Recurrent hepatitis C virus (HCV) infection after liver transplantation is a significant cause of morbidity, mortality and graft loss. Spontaneous clearance of recurrent HCV after liver transplant is a rarely reported phenomenon. We report a case of a 66-year-old woman who underwent liver transplantation for HCV cirr-hosis (treatment-naive genotype 2) under immunosuppression with tacrolimus, mycophenolate mofetil (MMF), and short-term corticosteroids. The patient developed histologically proved severe cholestatic recurrence of HCV hepatitis. Immunosuppression was reduced to tacrolimus monotherapy because of cytopenia. She subsequently became RNA negative at week 44 post-transplant while on tacrolimus and MMF despite no antiviral therapy. A spontaneous sustained virologic clearance was confirmed with subsequent HCV nucleotide testing. Only a few similar cases have been reported in the literature with uninterrupted immunosuppression and subsequent spontaneous clearance. Our experience, and the few other published cases in the literature, suggests that spontaneous clearance of HCV after liver transplantation is a rare but real phenomenon. Better understanding of this phenomenon may help to manage recurrent HCV disease after transplantation.
Hepatitis C virus (HCV) represents a major global health problem with an estimated prevalence of 2.2-3.0% worldwide (130-170 million people)1 and 12% in the USA.2 Most, 70-80% of acute HCV infections, persist and almost 30% of individuals with persistent infection develop chronic liver disease, including cirrhosis and hepatocellular carcinoma.3 Up to 45% of all liver transplants are done in patients with chronic hepatitis C-related liver disease. Recurrence of HCV infection in the transplanted organ is universal.4,5 Although the rate of fibrosis progression is variable, it is usually accelerated in immunocompromised liver transplant recipients compared with immunocompetent patients. About 20% of patients develop rapidly progressive fibrosis that may lead to graft failure within a few years of transplantation.
Spontaneous clearance of HCV infection in liver transplant recipients is very rare and not well understood. Very few cases have been reported in the literature. We report one such case of spontaneous clearance of a histologically proved recurrent post-transplant HCV. The patient was on tacrolimus and MMF at the time of viral clearance.
Case ReportA 66-year-old Caucasian woman underwent a cadaveric liver transplant in October 2008 for end stage liver disease secondary to treatment-naïve chronic hepatitis C, genotype 2a/2c and hepatocellular carcinoma (HCC). Hepatitis C virus infection was diagnosed pre transplant by serum anti-HCV and serum HCV RNA (detected by RT-PCR, Roche, Ampliprep/COBAS®, lower detection limit 43 IU/mL). Serology for HIV and hepatitis B were negative. Standard immunosuppression was given with tacrolimus, mycophenolate mofetil along with prednisone tapered over four months according to our standard protocol. The post transplant period was complicated by worsening cholestatic liver function test, new onset diabetes, recurrent sepsis and inferior vena cava thrombosis. Early liver biopsies (wk 6 & 8) were insignificant except very mild acute rejection (rejection activity index RAI score 3/9) which was not treated with additional corticosteroid. At week twelve, cholestatic enzymes, gamma glutamyl transferase (GGT) (normal less than 55U/L) and alkaline phosphatase (alk phos) (normal less than 200 U/L) peaked at 3031U/L and 1371U/L respectively. Subsequent biopsies confirmed recurrent HCV infection with only minimal portal fibrosis. Recurrent hepatitis C was reconfirmed with the presence of HCV RNA in the serum. Surveillance of cytomegalovirus (CMV) DNA by PCR was always negative. Because of pancytopenia (WBC 0.7giga/L ANC 0.3giga/L plat 34giga/L hgb 108g/L) MMF and protocol prophylactic ganciclovir for HCV recipients seropositive for CMV, was held at week 16. Tacrolimus monotherapy was continued with blood levels of 6-9 ng/mL. MMF was restarted at week 29 at a lower dose. The patient became deeply jaundiced and bilirubin peaked at week 22 at 283 (normal less than 18 μmol/L) and 170 (less than 5 μmol/L) for total and direct respectively. Subsequently, at week 23 she was treated with three 500 mg boluses of methylprednisolone for biopsy proved low grade acute rejection (RAI 4/9). Her ALT peaked at 308U/L (normal less than 40U/L) at week 37 followed by complete spontaneous normalization by week 44. Her HCV RNA was repeated at this time point and was undetectable. Three subsequent qualitative HCV RNA tests over a span of six months were also negative. At 15 months post transplant follow up, she remains asymptomatic and her liver biochemistry remains stable and unremarkable.
Literature ReviewA systematic literature search was performed of Medline (01/1999-12/2009) including the search terms: hepatitis C infection, spontaneous clearance and liver transplantation. Additional papers and reports were identified through a manual review of the reference lists of identified case reports and review articles.
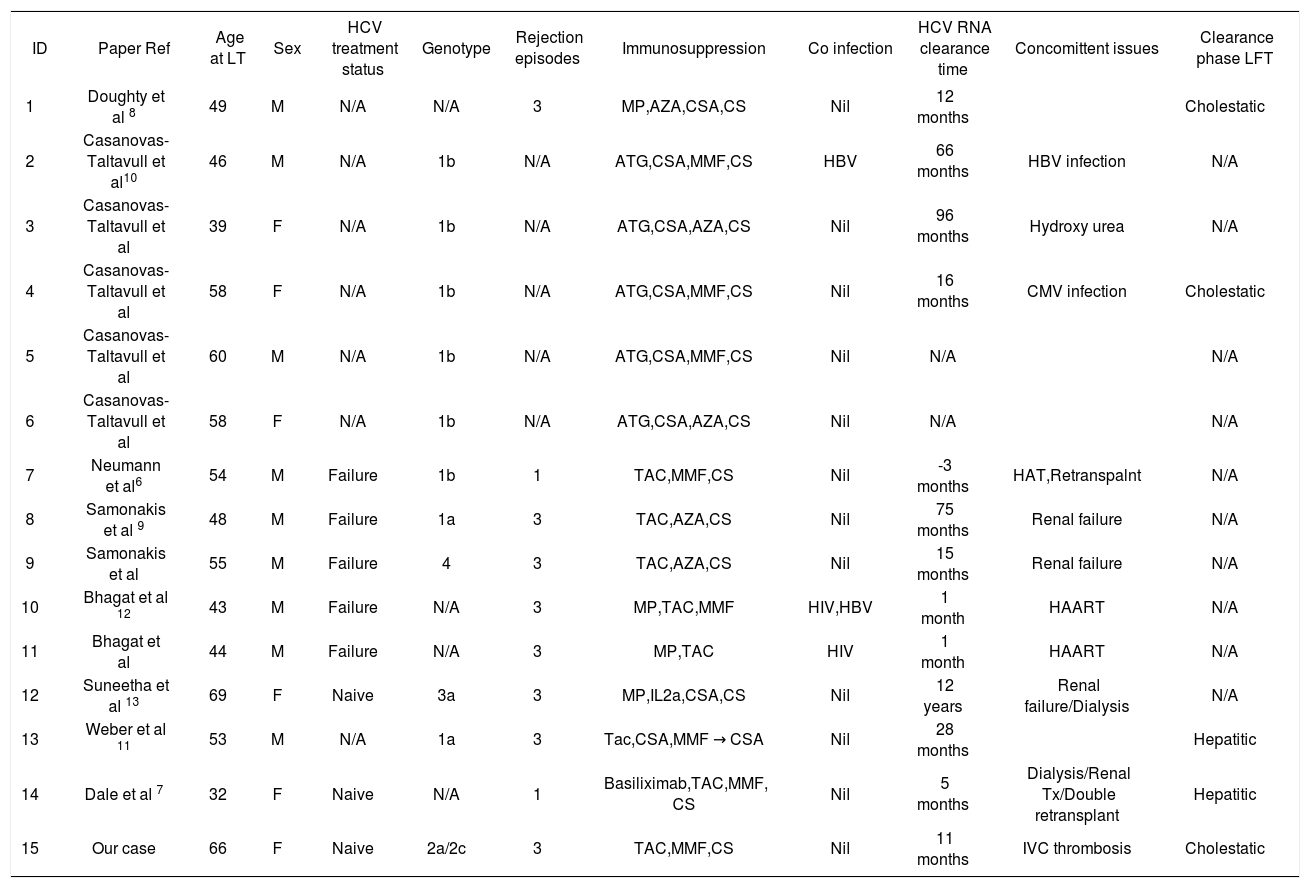
The medical literature on this subject reveals a small number of cases (n = 15 including our current case) reported in eight papers.6-13 Our review found a heterogeneous group of case series with incomplete published data. The largest series was reported by Cassanova et al10 of a total of 5 cases. The average age of all patients at time of transplant was 51 (ranges 32-69 years). Five of them had reported concomitant events: co-incidental de novo hepatitis B (HBV) infection, hydroxyurea treatment (for essential thrombocytosis), CMV infection, hepatic artery thrombosis and inferior vena caval thrombosis. Three cases had renal failure during clearance and one was on dialysis. Three cases were co infected with HIV or HBV. Only 2 out of the 14 cases reported previously had a cholestatic pattern of abnormal liver biochemistry during the viral clearance phase. Eight cases were on cyclosporine and seven were on tacrolimus. Five out of 15 cases were on anti-T cell depleting agent and change of immunosuppression was noted only in four of the cases. Seven cases had reported steroid pulses for acute rejection. Documented time for negative HCV RNA titer ranged from 14 to 78 months after transplantation (two cases not mentioned). Dale et al7 reported a case of spontaneous clearance of HCV RNA in a multiorgan transplant recipient. Only three reported cases including ours were interferon naïve pre-transplant. The literature search is summarized in Table 1.
Patient characteristics of reported cases of post liver transplant HCV spontaneous clearance.
| ID | Paper Ref | Age at LT | Sex | HCV treatment status | Genotype | Rejection episodes | Immunosuppression | Co infection | HCV RNA clearance time | Concomittent issues | Clearance phase LFT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Doughty et al 8 | 49 | M | N/A | N/A | 3 | MP,AZA,CSA,CS | Nil | 12 months | Cholestatic | |
| 2 | Casanovas-Taltavull et al10 | 46 | M | N/A | 1b | N/A | ATG,CSA,MMF,CS | HBV | 66 months | HBV infection | N/A |
| 3 | Casanovas-Taltavull et al | 39 | F | N/A | 1b | N/A | ATG,CSA,AZA,CS | Nil | 96 months | Hydroxy urea | N/A |
| 4 | Casanovas-Taltavull et al | 58 | F | N/A | 1b | N/A | ATG,CSA,MMF,CS | Nil | 16 months | CMV infection | Cholestatic |
| 5 | Casanovas-Taltavull et al | 60 | M | N/A | 1b | N/A | ATG,CSA,MMF,CS | Nil | N/A | N/A | |
| 6 | Casanovas-Taltavull et al | 58 | F | N/A | 1b | N/A | ATG,CSA,AZA,CS | Nil | N/A | N/A | |
| 7 | Neumann et al6 | 54 | M | Failure | 1b | 1 | TAC,MMF,CS | Nil | -3 months | HAT,Retranspalnt | N/A |
| 8 | Samonakis et al 9 | 48 | M | Failure | 1a | 3 | TAC,AZA,CS | Nil | 75 months | Renal failure | N/A |
| 9 | Samonakis et al | 55 | M | Failure | 4 | 3 | TAC,AZA,CS | Nil | 15 months | Renal failure | N/A |
| 10 | Bhagat et al 12 | 43 | M | Failure | N/A | 3 | MP,TAC,MMF | HIV,HBV | 1 month | HAART | N/A |
| 11 | Bhagat et al | 44 | M | Failure | N/A | 3 | MP,TAC | HIV | 1 month | HAART | N/A |
| 12 | Suneetha et al 13 | 69 | F | Naive | 3a | 3 | MP,IL2a,CSA,CS | Nil | 12 years | Renal failure/Dialysis | N/A |
| 13 | Weber et al 11 | 53 | M | N/A | 1a | 3 | Tac,CSA,MMF → CSA | Nil | 28 months | Hepatitic | |
| 14 | Dale et al 7 | 32 | F | Naive | N/A | 1 | Basiliximab,TAC,MMF, CS | Nil | 5 months | Dialysis/Renal Tx/Double retransplant | Hepatitic |
| 15 | Our case | 66 | F | Naive | 2a/2c | 3 | TAC,MMF,CS | Nil | 11 months | IVC thrombosis | Cholestatic |
N/A: Not available data/not reported. MP: Mmethyl prednisone. AZA: Azathioprine. CSA: Cyclosporine. CS: Prednisone. ATG: Antithymocyte globulin. MMF: Mycophenolate mofetil, Tac: Tacrolimus. IL2a: Interleukin 2 receptor antibody. HBV: Hepatitis B. HIV: Human immunodeficiency virus. HAART: High activity anti-retroviral therapy. LFT: Liver biochemical pattern.
Spontaneous clearance of HCV has been described in different phases of the natural history of this virus and associated with different concomitant events. In acute de novo HCV outside of transplantation, 10-50% of acute HCV infections resolve spontaneously.14 Symptomatic disease has been described as positive predictor, whereas co infection with HIV and patients on immunosuppressive medications are more likely to develop chronic infection.15 Cellular immune responses play a crucial role. Development of robust and multispecific CD4+ and CD8+ T-cell responses in blood and liver have been shown to be highly associated with spontaneous resolution,16 however, no association is found with HCV-RNA levels or genotype. In chronic HCV infection, the rate of spontaneous clearance of virus in chronic hepatitis C is very low. In a prospective study of patients with a history of intravenous drug use, Villano, et al.17 found that HCV RNA clearance occurred in only147 patients with a median time for clearance of 19 months from seroconversion (range 14 to 45). In a retrospective paediatric cohort study, Yeung, et el.18 reported 287 of spontaneous clearance with the first quartile of HCV clearance being 14.4 years. Route of transmission was not found to be a significant predictor.
In the setting of co-infection with hepatitis B (HBV) the rate of spontaneous clearance of chronic HCV infection appears to be higher.19 Sustained remission of chronic HCV has been reported in the setting of acute hepatitis B super infection.20 Soriano, et el. attributed viral interference phenomenon to be the cause of HCV clearance in patients with HIV/HBV co infection. Withdrawal of immunosuppressive medications with presumed “immune re-constitution” has also been reported to result in spontaneous clearance of chronic HCV, as Somsouk et al. reported a case of spontaneous clearance after withdrawal of immunosuppression in a renal transplant patient.21
In the liver transplant setting, HCV recurrence is well recognized and is regarded as almost universal after transplantation. Immunosuppression is thought to be the main determinant for the increased HCV replication. It can follow an accelerated course of liver disease associated with substantial morbidity, mortality and graft loss. This progression has been reported to be more pronounced in patients undergoing liver transplantation in recent years compared with those who underwent transplantation in the cyclosporine era22, however, as our experience and our medical literature review demonstrate, spontaneous clearance of recurrent HCV after liver transplantation is a rare but possibly real phenomenon. The balance between immunosuppression and effective virological immune response is probably crucial in terms of post-liver transplant viral clearance, although the effect of quasispecies remains speculative. In the non-transplant population homogeneity of the HCV quasispecies rather than genetic diversity is reported to be associated with acute resolving hepatitis as Farci, et el. reported patients showing “relative evolutionary stasis” in terms of quasispecies, were associated with resolution of acute HCV.23 In the post-transplant population, Doughty et al8 attributed spontaneous clearance to a rapid change in quasi-species in a patient with a high viral load and, similar to our patient, cholestasis. It was also speculated that cholestasis associated with a rejection episode may have induced local production of Th1 cytokines in the liver, leading to viral clearance12. In the non-transplant population, host genetic polymorphisms in IL2 have also been reported have a major role in successful clearance of HCV after anti-viral therapy.24 We note that our patient experienced change in immunosuppression and post-rejection cholestasis, which most likely affected the immunologic cytokine profile. These immunologic factors, in combination with infection with a HCV genotype (genotype 2a/2c) that is well recognized to be very responsive to antiviral therapy, may have culminated in conditions that allowed spontaneous clearance of HCV after liver transplantation.
ConclusionOccurrence of strong HCV-specific T cell responses may develop in HCV-infected patients after liver transplantation which can contribute to the resolution of HCV infection despite ongoing immunosuppression 13 Reduced immunosuppression or changes in the class of immunosuppression was reported in most of the cases, may be an important factor. Complete interruption of immunosuppression, however may not be the only factor to hasten this clearance as seen in our case and as well as cases described by Samonakis, et al.9 and Dale, et el.7 Prediction of spontaneous clearance phase by liver biochemical pattern (cholestatic vs. hepatitic) is also not consistent in reported cases. Our case represents one of the few reports of this “spontaneous” clearance of HCV in liver transplantation, without antiviral therapy; however, our literature review suggests that this phenomenon represents a real entity. Further studies are necessary to ascertain whether minimization of immunosuppression was the key factor or whether any other factors that may help viral clearance in this setting. Such information is long awaited in the context of the poorer outcome of recurrent HCV after liver transplantation.