Cases of viral hepatitis reported in Mexico are typically identified as hepatitis A, B and C. However, unspecified cases are reported annually. Hepatitis E virus (HEV) is an emergent agent that causes a self-limiting infection that can evolve to chronic in immunosuppressed individuals. In Mexico, HEV genotype 2 is considered endemic, though it's the prevalence is not well known. Therefore, the present study was designed to determine the prevalence of HEV among patients at the “Hospital Infantil de Mexico Federico Gomez”.
Materials and methodsThe study included 99 patients, anti-HEV antibody (IgG and IgM) were detected by indirect ELISA and viral genome was identified using RT-PCR technique. Two PCR products of positive cases were sequenced.
ResultsELISA results were positive in 3% and 6%, for IgG and IgM respectively, 54.5% prevalence was found by PCR. Low lymphocyte count (p<0.05) and malnutrition (p<0.005) were significant factors for high PCR prevalence and could increase the possibility of infection. Two samples were sequenced and confirmed the presence of HEV genotype 3.
ConclusionsThis report reveals the incidence of HEV in pediatric patients in Mexico. Moreover, the identification of HEV genotype 3 in human samples suggests a potential zoonotic risk that requires further research.
Hepatitis E is a public health problem in many developing countries and it is an emerging disease in industrialized nations [1–4]. Clinical presentation can range from asymptomatic to acute hepatitis (with 1% of mortality) [5,6]. However, among pregnant women, mortality is higher because the disease can progress to a fulminant hepatic failure in up to 20–30% of those infected [7–9]. The chronic presentation of Hepatitis E is observed in cases of underlying hepatic disease and in immunosuppressive conditions, such as in transplant recipients and patients with hematological illnesses and oncological diseases or immunosuppressive viral diseases, some cases can be due to blood transfusions [10–12]. Also in patients with chronic hepatitis caused by the Hepatitis C virus (HCV) can be observed. Sometimes Hepatitis E patients with impaired liver function may develop other symptoms, such as neurological disorders [13–15]. The Infection by Hepatitis E virus (HEV) in children is frequent but typically asymptomatic or manifested only as a very mild disease without jaundice, and usually it often goes undiagnosed [5,16]. Two weeks after infection, the virus is excreted in the feces. Clinical symptoms include fever, pain, myalgia, anorexia, jaundice, pruritus, clear stools and dark urine. A mild, transient increase in transaminase levels is observed in some, but not all, cases and finally, viral RNA can be identified in the serum [4,17,18].
The HEV is a single-stranded, positive-sense RNA virus classified in the family Hepeviridae, genus Orthohepevirus. Orthohepevirus A includes genotypes that can affect humans (genotypes 1–4), pigs (3, 4), camels (7), boars (3–6), and other mammals (3–5) [9,19–21]. Of the 4 genotypes reported in humans, genotype1 is distributed mainly in Asia, genotype 2 is found primarily in Mexico (strain 14 or strain Mexico) and some regions in Africa, the genotype 3 is distributed globally, and genotype 4 has been linked to sporadic cases in Japan, China and Taiwan [22,23]. Genotypes 1 and 2 have been reported exclusively in humans and are considered possible risk factors for infection after hemodialysis treatment and post-transfusion of hematic derivatives [24]. Genotypes 3 and 4 also infect animals, mainly pigs, and zoonotic transmission to humans has been reported [25–28]. The World Health Organization reports that in 2014 there were 20 million cases of HEV infection, including more than 3 million acute cases, and those 56,600 deaths related to this virus occurred worldwide.
In Mexico, the cases reported of viral hepatitis typically consist of hepatitis A, B and C; however, many unspecified cases of viral hepatitis are reported annually. Panduro et al. reported 8982 cases (in a one-year period) in which the etiologic agent was not identified [29]. Few research groups have reported seroprevalence data on HEV in humans in Mexico. Alvarez-Muñoz et al. reported age-dependent prevalence values of 1.2–15% in a study conducted in the State of Hidalgo [26]. There are additional reports, but all of them were performed in adults [8,25,29–31]. The information on the prevalence of HEV in pediatric patients is scarce. Therefore, the present study was designed to determine the prevalence of HEV infection among patients at the Hospital Infantil de Mexico Federico Gomez (HIMFG) using ELISA, (immunoglobulins [Ig] G and M) and RT-PCR.
2Materials and methods2.1Samples and case characteristicsA longitudinal/transversal clinical and virologic study was conducted between 2012 and 2014. Serum samples were collected from 99 patients enrolled in the research protocol HIM/2012/009 approved by the Committees on Ethics and Research at the HIMFG following the rules of the Declaration of Helsinki of 1975. All patients were attended at the HIMFG over a two-year period from March 2012 to March 2014. The HIMFG is located Mexico City (CDMX) and it is both a tertiary referral center for patients from all states in Mexico and a Research Institute. The characteristics of the patients, such as gender, laboratory data (i.e., transaminase levels, total bilirubin, serology results for other hepatitis viruses [A, B, C]), percentage of lymphocytes, blood transfusion and weight/high ratio in order to obtain the nutrition status of each patient are presented in Table 1. Also the place of residence/origin and the clinical service which each patient ingresses to the HIMFG were recorded.
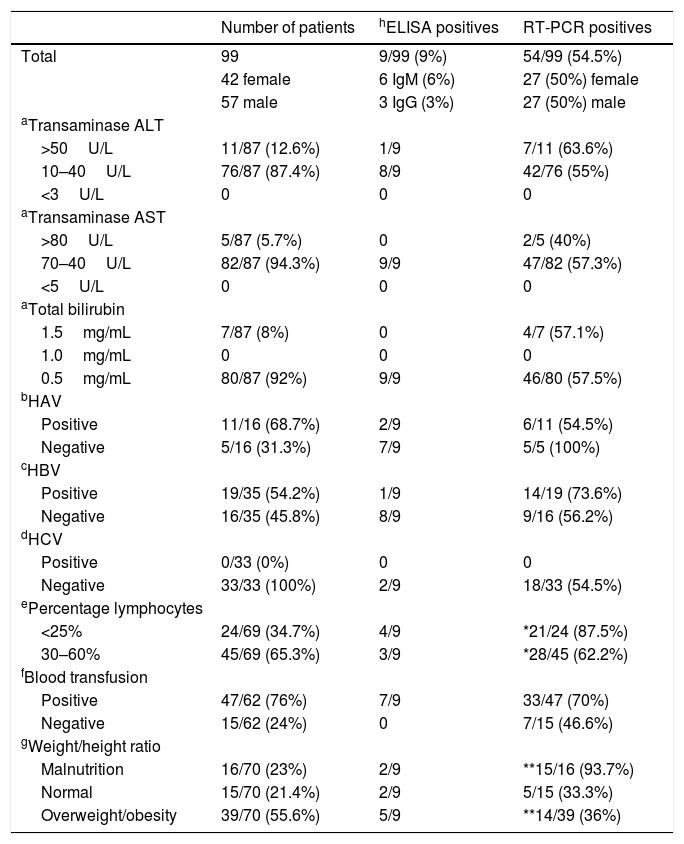
Relevant clinical characteristics of patients from the “Hospital Infantil de Mexico Federico Gomez”, serological and molecular prevalence.
| Number of patients | hELISA positives | RT-PCR positives | |
|---|---|---|---|
| Total | 99 | 9/99 (9%) | 54/99 (54.5%) |
| 42 female | 6 IgM (6%) | 27 (50%) female | |
| 57 male | 3 IgG (3%) | 27 (50%) male | |
| aTransaminase ALT | |||
| >50U/L | 11/87 (12.6%) | 1/9 | 7/11 (63.6%) |
| 10–40U/L | 76/87 (87.4%) | 8/9 | 42/76 (55%) |
| <3U/L | 0 | 0 | 0 |
| aTransaminase AST | |||
| >80U/L | 5/87 (5.7%) | 0 | 2/5 (40%) |
| 70–40U/L | 82/87 (94.3%) | 9/9 | 47/82 (57.3%) |
| <5U/L | 0 | 0 | 0 |
| aTotal bilirubin | |||
| 1.5mg/mL | 7/87 (8%) | 0 | 4/7 (57.1%) |
| 1.0mg/mL | 0 | 0 | 0 |
| 0.5mg/mL | 80/87 (92%) | 9/9 | 46/80 (57.5%) |
| bHAV | |||
| Positive | 11/16 (68.7%) | 2/9 | 6/11 (54.5%) |
| Negative | 5/16 (31.3%) | 7/9 | 5/5 (100%) |
| cHBV | |||
| Positive | 19/35 (54.2%) | 1/9 | 14/19 (73.6%) |
| Negative | 16/35 (45.8%) | 8/9 | 9/16 (56.2%) |
| dHCV | |||
| Positive | 0/33 (0%) | 0 | 0 |
| Negative | 33/33 (100%) | 2/9 | 18/33 (54.5%) |
| ePercentage lymphocytes | |||
| <25% | 24/69 (34.7%) | 4/9 | *21/24 (87.5%) |
| 30–60% | 45/69 (65.3%) | 3/9 | *28/45 (62.2%) |
| fBlood transfusion | |||
| Positive | 47/62 (76%) | 7/9 | 33/47 (70%) |
| Negative | 15/62 (24%) | 0 | 7/15 (46.6%) |
| gWeight/height ratio | |||
| Malnutrition | 16/70 (23%) | 2/9 | **15/16 (93.7%) |
| Normal | 15/70 (21.4%) | 2/9 | 5/15 (33.3%) |
| Overweight/obesity | 39/70 (55.6%) | 5/9 | **14/39 (36%) |
ALT (alanine transaminase) range: 3–50U/L, AST (aspartate transaminase) range: 5–80U/L, total bilirubin range: 0.5–1.5mg/dL.
Human anti-HEV antibodies (IgG and IgM) ELISA Kits (Direct ELISA) from LifeSpan BioSciences (USB Biological and Biological Reagents, USA) were used following the manufacturer's instructions. The absorbance was determined using a MultiskanAscent spectrophotometer (Labsystems), this immunoassay has 98% specificity and 95% sensitivity. To corroborate and expand the detection of positive samples, anti-HEV antibodies were also detected using another commercial (IgG) ELISA kit, this one from Wantai Biopharmaceutical, Inc. (Beijing, China), also following the manufacturer's instructions. The sensitivity of the Wantai HEV-IgG is 97.9% and it has a specificity of 99.9%. The cut-off value was calculated as the mean absorbance of the negative control plus 0.16, as indicated by the manufacturer. All serum samples with an absorbance value above cut-off value were considered positive.
2.3HEV-RNA extraction from patients’ seraFor RNA extraction, 1mL of serum from each patient was used, and extraction was performed with TRIzol reagent (ambion/RNA lifetechnologies™) following the manufacturer's instructions. The RNA obtained was resuspended in 20μL of nuclease-free water, and total RNA concentration was quantified by a Genoa Nano spectrophotometer (JENWAY) using a sample size of 2μL. The RNA was stored at −70°C until use.
2.4DNA synthesiscDNA synthesis was performed with random hexamer primers in the following reaction mixture: 1μL of buffer (5×), 4μL of RNAse inhibitor (20U/μL), 1μL of dNTPs (10mM), 2μL of reverse transcriptase (20U/μL), and 1μL (1000ng) of RNA in a final volume of 20μL. A First Strand cDNA Synthesis kit (Thermo Scientific, #K1612) was used under the following conditions: at 37°C for 1h, 70°C for 10min, and 20°C for 1min. The reaction was performed using a BIO-RAD T100™ Thermal Cycler.
2.5PCR amplificationA PCR amplification of the 198pb fragment of the HEV capsid gene (open reading frame 2 [ORF2]) was performed. The sequences of the primers used were those reported by De La Caridad Montalvo Villalba et al. [32] as follows: FHEV: GAC AGA ATT AAT TTC GTC GGC TGG (position: 6265–6288) and RHEV: CTT GTT CAT GCT GGT TGT CAT AAT C (position: 6437–6461).
The Taq DNA Polymerase Kit (Thermo Scientific, #EP0402) was used with the following reaction mixture: 5μL of Taq Buffer 5×, 5μL of Mg2+ (MgCl2), 1μL of 10-mM dNTPs, 0.25μL of 5-U/μL Taq polymerase, 5μL of cDNA, and 3μL of each 10-μM primer in a final volume of 50μL. The amplification program was performed as follows: initial denaturation at 94°C for 5min; 38 cycles of denaturation at 95°C for 30s, alignment at 55°C for 45s, and elongation at 72°C for 60s; followed by a final extension cycle at 72°C for 5min and at 20°C for 5min. A BIO-RAD T100™ Thermal Cycler was used. The amplification product was separated by electrophoresis on a 14% polyacrylamide gel for 70min at 110V. Silver staining was used to reveal the amplification product.
2.6Semi-nested PCR for HEV ORF2 gene amplificationTo further expand the study, the samples with higher RNA concentrations were selected for a second PCR, this one performed with the Taq DNA Polymerase Recombinant kit (Invitrogen®) according to the manufacturer's recommendations and using the (semi-nested) protocol described by de Deus et al. [33]: The sequences of the primers used were those: ORF2 F1 (TTV GGG CTY GAC TTT GC) and ORF2 R1 (CCR AGA AGY GTA TCA GC) in the first round, and ORF2 F1 and ORF2 R2 (CCR CGR CCC ACC TCA CCA AC) in the second round. The expected 216bp product was amplified under the following conditions: at 95°C for 9min, and 39 cycles at 94°C for 1min and at 72°C for 2min, followed by a final cycle at 72°C for 7min. The reaction mixture contained 63μL of PCR-grade water, 10μL of 10× buffer, 4μL of MgCl2 (50mM), 4μL of dNTPs, 2μL of each primer, 1μL of enzyme, and 10μL of cDNA.
2.7Sequence and phylogenetic analysisThe expected PCR products of three samples were purified using E-Gel SizeSelect Gels (Thermo Fisher Sc) and directly sequenced by the Sanger method at the Instituto de Investigaciones Biomedicas (UNAM). The sequences of both DNA strands of the PCR products were determined.
Nucleotide sequences were analyzed using BioEdit and MEGA7 packages. The analysis involved 40 nucleotide sequences, including the reference strains proposed by Smith et al. [21], for every genotype and sub-genotype. Sequences were identified by country (first two letters), host (third and fourth letters), genotype, sub-genotype (if available), accession number, and year of sampling.
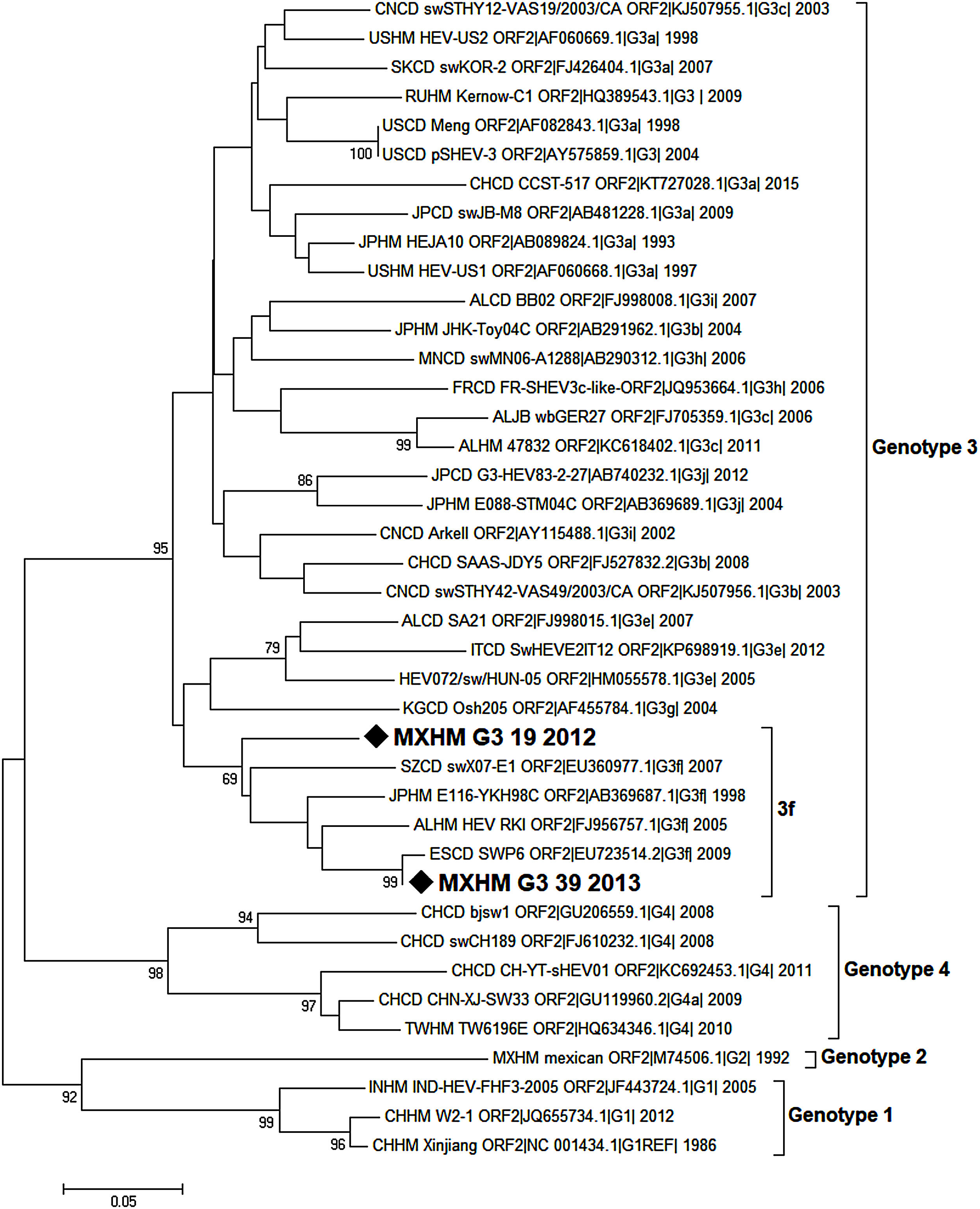
Phylogenetic analysis was inferred by the Neighbor Joining method based on the Tamura-Nei+G model with bootstrap tests of 1000 replications (only values >65 are shown). This analysis was conducted with a 209 nucleotide fragment of the capsid protein of HEV. The strains isolated in the present study are indicated with a black diamond.
2.8Relationship between positive cases by serology and PCR and patient dataOnce the HEV results were obtained by serology and RT-PCR, the positive percentages were calculated and grouped according to the specific clinical characteristics of each patient: Alanine transaminase (ALT) and Aspartate transaminase (AST), total bilirubin, serology to another hepatitis viruses, percentage of lymphocytes, blood transfusion antecedent, weight/high ratio in order to obtain the nutrition status (malnutrition, normal nutrition or overweight/obesity). Also, was recorded the residential/origin place of each child and the clinical service where it was performed the ingress in each patient.
2.9Statistical analysesDifferences between positive and negative cases were compared using the Fisher's and chi-square tests. Results were significant (p<0.05) for both ELISA (IgG vs. IgM) and RT-PCR.
3Results3.1HEV prevalence according to serologyThe present study was focused on pediatric patients. Among the 99 serum samples analyzed by ELISA, we identified three samples that were positive for IgG: 2 males and 1 female for a prevalence of 3%. We also found five samples that were positive to IgM (6%): 6 males that could represent patients with primo infection. Unfortunately, we were unable to do follow-up on these patients to prove their seroconversion because they never returned to the HIMFG. The differences between the patients positive for HEV by IgG and IgM were not significant (p>0.05) (Table 1).
The reasons for hospital admission of the patients in this study cohort varied because they did not present clinical hepatitis. No relationship was observed between HEV positivity and elevated levels of transaminases and total bilirubin (Table 1). The seroprevalence reported in developed countries is from 0.3 to 53%. Many studies indicate that published data depend significantly of the serological assays used and the quality of the technical performance. Therefore, we analyzed 45 samples with ELISA Kits Wantai. This procedure revealed one positive sample that was previously determined to be negative, as well as two samples that had values not considered positive (i.e., below the cut-off vale) analyzed by the LifeSpan BioSciences assay.
3.2Prevalence of HEV by RT-PCROf the 99 samples analyzed by RT-PCR, 54 showed the 198bp fragment of the HEV capsid gene, indicating a prevalence of 54.5% (Table 1), this difference respect the serological prevalence, 3%, was significant (p<0.05).
3.3Prevalence of HEV by RT-PCR and transaminases and total bilirubin levelsBecause the HEV infection can perturb hepatic function, the evaluation of hepatic activity was recorded in the cases where this information was available. Results did not show difference between the positivity in both serological and PCR for HEV and any alteration in both transaminases (ALT and AST) and total bilirubin (Table 1). Differences between positive and negative cases were not significant (p>0.05).
3.4Distribution of positive cases to HEV according to another hepatitis infectionThe co-infection with another hepatitis viruses (A, B and C) did not revealed significant differences (p<0.05), besides were more positive cases to Hepatitis B virus (HBV) and HEV. In pediatric patients, hepatic disease is usually associated with Hepatitis A virus (HAV) infection and HEV is not commonly tested. Our findings demonstrate the importance to perform an HEV diagnosis in all hepatic disease cases, especially when the causative agent is not identified.
3.5Distribution of positive cases to HEV according to levels of lymphocytesThe immunological status is an important factor that participates in immune response to infectious agents. Table 1 shows that almost all cases with less to 25% of lymphocytes were PCR positive to HEV. The differences with the normal levels of lymphocytes, 30–60% were significant (p<0.05).
3.6PCR prevalence cases and blood transfusion70% of blood transfusion patients were positive to HEV by PCR (Table 1) but this difference was not significant vs. the negative cases (p<0.05), however this result reveals the importance in to detect HEV and includes as blood test in blood transfusion. Seven of out 9 of the ELISA positive cases were patients that received blood transfusion.
3.7PCR prevalence and nutrition statusWeight and height data were considered in order to get the W/H index and with the help of tables for Mexican children, the nutritional status was obtained.
The nutritional status reveals mainly three stages: malnutrition, normal nutrition and overweight/obesity. Results shown in Table 1 reveal that malnutrition has almost all cases PCR positive and the differences were significant only in comparison between malnutrition and overweight/obesity group (p<0.005).
3.8PCR prevalence, residential place and clinical serviceThe majority of patients (83%) resided in CDMX and Estado de Mexico, from these patients, 47.3% (9/19 cases) and 50% (32/63 cases) were positive by PCR, respectively. The positivity by PCR according to clinical service, was 80% (16/20 cases) for oncology, 62% (13/21 cases) for neurology, 45% (5/11) for gastroenterology, 43% (18/42 cases) for nephrology, for infectology 25% (1/4 cases) and 100% (1/1) for hematology patients. The patients that received blood transfusions were mainly from oncology and nephrology services, which correlate to more cases PCR positive to HEV.
3.9Viral genotype identificationOnly a few samples were subjected to the semi-nested PCR protocol because most of them had been used for the ELISA and RT-PCR assays. The samples analyzed using the de Deus protocol [33] included three positive cases, two of them coincided with the positive results obtained by the first PCR performed. One sample that was negative according to the first PCR protocol was identified as positive by the second. The amplification products of the 216bp of two samples were sent for sequencing. Sequences were obtained from two patients: one aged 8 years old (MXHM_G3_39_2012, GenBank Accession Number MG570165.1, protein_id=“AXL97218.1”), the other 9 years of age (MXHM_G3_19_2013, GenBank Accession Number MG570166.1, protein_id=“AXL97219.1”). They exhibited homology with HEV genotype 3, sub-genotype f (Fig. 1).
Phylogenetic analysis of HEV positive samples from the HIMFG. The analysis included 70 nucleotide sequences (CD-HIT 98%). The evolutionary inference was performed according to the Maximum Likelihood method using the Tamura-Nei model with a discrete gamma distribution and permissible values for invariant regions. 1000 bootstrap replicates were run. Branches were clustered for graphical purposes; first number indicates number of sequences, genotype and sub-genotype, years of detection. The sequences found in this study are identified with diamonds and bold letters (GeneBank accession number MXHMg3_19|_2012, and MXHMg3_39|_2013).
Data availability: Obtained sequences are publicly available in Genbank with accession numbers MG570165.1d for strain MXHM_G3_39_2012, and MG570166.1 for strain MXHM_G3_19_2013.
The objective of this study was to determine the prevalence of HEV infection among patients at the HIMFG using ELISA and RT-PCR. The prevalence was 3% (3/99, IgG). However indirect ELISA identified 6 positive cases to IgM: (6%). This low prevalence is comparable to results obtained in other studies focusing on pediatric cases, such as 2.1% in Turkish children from an open community, and a lowest one 0.89% by IgG in children from a Turkish community with low socioeconomic status [34,35]. Japan reports that 3.3% of children with acute hepatitis and 2.6% of the open population are HEV IgG positive [36]. Seroprevalence rates for HEV across European countries varies considerably depending on the cohort studied, the serology assay used and even between geographic regions within countries. Germany, that has stricter sanitary controls, reports a prevalence of 1%, in a study conducted there between 2008 and 2010 with a group of children, this finding contrasts to those recorded in countries with less sanitary control of water services, such as India, which reported a prevalence of 16.3% among children with viral hepatitis [37,38].
Given that the vast majority of HEV infections (>90%) are asymptomatic, liver enzyme abnormalities detected during routine monitoring may provide the only prompt to consider the diagnosis. No relationship has been found between HEV infection and changes in laboratory parameters, such as liver function tests or total bilirubin. Attempts have been made to determine a threshold for HEV testing in immunocompetent patients based on ALT levels ranging from 100 to 300IU/L, but no clear consensus has been reached [39]. Indeed, reasonably consistent elevated alanine aminotransferase levels have only been reported in adult patients [26].
Interestingly, in this report we shown that in cases where the serology was known for other hepatitis viruses, such as A, B or C, most of all results were negative. This contrasts to reported in children from Istanbul, Turkey, HBV prevalence values of 18.6–15.4% were reported in the same age group [34,35]. Other studies in children with acute viral hepatitis describe prevalence of 64.5% for HAV and 7.6% for HBV [38]. Due to during childhood, hepatic disease is mainly associated with HAV infection and HEV is not commonly tested as a causative agent, our findings shown the importance to HEV detection.
Our results reveal that, according to the kit's manufacturer-recommended cut-off values (IgM and IgG HEV), no cases were considered positive. However, in some instances it was possible to observe the presence of viral RNA. These data correlate with those reported by other authors, IgG antibody detection does not necessarily coincide with the detection of the viral genome. Another possibility is that the amounts of antibodies in those samples were below the detection level of the kit. As many studies have indicated that published data depend strongly on the assays used and the quality of their technical performance, we analyzed 45 samples using the ELISA Kit (Wantai), which revealed one positive sample that was previously determined to be negative by the LifeSpan BioSciences assay, as well as two undetermined samples, which were sequenced and found to be positive for HEV3. Also, persistent HEV infection was described with detectable HEV RNA during more than 3 months. This condition has been reported in solid transplant recipients, in cases of HIV, in patients receiving immunomodulatory therapies, and in those treated with chemotherapy [39].
Viral genome was detected using RT-PCR. All nine positive samples by serology were patients with clinical history of blood transfusion, important fact that confirms the inclusion of HEV determination in blood test before a blood transfusion, in an at-risk immunosuppressed patient, the HEV detection should be considered even where when liver function tests are only modestly elevated. It is proved from the data of higher prevalence of HEV in patients from oncology and nephrology services (80% and 43%, respectively) which almost all received a blood transfusion.
As mentioned previously, several caveats must be considered when interpreting HEV serology; as reported immunosuppressed patients may not activate an antibody response despite detectable HEV viremia. HEV3 causes chronic hepatitis in 70% of immunosuppressed individuals in whom viral RNA can be detected for 6 months before seroconversion [40,41], for this reason, HEV RNA polymerase chain reaction should always be performed when HEV is suspected [39]. It is important to mention that we can document in 21 out 24 PCR positive patients the data of less of 25% percentage of lymphocytes that contrasts to 28 out 45 patients with above 30%. This data (less of 25% of lymphocytes) could refer an immunosuppressed state. One case was IgG+ and another one was IgM+. Furthermore, the nine-serology positive patients had blood transfusions. These could be reasons to explain why detection of antibodies may not coincide with the detection of the viral genome; moreover, no correlation between these samples should be expected. In cases of acute infection, meanwhile, monitoring of HEV RNA viral load may be helpful for the early identification of patients who are at risk of developing chronic infection, especially when the patients involved are similar to those reported in this study: patients that have ailments such as kidney disease, leukemia or lymphoma. Thus, clinicians should be aware of the potential for HEV to present as either acute or chronic infection, with mild or absent clinical symptoms.
A study performed in Mexico in 1999 reported a prevalence of 10.5% using the ELISA method in patients aged 1–29 years [26]. Recently, HEV3 genotype was reported in 15.79% of patients with chronic liver damage in Mexico [42]. In other countries, the prevalence values determined among adults using ELISA-IgG varied: 4.7% in Scotland, 6.8% in Germany, 9.4% in Greece, 11% in northern England, 27% in Holland, and 52.5% in southern France, which is considered a hyper-endemic area [43]. These findings confirm the observation of Krumbholz et al. who established the existence of a very low number of HEV infections among patients who were asymptomatic in their study. In the present study, the prevalence measured among pediatric patients was 3–6%, which is higher in pediatric patients than other studies, as in Germany, the highest seroprevalence reported was 1.5% among individuals aged 15–17 years [37]. However, there is a discrepancy in this trend relative to the values reported by Sidal et al. [35] of 3.7% in children aged 6 months to 4.9 years vs. 0.3% among children aged 10–15 years. Our work suggests that no preference exists in contracting HEV because the source of the contagion could be something as widely-used as simple water [44].
The two methods tested in the present study are complementary. RT-PCR can detect the presence of the virus even before the induction of antibodies, while ELISA can detect primo infection (IgM) and memory antibodies (IgG). Therefore, using both methods to detect HEV is recommended.
In this study, we determined the presence of HEV zoonotic genotype 3 in Mexican pediatric patients. As mentioned above, genotype 3 can produce infection in animals, and some studies have reported the presence of viral particles and RNA in city sewage systems [44,45]. However, no subsequent studies have confirmed the risk of HEV infection through this type of water pollution in industrialized countries. Clearly, systematic studies to determine the risk posed by water conditions in Mexico are indispensable, especially where the possibility of zoonotic transmission is suspected.
Furthermore, because of an unspecific range of symptoms has been described for HEV infection, not only including signs of viral hepatitis, but also neurological and other extra-hepatic manifestations blood transmission must be screening as in nosocomial transmission of HEV [46], and in our study 21% of samples were from neurology service. The fact that the 9 patients positive to both IgM and IgG had blood transfusion highlights the importance of studying that transmission route.
5ConclusionsThe two methods tested in the present study, ELISA and RT-PCR can detect primo infection (IgM) and memory antibodies (IgG), and the presence of the virus even before the induction of antibodies (RT-PCR). Therefore, using both methods to detect HEV is highly recommended. Likewise, the presence of the viral genome in patients with clinical history of blood transfusion is an important fact that confirms the inclusion of HEV determination in blood test before a blood transfusion. There is the possibility of transmission of HEV genotype 3 by zoonosis according to two cases studied.AbbreviationsHEV Hepatitis E virus Hepatitis C virus enzyme-linked immunosorbent assay reverse transcription polymerase chain reaction techniques Hospital Infantil de México Federico Gómez alanine transaminase aspartate transaminase Hepatitis B virus Hepatitis A virus open reading frame 2
This research was funded by Federal Funding Project grant number HIM/2012/009/SSA 1015, and by Mexico's Consejo Nacional de Ciencia y Tecnología grant number CB 221186 HEV.
Conflict of interestThe authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.