4 billion [4]. The gold standard for the diagnosis of NASH remains a liver biopsy. NASH is defined as the presence of >4% hepatic steatosis and inflammation with hepatocyte injury (ballooning), with or without fibrosis [5]. Some studies have indirectly assessed the prevalence of NASH. The prevalence of NASH among NAFLD patients who underwent a random liver biopsy was 6.67%. The prevalence of NASH among NAFLD patients with a clinical indication for a liver biopsy was 59.10%. Given these estimates, the average prevalence of NASH was estimated to be between 1.5% and 6.45% [6].
Since liver biopsy is not feasible in the general population, there are no studies that can accurately assess the incidence or prevalence of NASH.
Concomitant metabolic syndrome further increases the risk of steatohepatitis and fibrosis. To underscore the impact of metabolic syndrome, more recently, a consensus of international experts proposed changing the name of NAFLD to metabolic (dysfunction)-associated fatty liver disease (MAFLD) [7]. However, the use of the term MAFLD would not include the NASH population, who are at the highest risk of progression. While no medications are currently approved to treat NAFLD, once NASH is diagnosed, the American Association for Study of Liver Diseases (AASLD) updated guidelines recommend treatment with vitamin E for patients without diabetes mellitus and pioglitazone in patients with NASH and diabetes [8]. Therefore, it is essential to determine the prevalence of NASH in the general population and evaluate its association with metabolic syndrome.
Previously, since imaging modalities were not yet readily available in the United States, the NAFLD Fibrosis score was the only tool to assess fibrosis. However, ultrasound-based elastography, magnetic resonance elastography, and the fibrosis-4 score are other screening tools now available for fibrosis staging. Currently, all major international societies recommend against systematic screening in the general population [1,9-11]. ([1], European Association for the Study of the Liver [9] 2016 Jun, [10,11]) In our study, we aim to assess the prevalence of NASH over the last decade in a large cohort of patients derived from multiple institutions in the United States. We also aim to quantify the association between metabolic risk factors and NASH to guide fibrosis screening efforts in high-risk NAFLD patients.
2Materials and methods2.1Study designThis is a retrospective analysis of a large electronic health record (EHR)-based commercial database called Explorys (IBM). Explorys platform aggregates electronic health records from 26 nationwide major healthcare systems spread over 50 states in the US. It stores over 60 million unique patient records. Patient information is then de-identified, standardized, and stored in a cloud database [12]. The Explorys platform uses systemized nomenclature of clinical medical terms (SNOMED-CT) for medical terms, diagnoses, and procedures. For diagnoses, International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes are mapped into the SNOMED-CT hierarchy [12]. This platform is Health Insurance Portability and Accountability Act (HIPAA) and Health Information Technology for Economic and Clinical Health Act (HITECH) compliant. The Cleveland Clinic Institutional Review Board deemed studies using Explorys as the recorded dataset, exempt from approval because all patient information is de-identified. Explorys protects patient confidentiality by approximating each population count to 10. All counts between 0 and 10 are treated equally.
2.2Patient selectionAdult subjects (age above 18 years) with active electronic health records since 1999 were identified using the search tool in Explorys. Using the SNOMED-CT diagnosis “Non-alcoholic steatohepatitis,” we identified patients diagnosed with NASH each year between 2010 and 2020. Patients with the diagnosis of “fatty liver disease,” “alcoholic hepatitis,” and “alcoholic fatty liver disease” were excluded. We collected cross-sectional information on patient demographics such as gender, race, and comorbidities known to be associated with NASH, such as hypertension (HTN), diabetes (DM), obesity (defined as BMI >30), and hyperlipidemia (HLD) by searching the database for their respective SNOMED-CT terms.
2.3Statistical analysisThe prevalence is calculated on a year-by-year basis by determining the proportion of patients with NASH in their respective years. To assess the statistical significance of the year-by-year trend, we used the Cochran-Armitage test. Categorical variables were presented as numbers and percentages and were compared using the Pearson χ2 test. Odds ratios are presented with 95% confidence intervals (CI). A univariate binary logistic model was constructed using NASH as the dependent variable and other variables as independent variables. A multivariable model adjusting for all covariates mentioned in univariate variables was added to adjust for possible confounding. All statistical analysis was done using R version 3.6.3 (The R Foundation). Significance was defined as the 2-tailed value of P < 0.05.
2.4Regulatory approvalsThis study was exempt from approval by the Cleveland Clinic Institutional Review Board as the dataset obtained from the Explorys platform is de-identified.
3ResultsThe total population in the Explorys cohort is determined each year between 2010 and 2020. It has increased from around 10 million in 2010 to about 62 million in 2020, representing a growing number of health centers using electronic medical records databases incorporated into Explorys universal database. The number of patients diagnosed with NASH increased from 151,200 in 2010 to 1,867,000 in 2020. Table 1 summarizes the demographic characteristics of the cohort and NASH risk factors.
Demographics and risk factors of NASH in United States 2010-2020
The annual prevalence rate has significantly increased from 1.51% in 2010 to 2.79% in 2020 (chi-square statistic 441441.19, P-value < 0.0001) (Figure 1). The proportion of patients with NASH by gender was consistent during this time period; approximately 54.1% Female vs 45.9% Male. Using Multivariable logistic regression model, this difference was not found statistically significant (OR 1.04 [0.91-1.11]) Table 2. Caucasian were found to have higher odds of having NASH in comparison with non-Caucasian (OR 1.42 [1.31-1.54]) Table 2. Looking at risk factors of NASH, as expected, NASH is strongly associated with DM and Obesity (OR 18.61 [17.35-19.94]) and (OR 20.97 [17.87-23.21]) respectively. Other individual components of metabolic syndrome were associated with NASH to a lesser degree; HTN (OR 3.24 [3.20-3.28]) and HLD (OR 4.93 [4.85-4.01]).
Multivariate analysis of NASH risk factors
OR: Odds ratio; CI: Confidence Interval
In this retrospective study, utilizing an extensive database of multiple institutions, we found the prevalence of NASH steadily rising from 1.51% to 2.79% of the population aged 20-80 years between 2010 and 2020. This finding concurs with Neuschwander-Tetri et al. in his summary report of AASLD single topic conference, estimating that 2-3% of adults in the US meet diagnostic criteria for NASH [13]. Considerable variations were observed in the prevalence of NASH by sex and by race/ethnicity, with men and Caucasians being disproportionally affected. We also found solid independent associations between diabetes mellitus, hyperlipidemia, hypertension, and obesity with NASH.
The exponential increase in the global obesity pandemic has made NASH the leading cause of cirrhosis and is on a trajectory to becoming the leading indication for liver transplantation. [14,15] The largest study using ultrasound paired with hepatic histology evaluating asymptomatic middle-aged patients revealed NASH's 12.2% prevalence rate [16]. On autopsy studies, steatohepatitis was found in 18.5% of markedly obese but only 2.7% of lean persons [17]. High rates of NASH among obese patients were subsequently confirmed in a study of patients undergoing bariatric surgery, where NASH frequency was reported as high as 37%. [18] Obesity has been linked to gut-derived endotoxin in humans [19,20]. The endotoxin causes upregulation of inflammatory pathways by activating Kupffer cells and releasing pro-inflammatory cytokines. Yang et al. showed that steatosis is susceptible to endotoxin-induced hepatocyte damage and predisposes to NASH development [21]. Since prevalence of obesity has been steadily increasing in the US over the last two decades, [22] our study found a steadily increasing prevalence of NASH alongside. After adjusting for covariates, we found very high association rates between obesity and NASH (aOR 20.97).
NASH is often diagnosed in the middle age during the fourth to sixth decades of life, although cases in the pediatric population are also being reported with increasing frequency [23]. A meta-analysis demonstrated a 7.6% pooled mean prevalence of pediatric Non-Alcoholic Fatty Liver Disease (NAFLD) in the general population [24]. In our study, more than 50% diagnosed with NASH were between 50-70 years of age. NASH has also been reported to be more common in men than women, with a late peak in disease prevalence reported in post-menopausal women [25]. In our study, on multivariate analysis after adjusting for all confounders, we did not find a significant difference in NASH prevalence in men compared with women. The Dallas Heart Study reported that the Hispanic population had a higher prevalence of NAFLD (45%) compared with Caucasians (33%) and African-Americans (24%). A systematic review and meta-analysis confirmed the high prevalence of NAFLD among Hispanics [26]. The NASH Clinical Research Network found that Hispanics with NASH were younger, less active, and consumed a diet high in carbohydrates than Caucasians [27]. On the contrary, we report a small but relatively higher risk of NASH among Caucasians compared with other races. This is likely due to the rapid increasingly rates of metabolic syndrome. Since 1975, the rates of obesity have tripled. In 2016, 39% of adults in the United States were overweight, and 13% were obese [28].
The prevalence of NASH in diabetes mellitus is 22%. Since NAFLD is considered the hepatic manifestation of metabolic syndrome, the subset of patients who meet the histopathologic criteria for NASH are at the most significant risk for mortality and morbidity [29,30]. Hence, not surprisingly, we reported an 18-fold increased risk of NASH in people with diabetes. Although insulin resistance and hyperinsulinemia are pivotal to the development of steatosis, the consensus is lacking on the subsequent pathogenesis that leads to steatohepatitis and fibrosis. The excess and dysfunctional visceral adipose tissue promotes insulin hypersecretion secondary to insulin resistance. Adiponectin, an anti-inflammatory cytokine, is downregulated in obesity, insulin resistance, diabetes mellitus, and metabolic syndrome [31]. Consequently, higher leptin and lower adiponectin levels are associated with NASH and fibrosis [32].
Free-fatty acids are hepatotoxic and are particularly harmful to intracellular organelles. They promote activation of inflammatory response causing NASH and, through activation of hepatic stellate cells, promote fibrosis [33,34]. The hedgehog signaling pathway promoting wound healing in the liver is activated in response to lipotoxicity, promoting portal inflammation, hepatocellular ballooning, and fibrosis [35]. We found an increased association of dyslipidemia in patients with NASH (aOR 4.93).
Exploring the data in more depth, we found a disproportionate increase in the prevalence of NASH in comparison to the studied risk factors. This may reflect increased awareness of the hidden epidemic of NAFLD (and its subtype NASH) as an important disease among Primary Care Physicians and Endocrinologists in high-risk individuals. Moreover, evaluation of fatty liver disease became more accessible in recent years. The gold standard diagnosis of NASH remains a liver biopsy with strict pathologic criteria [1]. However, this is limited by cost, procedure-related morbidity and mortality, and sampling error. In the last decade, introduction of non-invasive tests including imaging modalities like ultrasound-based transient elastography, and Magnetic Resonance Elastography (MRE) as well as biochemical markers-based predictive scoring panels like fibrosis-4 index (FIB-4) and the NAFLD fibrosis score (NFS) have improved the diagnosing strategy of NAFLD, and in extent, the appropriate referral for liver biopsy.
Our study has limitations. The database used is an EMR-derived data collection from select health care systems. As such it is vulnerable to selection data and coding entry, missing data, and follow-up limitations and biases. Validation of the SNOMED-CT diagnostic codes was not possible because the patient information in the database was de-identified. However, SNOMED-CT has more concepts coded per clinical document than ICD-10, [36] making it more accurate in enlisting relevant clinical information. Moreover, although Explorys uses a master-patient identifier to match the same patient across different health care institutions and combine the data, [37] a few patients may have received care in multiple institutions within Explorys health care partners and thus could have been counted multiple times. However, this is countered by the fact that Explorys uses a robust patient matching algorithm [37], the effect of this error might be minimal and might affect the study groups equally. In addition, due to ICD-10 limitations, Explory's cannot confirm if the diagnosis of NASH was biopsy-proven or based on imaging studies. Nevertheless, the driving strength of our study was the large population size representing disease burden at multiple healthcare institutions. The results are therefore generalizable to the population nationwide.
5ConclusionIn conclusion, in the past decade, the prevalence of NASH has increased by almost 100%. NASH disproportionately affects Caucasians, males, people aged 50-70, and individuals with diabetes, obesity, dyslipidemia, and hypertension. The rising prevalence of metabolic syndrome in the United States is expected to further contribute to the burden of liver disease, notably NASH and its complications such as cirrhosis, hepatocellular carcinoma, and the need for a liver transplant. Significant effort is needed to identify and treat patients with metabolic syndrome early in the disease. For patients with NAFLD, regular follow-up and multidisciplinary management of the disease can prevent further inflammation and irreversible fibrosis. Further research in developing tools to detect NASH among the general population may be beneficial.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsOH: Study concept, data interpretation, manuscript writing and editing. AE: Manuscript editing, critical revision. AM: Manuscript editing, critical revision. KAA: Manuscript editing, critical revision. ST: Manuscript editing, critical revision. IA: Study concept, data interpretation, manuscript editing. All authors have read and agreed to the published version of the manuscript.