Decompensated liver cirrhosis has a dismal prognosis, with an overall survival of 2-4 years, which is worse than for many oncological diseases. Albumin is an important tool in the management of patients with cirrhosis, since it decreases for less than half the risk for post-paracentesis cardiocirculatory dysfunction and mortality associated with spontaneous bacterial infection, as well as, it triplicates the response to terlipressin in patients with hepatorenal syndrome. Recently, research on albumin has been a hot topic, with important new insights such as the characterization of the pleiotropic effects of albumin (which surpass its oncotic properties) and the concept of effective albumin concentration. In fact, patients with liver cirrhosis present posttranslational modifications on albumin that compromises its function. Those modified albumin forms were proved to have prognostic value and its knowledge may change the paradigm of albumin treatment. In this review, we critically summarize the latest evidence on the potential benefits of albumin in patients with end-stage liver disease.
Liver cirrhosis is the 14th most common cause of death, being responsible for over a million deaths per year worldwide.1,2 Survival significantly decreases when the disease progresses to a decompensated phase.1 In fact, survival of patients with decompensated cirrhosis is 2 to 4 years, which is worse than survival associated with many oncologic diseases.3 Ascites is the most frequent decompensation, and once it develops, about one half of the patients will be dead in 5 years.4 The development of renal failure associates with further increase in mortality, with more than 60% of the patients being dead in one year.5
Advanced cirrhosis is associated with a decrease in plasmatic albumin.6 Patients with cirrhosis have impaired hepatocellular function and reduced albumin synthesis, which can reach a 60-80% reduction in advanced cirrhosis.7 Protein levels further decrease due to the dilution effect from water and salt retention, and to the sequestration of circulating albumin in extracellular space and ascitic fluid.8, 9 Importantly, albumin is a major prognostic factor, being a significant predictor of death in over a hundred studies in patients with cirrhosis.6 Albumin is a component of the most important and widely used prognostic score in cirrhosis, the Child-Pugh-Turcotte score.10
Recently, a new concept of effective albumin concentration indicates that not only serum albumin decreases in liver cirrhosis, but the quality of albumin also changes. In fact, in patients with liver cirrhosis, albumin undergoes several reversible and irreversible posttranscriptional changes (for example oxidation) that change its properties.11–14
Treatment with albumin has been widely used in liver cirrhosis due to its oncotic properties, in order to expand plasma volume and to increase effective circulatory volume, and hence to abrogate the cardiocirculatory changes associated with portal hypertension.7 However, recently, other potentially beneficial albumin functions have been reported, such as its binding capacity, anti-oxidant and anti-inflammatory properties, modulation of hemostasis, vasodilatation and acid base homeostasis.15–19 There are three approved indications for the administration of human albumin solutions in cirrhosis:
- •
After large volume paracentesis to prevent paracentesis-induced circulatory dysfunction (PICD);
- •
Spontaneous bacterial peritonitis (SBP); and
- •
Other proposed indications for albumin use in cirrhosis are still controversial, such as: bacterial infections other than SBP,15 hepatic encephalopathy21 and chronic ascites.22
In this article, we aimed at reviewing the most relevant information and new insights about albumin and liver cirrhosis in a critical point of view, highlighting consensual and controversial available data.
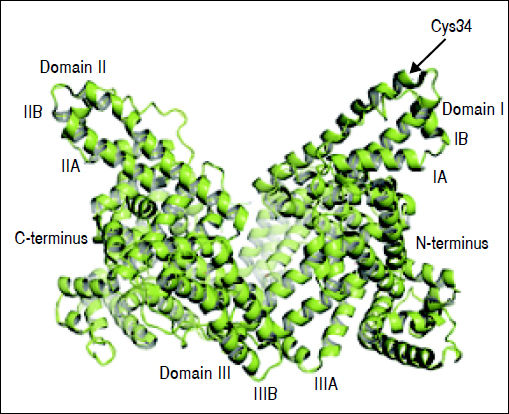
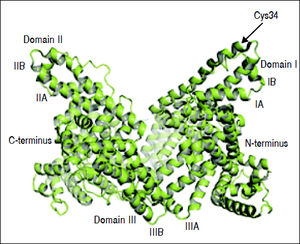
Structure and Function of AlbuminAlbumin is a 66.5 kDa negatively charged protein with high solubility and stability, encoded on chromosome 4.15,23,24 It has a single polypeptide sequence formed by 585 amino acids, a relative abundance of charged amino acids lysine, arginine, glutamine and aspartic acid, as well as, one tryptophan and 35 cysteine residues.25 Thirty-four of those 35 cysteine residues form 17 disulfide bridges that will determine the albumin structure, leaving the cysteine-34 residue free and available for reaction with other molecules. The secondary structure consists of 55% a-helix and 45% β-structure.26 At the tertiary structure, the protein has a heart-like shape, possessing three homologous domains I-III27 (Figure 1).
Albumin structure. Albumin has a single polypeptide sequence formed by 585 amino acids. At position 34, the cysteine residue is free and available for reaction with other molecules. The protein has a heart-like shape, possessing three homologous domains I-III, each domain is divided into A and B subdomains.
Albumin is the most abundant protein in plasma, with a concentration of 30–50 g/L, which corresponds to 50% of all plasmatic proteins.13,25 Despite albumin’s plasmatic abundance, the majority of albumin is not in circulation, as 60% is stored in the interstitial space.23,28 In fact, although the half-life of albumin is about 17 days, albumin only lasts 16–18 h in circulation.23,24 The transcapillary escape of albumin can be reverted, as albumin can eventually return into the plasma component via lymphatic return to maintain steady plasma concentrations.29
Albumin is predominantly synthesized in the liver, although albumin mRNA was detected in extra-hepatic localizations, such as pancreas, kidney and brain.30 Nahon, et al. found mature albumin mRNA in kidney, pancreas, heart and lung of newborn rats and in kidney and pancreas of adult rats. Furthermore the authors described a cell population in the rat kidney that was found to actively transcribe albumin gene.31 Moreover, albumin protein and mRNA were found in mouse retina, suggesting that albumin is also synthesized in the eye.32
Hepatic albumin synthesis rate is 150 mg/kg/day, corresponding to 10–15 g per day, and 10% of hepatic protein synthesis.23,24 Hepatic albumin synthesis can increase up to 4 times in response to hypoalbuminemia,33 and stimulation by insulin,34 glucocorticoids,35 or growth hormone.36 Conversely, chronic (but not acute) acidosis37,38 and proinflammatory cytokines such as tumor necrosis factor (TNF)-a, interleukin (IL)-6 and -1β inhibit albumin synthesis.39 Degradation of albumin can occur in any tissue, but the majority occurs in the liver, kidney and muscle.40 Plasma albumin concentration is the result of the balance between albumin synthesis, exchange between intravascular and interstitial compartments, albumin degradation by catabolism, and renal or intestinal loss.23
Serum albumin concentration has prognostic significance. In the general population, lower levels of albumin are associated with increased overall mortality.41–45 The reverse is also true, as in the normal albumin range, albumin levels higher than 43 g/L are associated with a 20%–40% decreased risk for all-cause mortality.46
Albumin contributes to 75% of the plasmatic oncotic pressure, which is disproportionally high since albumin only accounts for 50% of plasma proteins.28 That can be explained by albumin’s low molecular weight, which results in high osmotic activity per gram.23 Additionally, the osmotic activity of albumin increases by its negative charge, which holds osmotically active sodium cations (Gibbs-Donnan effect).47
Albumin has other important functions namely its binding capacity, anti-oxidant and anti-inflammatory properties.15,16 It has three essential functional domains:
- •
A metal binding domain.
- •
Domains that bind to other substances, and
- •
A domain that confers stability.28
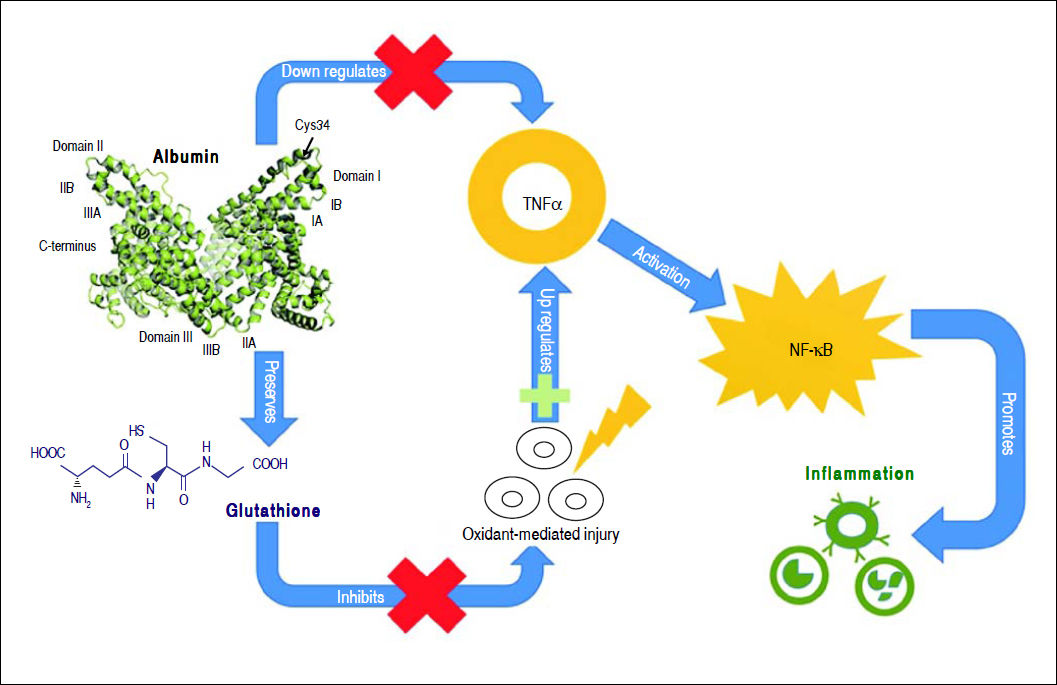
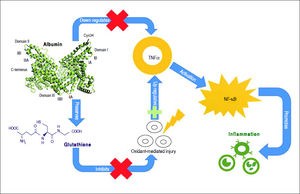
Albumin can bind to a variety of substances such as drugs, long-chain fatty acids, bilirubin, bile acids, endotoxin, hormones, and eicosanoids, modulating their biologic activity, distribution and clearance.23 Moreover, albumin has anti-oxidant properties as it binds to highly toxic reactive metal species,48 and its thiol groups from cysteine-34 residue act as potent scavengers of reactive oxygen species.49 Albumin also has anti-inflammatory properties, as it inhibits inflammatory mediators, such as TNF-α and C5a.50,51 Albumin can inhibit inflammation through several mechanisms. It inhibits TNF-α expression directly through transcription down-regulation52,53 and indirectly by preserving cellular glutathione and thus protecting cells against oxidant-mediated injury,1 which is a trigger for inflammatory responses. TNF-α down-regulation by albumin inhibits the activation of pro-inflammatory nuclear factor-kappa B (NF-κΒ) pathway51 and recruitment of leukocytes,51,54 further limiting inflammation (Figure 2).
Moreover, albumin interferes with hemostasis inhibiting platelet aggregation and promoting vasodilation,17 for example through NO-albumin complexes.18 It may also play a role in acid-base homeostasis: on one hand, it behaves as a weak acid and, on the other hand, it can buffer non-volatile acids.19 Lastly, studies in animal models of liver cirrhosis, also found that albumin exerts a positive cardiac inotropic effect counteracting the oxidative stress and TNF-α effect in NFκB-íNOS pathway and b-receptor signalling.55
Forms of Albumin in Liver CirrhosisAlbumin can undergo several posttranslational modifications (PTM) under physiological or pathological conditions, such as oxidation,56,57 glycosylation,58–60 truncation at the C- or N-terminus,61,62 dimerization63 and carboxylation.64,65 These PTM result in important structural and functional heterogeneity of circulating albumin.66
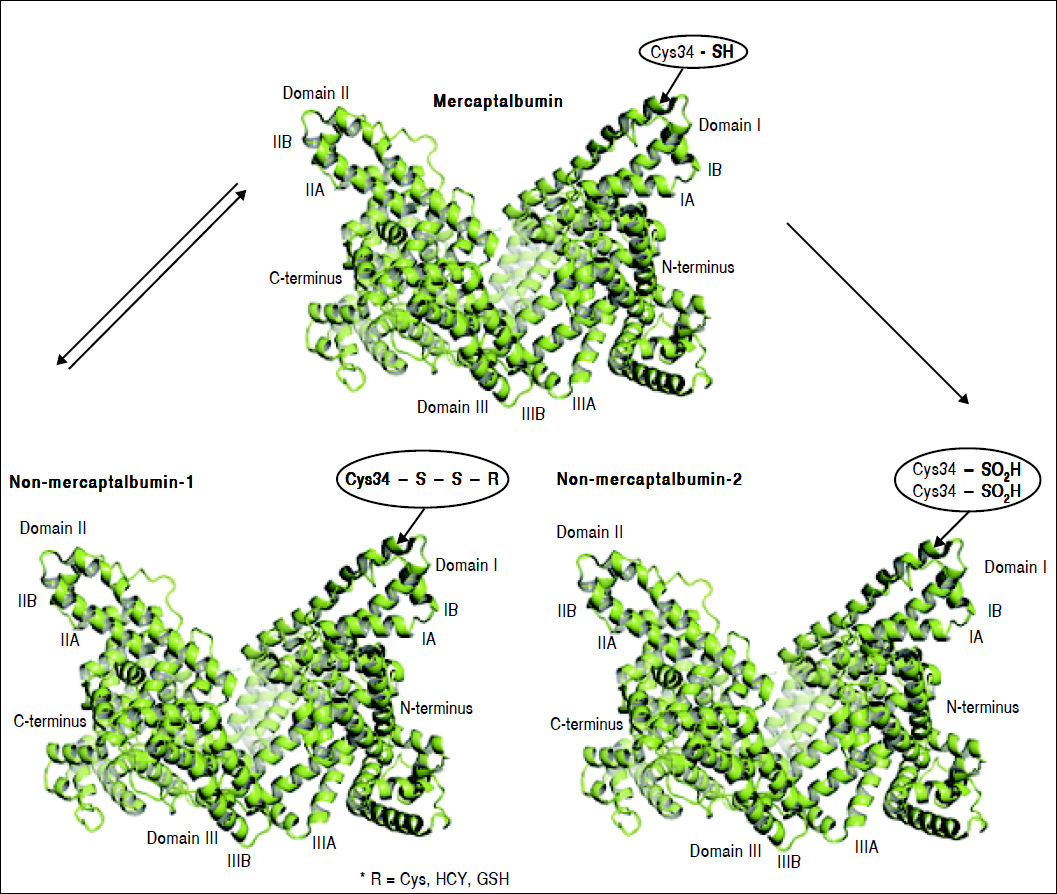
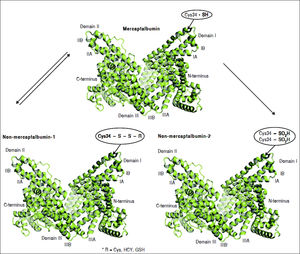
Oxidation of cysteine-34 residue is the most frequent PTM in albumin, and is a result of oxidative-stress induced protein damage.62 In healthy subjects, 70–80% of albumin circulates in its reduced form, dubbed mercaptoalbumin (HMA), which has a free sulfidril group at cysteine-34.11–15 Up to 25% of circulating albumin suffers reversible oxidative modifications through reversible binding via mixed disulfide bridges between cysteine-34 and compounds containing sulfidril groups such as cysteine, homocysteine and glutathione. This reversible oxidized form of albumin is dubbed non-mercaptoalbumin type 1 (HNA-1).67 Finally, up to 5% of circulating albumin suffers complete and irreversible oxidation of cysteine-34 in sulfonic or sulfinic acid, dubbed non-mercaptoalbumin type 2 (HNA-2)68 (Figure 3). These oxidized forms of albumin show compromised anti-oxidant properties.11,15 Oxidized albumin differs from native albumin pharmacokinetically and structurally, negatively impacting its function. Circulating life of oxidized albumin is lower than native albumin as a result of an increased plasma clearance by the liver and spleen.8 Ligand-binding properties to endogeneous and exogenous ligands and anti-oxidant properties of oxidized albumin are compromised relative to reduced albumin.69 Furthermore, oxidized albumin may itself have a noxious effect and exacerbate rather than ameliorate oxidative stress.70 In fact, Luna, et al. showed that with aging, oxidized albumin associates with endothelial damage through oxidative stress and increased apoptosis, suggesting that oxidized albumin may be a cardiovascular risk factor.71
Albumin forms. Mercaptoalbumin has a free sulfidril group at cysteine-34. Non-mercaptoalbomin-1 results from reversible oxidative modifications through reversible binding via mixed disulfide bridges between cysteine-34 and compounds containing sulfidril groups such as cysteine (Cys), homocysteine (HCY) and glutathione (GSH). Non-mercaptoalbumin-2 is a result of complete and irreversible oxidation of cysteine-34 in sulfonic or sulfnic acid.
Mercaptoalbumin has a free sulfidril group at cysteine-34. Non-mercaptoalbomin-1 results from reversible oxidative modifications through reversible binding via mixed disulfide bridges between cysteine-34 and compounds containing sulfidril groups such as cysteine (Cys), homocysteine (HCY) and glutathione (GSH). Non-mercaptoalbumin-2 is a result of complete and irreversible oxidation of cysteine-34 in sulfonic or sulfinic acid.
Liver cirrhosis, particularly when decompensated, associates with systemic inflammation and disturbed redox state,72,73 and hence associates with an increase in oxidized albumin forms HNA-1 and -2.11,13,14,74 Different groups found up to 50% increase in the proportion of HNA-1 and 200% of HNA-2 in patients with liver cirrhosis as compared with control healthy subjects.11,13,14,75 An increase in the percentage of oxidized albumin associated with severity of liver cirrhosis (as assessed by Child-Pugh-Turcotte class and MELD) and clinical decompensation such as development of ascites, HRS and bacterial infection.11,14,76 Finally, oxidized albumin correlated with short and long-term mortality in patients with liver cirrhosis, outperforming conventional prognostic tools such as MELD.11,75,76 Oettl, et al. found an optimal cut off of 12% HNA-2 proportion for predicting 1-month and long-term mortality in patients admitted for decompensated cirrhosis.75 More pronounced changes in oxidized albumin were also found in acute on chronic liver failure (ACLF) and alcoholic hepatitis,28,77,78 conditions with dismal prognosis.
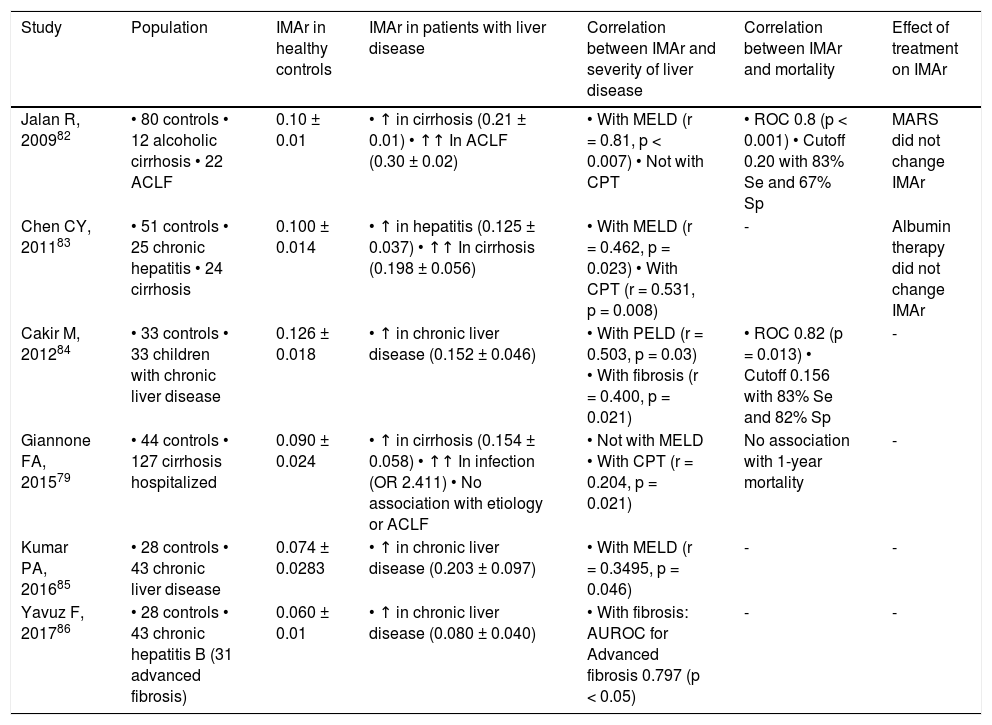
Other frequent PTM on albumin is ischemia-modified albumin (IMA) that consists in the functional alteration of the N-terminal portion of albumin molecule, resulting in a reduced capacity to bind cobalt.79,80 It occurs as a result of oxidative stress-induced N-terminal biochemical degradation.81 IMA and IMA/albumin ratio (IMAr) are increased in patients with cirrhosis, particularly in association with bacterial infection and in patients who progress to ACLF.79, 82–84 Corroborating the role of bacterial infection in the development of IMA, animal models of liver cirrhosis show increased IMA levels after injection of lipopolysaccharide.79 Importantly, IMA and IMAr strongly correlate with prognostic tools such as MELD.82,83 In fact, in patients with cirrhosis, IMAr higher than 0.02 strongly associates with mortality, which places IMAr as a potential prognostic marker in advanced liver disease.82 Of notice, neither albumin therapy nor molecular adsorbent recirculating system (MARS) has an effect on IMA/IMAr.82,83Table 1 summarizes the available evidence of IMAr in liver disease.
Evidence of IMAr in liver disease.
| Study | Population | IMAr in healthy controls | IMAr in patients with liver disease | Correlation between IMAr and severity of liver disease | Correlation between IMAr and mortality | Effect of treatment on IMAr |
|---|---|---|---|---|---|---|
| Jalan R, 200982 | • 80 controls • 12 alcoholic cirrhosis • 22 ACLF | 0.10 ± 0.01 | • ↑ in cirrhosis (0.21 ± 0.01) • ↑↑ In ACLF (0.30 ± 0.02) | • With MELD (r = 0.81, p < 0.007) • Not with CPT | • ROC 0.8 (p < 0.001) • Cutoff 0.20 with 83% Se and 67% Sp | MARS did not change IMAr |
| Chen CY, 201183 | • 51 controls • 25 chronic hepatitis • 24 cirrhosis | 0.100 ± 0.014 | • ↑ in hepatitis (0.125 ± 0.037) • ↑↑ In cirrhosis (0.198 ± 0.056) | • With MELD (r = 0.462, p = 0.023) • With CPT (r = 0.531, p = 0.008) | - | Albumin therapy did not change IMAr |
| Cakir M, 201284 | • 33 controls • 33 children with chronic liver disease | 0.126 ± 0.018 | • ↑ in chronic liver disease (0.152 ± 0.046) | • With PELD (r = 0.503, p = 0.03) • With fibrosis (r = 0.400, p = 0.021) | • ROC 0.82 (p = 0.013) • Cutoff 0.156 with 83% Se and 82% Sp | - |
| Giannone FA, 201579 | • 44 controls • 127 cirrhosis hospitalized | 0.090 ± 0.024 | • ↑ in cirrhosis (0.154 ± 0.058) • ↑↑ In infection (OR 2.411) • No association with etiology or ACLF | • Not with MELD • With CPT (r = 0.204, p = 0.021) | No association with 1-year mortality | - |
| Kumar PA, 201685 | • 28 controls • 43 chronic liver disease | 0.074 ± 0.0283 | • ↑ in chronic liver disease (0.203 ± 0.097) | • With MELD (r = 0.3495, p = 0.046) | - | - |
| Yavuz F, 201786 | • 28 controls • 43 chronic hepatitis B (31 advanced fibrosis) | 0.060 ± 0.01 | • ↑ in chronic liver disease (0.080 ± 0.040) | • With fibrosis: AUROC for Advanced fibrosis 0.797 (p < 0.05) | - | - |
IMA expressed as absorbance units (ABSU) estimated using cobalt binding test (ABT). IMAr normalized for albumin in g/dL. ACLF: acute on chronic liver failure. CPT: Child-Pugh-Turcotte class. IMA: ischemia-modified albumin. IMAr: IMA/albumin ratio. PELD: pediatric end-stage liver disease class.
Albumin dimerization is also increased in patients with cirrhosis, particularly in patients with severe ACLF.80,87 Dimerization occurs at cysteine-34 and can be found with the native or truncated forms of albumin, as homo or heterodimers. Homodimeric N-terminal truncated albumin form proportion can independently stratify 1-year mortality in patients with ACLF.80
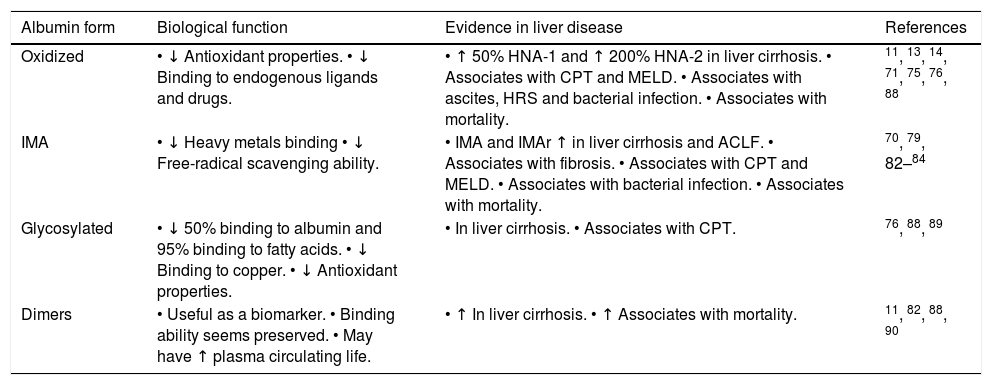
Many other different PTM in albumin were found increased in patients with liver cirrhosis, such as cysteinylated, glycated and truncated forms.11,77,87 As a consequence, native, non-modified albumin, is significantly reduced in patients with cirrhosis, particularly during clinical decompensation.11 The absolute reduction of native albumin or the reduction in the proportion of native to total albumin were able to predict mortality in patients with liver cirrhosis, outperforming total albumin concentration as a prognostic marker.11,82 Because modified albumin forms consistently present compromised function, it is clinically more relevant to estimate the concentration of fully functional native albumin concentration than total albumin concentration, and hence it has emerged the new concept of effective albumin concentration.11,28Table 2 summarizes the modified forms of albumin in liver disease.
Modified forms of albumin in liver disease.
| Albumin form | Biological function | Evidence in liver disease | References |
|---|---|---|---|
| Oxidized | • ↓ Antioxidant properties. • ↓ Binding to endogenous ligands and drugs. | • ↑ 50% HNA-1 and ↑ 200% HNA-2 in liver cirrhosis. • Associates with CPT and MELD. • Associates with ascites, HRS and bacterial infection. • Associates with mortality. | 11, 13, 14, 71, 75, 76, 88 |
| IMA | • ↓ Heavy metals binding • ↓ Free-radical scavenging ability. | • IMA and IMAr ↑ in liver cirrhosis and ACLF. • Associates with fibrosis. • Associates with CPT and MELD. • Associates with bacterial infection. • Associates with mortality. | 70, 79, 82–84 |
| Glycosylated | • ↓ 50% binding to albumin and 95% binding to fatty acids. • ↓ Binding to copper. • ↓ Antioxidant properties. | • In liver cirrhosis. • Associates with CPT. | 76, 88, 89 |
| Dimers | • Useful as a biomarker. • Binding ability seems preserved. • May have ↑ plasma circulating life. | • ↑ In liver cirrhosis. • ↑ Associates with mortality. | 11, 82, 88, 90 |
Patients with cirrhosis present with absolute hypoalbuminemia, which has been mainly attributed to a decreased synthetic capacity,91 but it is believed to be multifactorial.92 In liver dysfunction, albumin synthesis is decreased as a result of liver dysfunction and abnormal portal blood flow distribution.13.20 The decrease in effective intravascular blood volume frequently seen in cirrhosis, leads to compensatory activation of the renin-angiotensin system and sympathetic nervous system, and to increased release of antidiuretic hormone, which culminates in sodium and water retention, as well as, in reduction of renal perfusion and glomerular filtration rate.93 This mechanism is responsible for the development of ascites and hepatorenal syndrome (HRS). Human albumin was first introduced in the management of cirrhotic patients with hypoalbuminemia and ascites in the 1950s, due to its expanding volume capacities.13,91 However, we now know that the beneficial effects of albumin surpass its oncotic properties.13,22
There are three approved indications for the administration of human albumin solution in patients with cirrhosis:
- •
After large volume paracentesis (LVP) to prevent paracentesis-induced circulatory dysfunction (PICD).
- •
Spontaneous bacterial peritonitis (SBP).
- •
There are other controversial proposed indications for albumin expansion, such as non-BSP infections,96,97 ascites22 and encephalopathy.21,98
Prevention of paracentesis-induced circulatory dysfunctionAfter LVP, intra-abdominal pressure abruptly drops, which increases venous return to the right atrial pressure, with consequent increase in cardiac output and stroke volume.99 However, this transient increase in cardiac output induces an excessive drop in peripheral vascular resistance, while increasing intrahepatic vascular resistance.100 As a consequence, blood is sequestrated into the peripheral circulation resulting in a decrease in effective circulating volume and in arterial pressure.100–103 Interestingly, this impairment in effective blood volume does not associate with an increase in transvascular escape of albumin.102 A persistent reactivation of renin angiotensin aldosterone system (RAAS) occurs as a counteracting mechanism that can last for months,101 which can result in recurrence of ascites, hyponatremia, HRS and decreased survival.101,104–107 Paracentesis-induced circulatory dysfunction (PICD) has been defined as at least 50% increase in plasma renin activity up to level higher than 4 ng/mL/h at the 6th day after paracentesis.104 PICD occurs in one third of patients submitted to LVP and does not revert spontaneously.105
The first study showing a benefit of albumin administration in the prevention of PICD was performed, more than 30 years ago, by Gines, et al.104 Albumin infusion abrogated an increase in plasma renin activity after paracentesis, and prevented hyponatremia and deterioration in renal failure. The rational for the use of albumin is expansion of plasma volume avoiding a decrease in effective blood volume, which occurs in the absence of an increase in albumin transvascular escape.102 One decade later, the same group showed that this strategy of albumin infusion would only be beneficial when more than 5 liters of ascites are removed (decreasing by 50% the risk of PICD), since for lower volume paracentesis the risk of PICD is similar to the spontaneous deterioration in untreated cirrhotics with ascites, i.e. about 10%.105 Since then, several studies and different meta-analysis compared the efficacy of albumin vs. other volume expanders such as dextran, gelatin, hydroxyethyl starch and hypertonic saline. In 2012, a meta-analysis of 17 trials comprising 1,225 patients, showed that albumin was superior to the other strategies, decreasing in 57% to 75% the risk for PICD, in 42% the risk for hyponatremia and in 36% the risk for mortality.101 Subsequent meta-analysis confirmed the superiority of albumin in reducing PICD and hyponatremia, while showing insufficient evidence for mortality prevention.108,109 However, the strength of most recent meta-analysis has been questioned since they included different albumin protocols as well as studies comparing albumin with no treatment.101
According to AASLD and EASL guidelines, LVP is the first-line therapy in patients with large ascites (grade 3 ascites) and should be performed together with the administration of albumin to prevent PICD, when more than 5 liters of ascites are removed.94,95 The recommended dose of albumin is 8 g/L of ascitic fluid removed, which was the most frequently used dose in clinical trials. Two small studies compared the standard albumin dose with half the dose (4 g/L of ascitic fluid removed) and found no difference in adverse outcomes: prevalence of PICD, hyponatremia, renal impairment, ascites recurrence or 6 months survival.110,111 For the time being, however, this dose cannot be recommended because these findings have not yet been replicated.
A different strategy studied to prevent PICD is the administration of vasoconstrictors, since the decrease in effective blood volume is a direct consequence of excessive peripheral vasodilation. The level of evidence is still low, however while midrodine seems less effective than albumin,112–115 small studies did not demonstrate differences between noradrenaline116 or terlipressin116–118 as compared with albumin infusions.
Treatment of bacterial spontaneous peritonitisAbout one third of patients with SBP develop renal dysfunction119 due to a rapidly progressive impairment in systemic hemodynamics as a consequence of activation of vasodilation systems by proinflammatory cytokines.101,120 These patients present worse outcomes including decreased survival.101
The first randomized study assessing the effect of albumin administration in renal function and mortality, in patients with SBP, was performed in 1999. This study included 126 patients, which were treated either with antibiotics alone or in association with human albumin solution at a dose of 1.5 g/kg of body weight at the time of diagnosis, followed by 1 g/kg of body weight on the third day.121 In the group not treated with albumin, 33% of the patients developed renal impairment as compared with only 10% in the albumin group.121 Furthermore, albumin administration associated with a 50% decrease in 3 months mortality (41% vs. 21%).121 Other studies replicated these results. In fact, a meta-analysis of 4 studies including 288 patients, showed 80% reduction in the risk of renal impairment (30.6% vs. 8.3%) and 66% reduction in the risk of mortality (35% vs. 16%).122
More recent studies tried to select a high-risk population of patients with SBP in order to limit albumin administration. Sigal, et al.123 studied 38 episodes of SBP and defined high-risk patients the ones who presented plasma bilirubin > 68.4 µmol/l (4 mg/dL), creatinine > 88.4 µmol/l (1 mg/dL) or blood urea nitrogen > 30 mg/dL.123 Low-risk patients were not treated with albumin, and none developed renal failure nor died.123 Another study analyzed 216 episodes of SBP during a 7-year period and did not treat with albumin low-risk patients, while in the high risk patients albumin was given at the discretion of the attending physician.124 Low-risk patients presented much lower risk of renal failure (4.7% vs. 18.6%) or inhospital mortality (3.1% vs. 28.8%) as compared with high-risk patients treated with albumin.124
Since the recommended dose comes from arbitrary doses used in the first study performed in 1999,121 a more recent pilot study included 46 patients with SBP and compared the standard albumin dose with a reduced protocol of 1 g/kg at admission and 0.5 g/kg of albumin on the third day.125 No significant differences were found between albumin doses in respect to development of renal failure and HRS, in-hospital mortality and mortality up to 3 months.125
A small study in high-risk patients with SBP compared 4 different strategies: administration of albumin, terlipressin, terlipressin plus albumin or midrodine. Though they did not find different outcomes in the albumin or terlipressin groups, patients treated with midrodine developed higher circulatory dysfunction, higher incidence of renal failure and a non-significant trend towards higher mortality.126
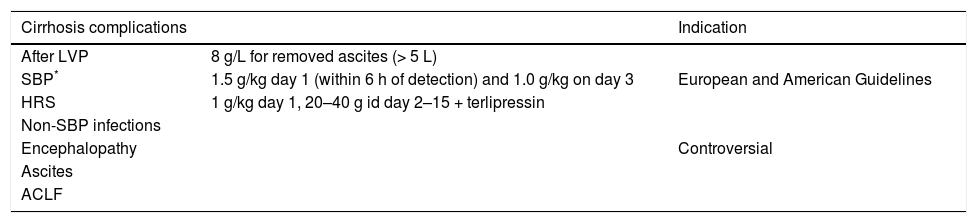
Until further development, evidenced-based current guidelines recommend that patients with ascitic fluid polymorphonuclear leukocyte counts > 250 cells/mm3 and clinical suspicion of SBP who also have a serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4mg/dL should receive 1.5 g albumin per kg body weight within 6 h of detection and 1.0 g/kg on the third day.94,95
Prevention of hepatorenal syndromeIn patients with advanced cirrhosis and portal hypertension there is a reduction in the effective arterial blood volume as a result of splanchnic arterial vasodilation and cardiac dysfunction.115 RAAS is activated, as a compensatory response, and, over time, severe intrarenal vasoconstriction, renal hypoperfusion and, ultimately, renal failure can develop.15 HRS is defined as a functional renal failure, unresponsive to plasma volume expansion that occurs in patients with end-stage liver disease.15,94,127 There are two types of HRS: type 1, a rapid deterioration of renal function, defined as an increase in serum creatinine to a value twice the baseline and > 2.5 mg/dL within 2 weeks; type 2, a slow and gradual deterioration of renal function with serum creatinine above 1.5 mg/dL.15,94 Current guidelines recommend the administration of both vasoconstrictors and human albumin in the treatment of HSR.94,128
The evidence for the use of albumin comes from a small study that included 21 patients with HRS (16 with type 1 HRS, 5 with type 2 HRS) that compared treatment with terlipressin alone versus terlipressin with albumin, until complete response was achieved (defined as serum creatinine level < 1.5 mg/dL) or for 15 days.129 Albumin administration was the only predictive factor of complete response. In fact, treatment with albumin was associated with a 3-fold increase in complete response (77% in patients receiving terlipressin and albumin vs. 25% in those receiving terlipressin alone, p = 0.03). Importantly, complete response was associated with improved survival.129
The efficacy of this combined therapy results from the vasoconstriction effect induced by terlipressin on the splanchnic vascular district and the expansion caused by albumin, which together improves effective hypovolemia and ultimately restores renal perfusion.15,94,127,129–131
Controversial indications for albumin therapy in cirrhosisThe beneficial effect of human albumin was also assessed in patients with cirrhosis and bacterial infections other than SBP.15 In fact, non-SBP infections also associate with an increased risk for renal failure, which occurs in more than one third of the patients.132 However, the risk seems to be particularly significant in sub-diaphragmatic infections.133 Guevara, et at. evaluated 110 patients with cirrhosis and non SBP infections (such as respiratory, urinary and skin infections) and found a trend to lower frequency of type 1 HRS in patients treated with antibiotics and albumin compared to those treated only with antibiotics, after 14 days.96 Importantly, although no differences between the two groups were found on 3 months survival, when adjusted for factors with independent prognostic value, treatment with albumin was an independent predictive factor for survival.96 A more recent clinical trial on 193 Child-Pugh-Turcotte B or C cirrhotic patients with non-SBP infection found albumin treatment to delay, rather than to prevent, renal failure. No effect of albumin was seen on 3-months mortality, and 8% of patients developed pulmonary edema after albumin infusion.97 Therefore, until more robust evidence is provided, there is not enough evidence to support albumin administration in non-SBP infections.115
Albumin was also proposed for the treatment of hepatic encephalopathy. A study that included 56 patients with cirrhosis and hepatic encephalopathy, compared 2 strategies to expand plasma volume: albumin infusion (N = 26) or expansion with a crystalloid (N = 30). Both strategies associated with similar resolution of hepatic encephalopathy, however, there was significant improvement on 3-months survival in the albumin-treated group.21 A recent study on 120 patients with hepatic encephalopathy treated with either lactulose alone or lactulose plus albumin found that the association with albumin correlated with a 50% increase in the resolution rate of encephalopathy as well as 50% decrease in mortality.98 Further studies are needed before recommendations for albumin administration in hepatic encephalopathy can be made.
Lastly, Romaneli, et al. evaluated the effect of albumin administration (albumin plus diuretics vs. diuretics alone) in the treatment of patients with ascites, with a median follow-up of 84 months.22 In this trial, albumin-treated patients had lower probability of ascites recurrence (51% vs. 94%, P < 0.0001) and significantly greater cumulative survival rate (Breslow test = 7.05, p = 0.0078). Interestingly, the difference in survival was only evident after 16 months of therapy. More recently, Bernardi, et al. presented in EASL 2017 a study on the long-term effect of weekly albumin administration versus standard of care in more than 400 patients with cirrhosis and ascites. Albumin treatment not only reduced in half the necessity of paracentesis and the risk for refractory ascites, but it was also associated with a decreased risk for other complications such as SBP and renal failure. Also, chronic albumin treatment was associated with a 38% reduced mortality risk (Table 3).
Summary of albumin indications in end stage liver disease.
| Cirrhosis complications | Indication | |
|---|---|---|
| After LVP | 8 g/L for removed ascites (> 5 L) | |
| SBP* | 1.5 g/kg day 1 (within 6 h of detection) and 1.0 g/kg on day 3 | European and American Guidelines |
| HRS | 1 g/kg day 1, 20–40 g id day 2–15 + terlipressin | |
| Non-SBP infections | ||
| Encephalopathy | Controversial | |
| Ascites | ||
| ACLF |
Albumin is the most abundant plasmatic protein. Liver cirrhosis associates with decreased levels of albumin as well as disturbed albumin function. Currently, there are three well established indications for albumin infusion in patients with liver disease:
- •
Prevention of cardiocirculatory and renal dysfunction after LVP.
- •
Treatment of SBP.
- •
Treatment of HRS (Table 1).
Colloidal albumin properties, and its impact on osmotic pressure, have been the rational for using albumin in these situations. Recently, however, potential beneficial effects of albumin surpass its oncotic effect, since it has been acknowledged its pleotropic nature, with important binding capacity to toxic substances and drugs, anti-inflammatory effects, modulation of hemostasis and acid-base homeostasis, among other properties. Therefore, further indications for albumin infusions in cirrhotic patients are under evaluation.
A relevant new concept is that of effective albumin concentration. Patients with advanced liver disease present posttranslational modifications on albumin such as oxidized HNA-1/2 and ischemia modified albumin, which perturb albumin function. Accordingly, not only a decrease in total albumin concentration, but specifically a decrease in native unmodified albumin (at expense of an increase in modified albumin forms) has important prognostic value, associating with decreased short and longterm mortality, in patients with liver cirrhosis. In the near future, determination of modified forms of albumin may enter clinical practice, similarly to determination of modified glycated hemoglobin in patients with diabetes mellitus. Also, the paradigm may shift from albumin replacement to albumin modification strategies in the treatment of patients with liver cirrhosis.
Albumin in liver cirrhosis is a hot topic for research, with expected exciting breakthroughs in the near future.
Abbreviations- •
CLF: acute on chronic liver failure.
- •
HMA: human mercaptoalbumin.
- •
HNA-1: human non-mercaptoalbumin type 1.
- •
HNA-2: human non-mercaptoalbumin type 2.
- •
HRS: hepatorenal syndrome.
- •
IMA: ischemia-modified albumin.
- •
IMAr: IMA/albumin ratio.
- •
LVP: large volume paracentesis.
- •
MARS: molecular adsorbent recirculating system.
- •
NF-kB: nuclear factor-kappa B.
- •
PICD: paracentesis-induced circulatory dysfunction.
- •
PTM: posttranslational modifications.
- •
RAAS: renin angiotensin aldosterone system.
- •
SBP: spontaneous bacterial peritonitis.
None.