Chronic liver disease is characterized by inflammation and fibrosis. Angiogenesis which leading to new vascu-lature may have prognostic value in disease progression. This study examined the implication of 5-lipoxy-genase pathway and angiogenic factors in hepatic fi-brosis progression and whether, the inhibition of arachidonic acid cascade product (cysteinyl leukot-rienes) can represent a potential target for therapy. Cholestasis and subsequent fibrosis was induced by common bile duct ligation and resection (BDL) for 5 weeks in rats. After surgery, Cysteinyl leukotrienes antagonist (montelukast) was orally and daily administrated (10 mg/kg) for 34 days. Sham operated and drug control groups received either saline or montelukast immediately after operation. BDL significantly increased liver hydroxyproline (Hp), nuclear factor kappa B (NF-Kβ), transforming growth factor beta (TGF-β), tissue inhibitor metalloproteinase (TIMP-1), vascular endothelial growth factor (VEGF), and reduced the level of matrix metalloproteinase 9 (MMP-9). On the other hand, montelukast treatment reversed all these biochemical parameters and ameliorated histopatho-logical changes which previously induced by BDL. Findings of the present study suggest that montelukast treatment may favor collagenolytic activity through modulating hepatic expression of TGF-β, NF-κβ, and MMP-9/TIMP-1 ratio. Amelioration of necroinflam-matory liver injury and fibrogenesis may support such assumption.
Liver fibrosis is a major feature of many chronic liver injuries including metabolic, viral, cholestatic and genetic disease.1 Chronic cholestatic liver diseases are characterized by defective bile acid transport from the liver to intestine caused by primary damage to the biliary epithelium.2 Fibrosis and subsequently cirrhosis are causal factors leading to morbidity and mortality related to liver disease.3 Collective evidences indicated that liver fibrosis incorporates uncontrolled inflammation as a part of its etiology. Kupffer cells, which act as resident macrophages in the body represent the primary inflammatory effectors which initiate the inflammatory cascade leading to tissue remodeling and fibrosis.4 As a result, such cascade leads to activation of hepatic stellate cells (HSCs) and initiation of unbalanced synthesis of collagen, proteoglycan and hyaluronate.5 Enhanced matrix synthesis and diminished breakdown of connective tissue proteins may lead to increased deposition of extracellular matrix and subsequent hepatic fibro-sis.6 Matrix metalloproteinases (MMPs) are zinc dependent endopeptidases that catalyze the degradation of extracellular matrix proteins, thereby controlling physiological processes (tissue remodeling and wound healing) as well as pathological ones (liver fi-brosis). Activity of MMPs is controlled by regulation of expression and secretion through proteolytic activation of proenzymes and the tissue inhibitors of metalloproteinases (TIMPs).7 Transcription of TIMP-1 is induced by pro-inflammatory cytokines and transforming growth factor beta (TGFβ1).8 An improper balance between MMPs and TIMP production is expressed as an important determinant of extracellular matrix deposition and breakdown.
Vascular endothelial growth factor (VEGF), an en-dothelial cell–specific mitogenic peptide is synthesized mainly due to hypoxia, endothelial cell damage, and tissue ischemia. It has a key role in vasculo-genesis and angiogenesis.9 VEGF also increases vascular permeability, in turn leakage of plasma proteins into the extra vascular space leading to edema and profound alterations in the extracellular matrix.10 A wealth of evidence indicated that intrahepatic inflammatory process, angiogenesis and up-regulation of vascular endothelial growth factor (VEGF) may play a central role in the current paradigm of liver fibrosis.11 Therefore anti-inflammatory strategies are one of the proposed therapeutic approaches to hepatic fibrosis.
Leukotrienes, as products of 5-lipoxygenase (5LO) pathway can increase microvascular permeability and acting as potent chemotactic agents. Cysteinyl leukot-rienes (CysLT), namely leukotrienes C4, D4, and E4 (LTC4, LTD4, LTE4) are secreted mainly by eosinophils, mast cells, monocytes and macrophages. They exert variety of actions which emphasize their importance as pathogenic elements in inflammatory states.12 Role of leukotrienes as mediators of the gastric damage induced by ethanol or other noxious substances was reported be-fore.13 Recent evidences indicated that 5-LO pathway has converging functions in liver inflammation, tissue remodeling, and fibrosis.14,15
Present study aimed to examine firstly the relative contribution of 5LO and CysLT as pro-inflammatory and angiogenic pathways in relation to hepatic inflammation and fibrosis progression. Secondly study the effect of leukotrienes inhibition through CysLT receptor antagonist (montelukast) on hepatic fibrosis induced experimentally by bile duct ligated and resected (BDL) technique in rats.
Materials and methodsExperimental animalsAdult male albino rats (220-250 g) were supplied by Egyptian Organization for Biological Products and Vaccine (Helwan, Egypt). Rats were housed in stainless steel cages at constant temperature of 25 ± 2C°, relative humidity of approximately 50%, illumination (12 h light/ dark) and had free access to standard pellet chow and water ad libitum. All experiments were carried out in accordance with protocols approved by the local experimental ethics committee.
Surgery and experimental designCholestasis was induced according to Yang et al.17 The common bile duct was doubly ligated at the hilum of the liver with 4-0 silk and transected between the two ligations (bile duct ligated and resected group, BDL). In the sham-operated rats, abdominal incision was made without ligation or transection of the bile duct. Four groups of rats were used. The animals of the first group (n = 10) were sham operated and saline treated for 34 days (Sham-operated control). Second group (n = 10) was sham operated and treated with oral CysLT receptor antagonist, montelukast (Merk Sharp and Dome, USA) suspended in saline using gum acacia (10 mg/kg/day)18 (Drug control). Third group (n = 15) was BDL. The last one (n = 15) was BDL and treated with montelukast orally and daily for 34 days on the second day of surgery. At the end of 5 weeks, all animals from each group were anaesthetized with ure-thane (1.3 mg/kg) and blood samples were collected, subjected to serum separation and divided into ali-quots. Fresh sera were tested for liver enzymes and other aliquots were stored at -20°C for later biochemical analysis. Rats were then killed by decapitation; the livers were collected. The wet livers were weighed and samples from each rat were excised carefully and processed for paraffin section preparation and directed for histological examinations. The remaining livers were frozen in liquid nitrogen and subjected to biochemical assessment.
Liver index calculationLiver index was calculated according to the formula of Yang et al.19 (liver weight/body weight) × 100.
Liver function tests and membrane oxidationAlanine aminotransferase (ALT),20 alkaline phos-phatase (ALP)21 total bilirubin (TB) and direct bilirubin (DB)22 were determined spectrophoto-metrically using commercial kits (Spinreact, Spain) lipid peroxide measured as malondialdyhde (MDA) were estimated as previously described.23
Liver TGFβ10.25 gm of liver tissue was homogenized in 1mL lysis buffer for protein extraction which contained 0.0625 mol/L tris buffer (pH 6.8), 2% sodium dodecyl sulphate (SDS), 3% beta mercaptoethanol, 10% glycerol, 100 mmol/L sodium fluoride, 10 μg/mL aprotinin and 1 mmol/L phenylmethylsulfonyl fluoride (Sigma, USA). Liver tissues were homogenized using a tissue homoge-nizer. After cell lysis the homogenate was centrifuged at 10,000 x g for 20 minutes at 4°C and the supernatant was examined for TGFβ1 using quantitative sandwich enzyme linked immunoassay using kits supplied from R&D Systems (Europe, Ltd. United Kingdom). We followed the instruction of the manufacturer.24
Collagen contentAcid hydrolysis of collagen liberated hydroxyproline (Hp) and was followed by oxidation with chloramine T. Reaction of oxidation product with p-dimethylami-nobenzaldhyde results in formation of colored complex measured in ELISA plate reader at 550 nm. Concentrations of Hp were calculated from plotted standard curve using Hp solution (0-200 μg/ml).25
Detection of NF-kB, MMP-9, TIMP-1, and VEGF gene expression by Reverse transcription-polyme-rase chain reaction (RT-PCR):RNA extractionTotal RNA was extracted from liver tissue by the acid guanidinum thiocyanate-phenol-chloroform method26 RNA content and purity were measured by a UV spectrophotometer. The A260/A280 ratio should be 1.8 to 2.0. The RNA was detected by agarose gel electrophoresis.
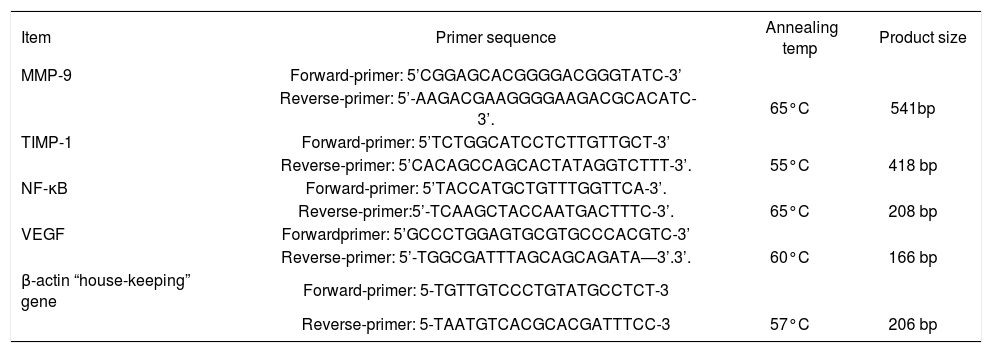
RT-PCR experimentsRT-PCR was done using the extracted RNA for detection of NF-κB, MMP-9, TIMP-1, and VEGF genes. β-actin expression level was determined as loading control for each sample. For amplification of the target genes, reverse transcription and PCR were run in two separate steps. Briefly, equal amounts of total RNA (6 μg) were denatured thermally and reverse transcribed by incubation at 42°C for 90 min with 12.5 U avian myelo-blastosis virus reverse transcriptase (AMV) (Promega Corp., Madison, WI), 20 U ribonuclease inhibitor RNa-sin (Promega Corp.), 200 nM deoxy-nucleoside 5’-triphosphate mixture, and 1 nM oligo-dT primer in a final volume of 30 μL of 1x AMV reverse transcriptase buffer. The reactions were terminated by heating at 97°C for 5 min and cooling on ice. The cDNA samples were amplified in 50 μL of 1x PCR buffer in the presence of 2.5 U Taq DNA polymerase (Promega Corp.), 200 nM deoxy-nucleoside 5’-triphosphate mixture, and the appropriate primer pairs (1 nM of each primer These sets of primers, annealing temperature and product size in Table I. PCR consisted in a first denaturing cycle at 97°C for 5 min, followed by a variable number of cycles of amplification defined by denaturation at 96°C for 1.5 min, annealing for 1.5 min, and extension at 72°C for 3 min. A final extension cycle of 72°C for 15 min was included.27
The oligonucleotide primers sequence of studied genes.
| Item | Primer sequence | Annealing temp | Product size |
|---|---|---|---|
| MMP-9 | Forward-primer: 5’CGGAGCACGGGGACGGGTATC-3’ | ||
| Reverse-primer: 5’-AAGACGAAGGGGAAGACGCACATC-3’. | 65°C | 541bp | |
| TIMP-1 | Forward-primer: 5’TCTGGCATCCTCTTGTTGCT-3’ | ||
| Reverse-primer: 5’CACAGCCAGCACTATAGGTCTTT-3’. | 55°C | 418 bp | |
| NF-κB | Forward-primer: 5’TACCATGCTGTTTGGTTCA-3’. | ||
| Reverse-primer:5’-TCAAGCTACCAATGACTTTC-3’. | 65°C | 208 bp | |
| VEGF | Forwardprimer: 5’GCCCTGGAGTGCGTGCCCACGTC-3’ | ||
| Reverse-primer: 5’-TGGCGATTTAGCAGCAGATA—3’.3’. | 60°C | 166 bp | |
| β-actin “house-keeping” gene | Forward-primer: 5-TGTTGTCCCTGTATGCCTCT-3 | ||
| Reverse-primer: 5-TAATGTCACGCACGATTTCC-3 | 57°C | 206 bp |
All PCR products were run on 2% agarose stained with ethidium bromide and visualized by UV transilluminator.
Semi-quantitative determination of PCR productsSemi-quantitation was performed using the gel documentation system (BioDO, Analyser) supplied by Biome-tra. according to the following amplification procedure. Relative expression of each studied gene (R) was calculated following the formula: R = Densitometrical Units of each studied gene/Densitometrical Units of β-actin.
Histopathological analysisFor light microscopic investigations, specimens from liver were fixed in 10% phosphate buffer formalin, dehydrated in alcohols and embedded in paraffin. Five micron tissue sections were stained with hematoxylin and eosin stain (H&E) for general histopathological examination28 and Masson’s trichrome stain for the determination of fi-brosis.29 Scoring of Histopathological changes were done as follow: (-) abscent; (+) mild; (++) moderate; (+++) severe, (++++) extremely severe.
Statistical analysisResults were expressed as means ± S.D. Statistical evaluation was done using one-way analysis of variance (ANOVA) according to Snedecor and Cochran30 and Tukey’s multiple comparison test. Values of P< 0.05 were considered significant.
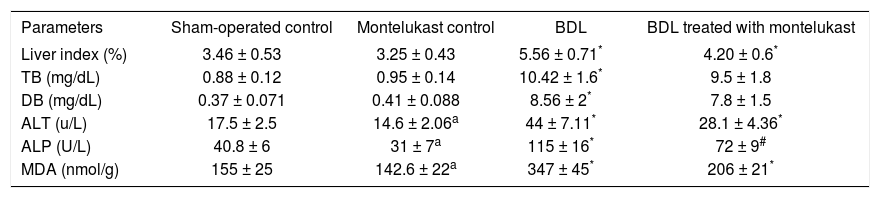
ResultsLiver index, liver function and membrane oxidationTable II shows that bile duct ligation and resection induced significant increase in liver index, TB and DB as well as the enzyme activities of ALT and ALP as compared to sham-operated control rats treated with either saline or montelukast (p<0.05). The MDA levels measured as a major degradation product of lipid peroxidation in liver tissues, were found to be significantly higher in BDL rats (124, 144%) as compared to those of the sham and drug control groups respectively. Cysteinyl leukot-rienes antagonist administration for 34 days significantly reduced liver index (25%), the levels of hepatic function markers (36%) and MDA content (41%). However, it had no effect on bilirubin levels.
Effects of bile duct ligation and montelukast on liver index, cholestasis (serum total and direct bilirubin), liver damage markers (serum ALT and ALP activities) and membrane oxidation (liver MDA).
| Parameters | Sham-operated control | Montelukast control | BDL | BDL treated with montelukast |
|---|---|---|---|---|
| Liver index (%) | 3.46 ± 0.53 | 3.25 ± 0.43 | 5.56 ± 0.71* | 4.20 ± 0.6* |
| TB (mg/dL) | 0.88 ± 0.12 | 0.95 ± 0.14 | 10.42 ± 1.6* | 9.5 ± 1.8 |
| DB (mg/dL) | 0.37 ± 0.071 | 0.41 ± 0.088 | 8.56 ± 2* | 7.8 ± 1.5 |
| ALT (u/L) | 17.5 ± 2.5 | 14.6 ± 2.06a | 44 ± 7.11* | 28.1 ± 4.36* |
| ALP (U/L) | 40.8 ± 6 | 31 ± 7a | 115 ± 16* | 72 ± 9# |
| MDA (nmol/g) | 155 ± 25 | 142.6 ± 22a | 347 ± 45* | 206 ± 21* |
Table III shows that hepatic fibrosis was evident in BDL rats as indicated by 2.8 and 3.4 folds increase in liver content of TGFβ1 and collagen (expressed as Hp) respectively compared to sham-operated control. Mon-telukast treatment dramatically reduced the previous indexes.
Hepatic fibrotic markers in BDL rats and following treatment with leukotriene antagonist montelukast.
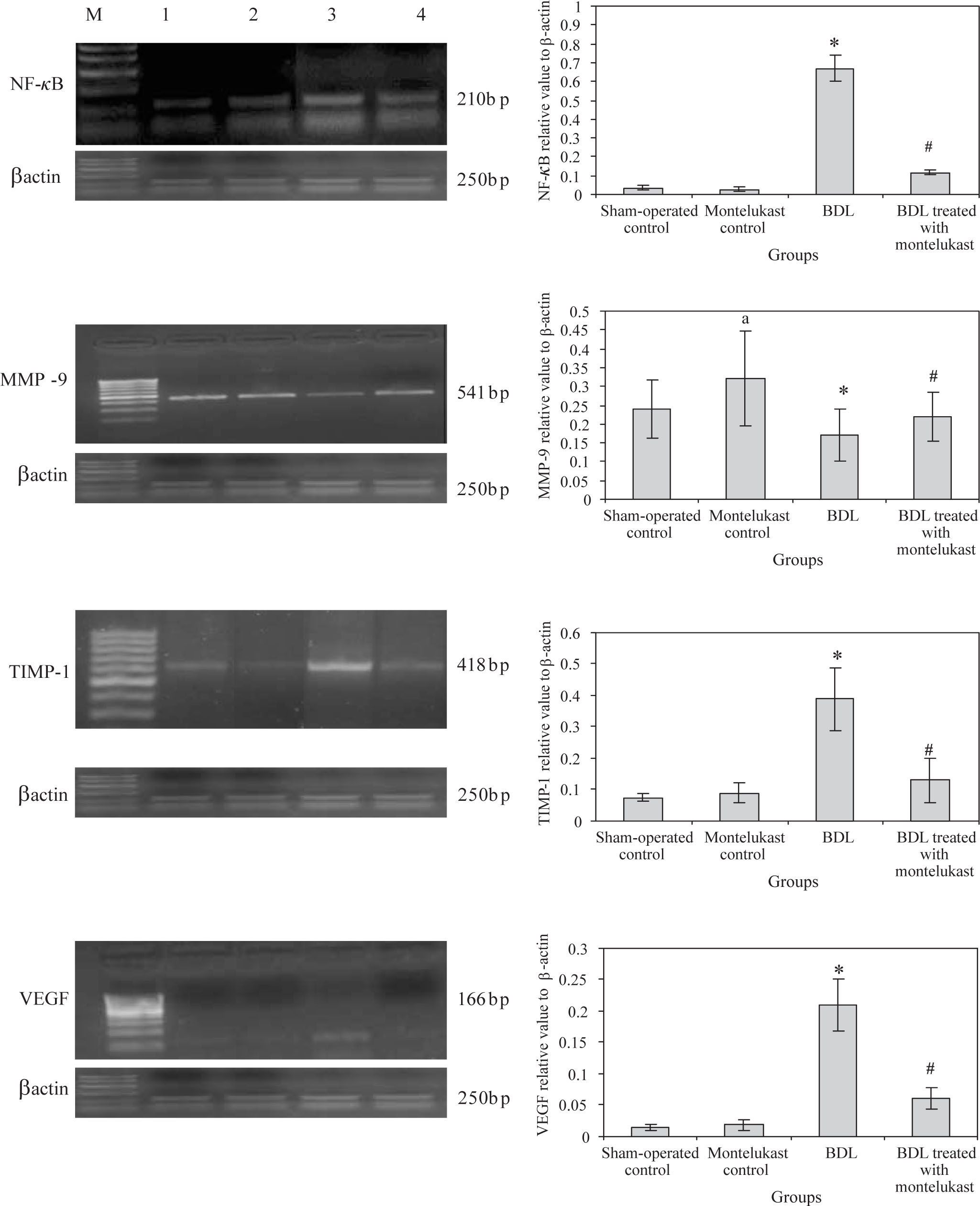
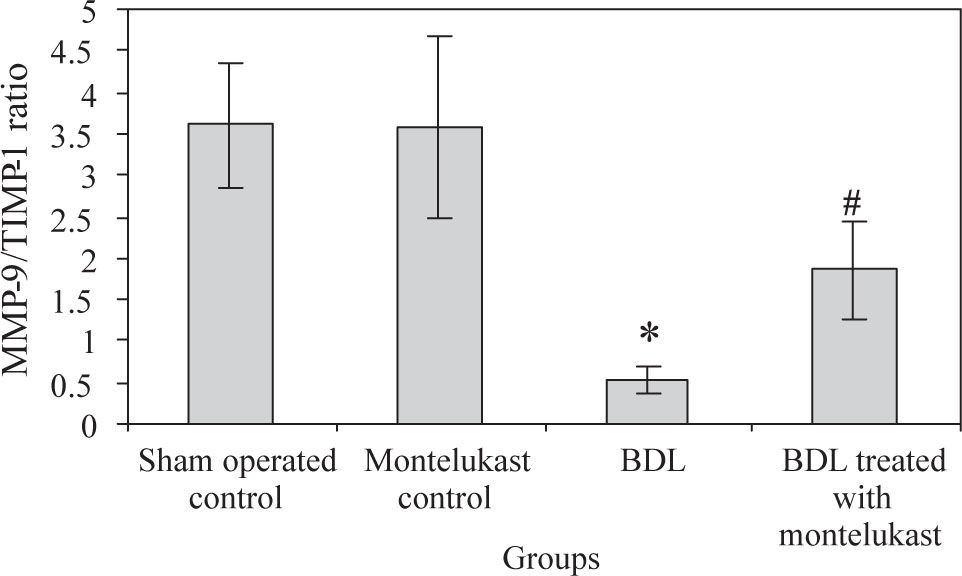
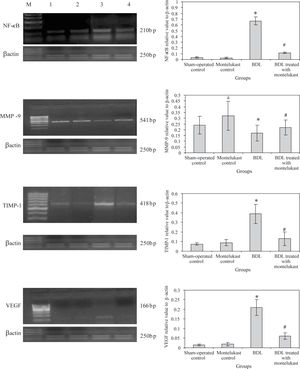
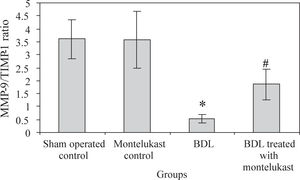
Liver from sham-operated control rats treated with either saline or montelukast expressed low levels of NF-κB, TIMP-1 and VEGF mRNA transcripts, whereas these parameters were significantly increased in BDL control group. However, the expression of MMP-9 mRNA transcript was higher in sham-operated and drug controls, it was dramatically reduced in BDL group. Montelukast therapy greatly reduced levels of NF-κB, TIMP-1 and VEGF mRNA transcripts and remarkably increased expression of MMP-9 (Figure 1). It also induced 3.4 folds increase in MMP-9 / TIMP-1 ratio as compared to BDL group (Figure 2).
mRNA expression levels of hepatic NF-kB, MMP-9, TIMP-1, VEGF, control gene (β-actin) in all rats (n=6 /group). Left panels representative RT-PCR in which Lane M: 100 bp DNA marker, Lane 1, 2, 3 and 4 are representative for sham operated control, monte-lukast control, BDL and BDL treated with montelukast groups respectively. Right panels illustrate the relative levels of mRNA of hepatic NF-κB, MMP-9, TIMP-1, VEGF to β-actin using RT-PCR. Values are mean ± SD of six observations *p < 0.001 vs sham-operated control and montelukest control, #p < 0.01 vs BDL group.
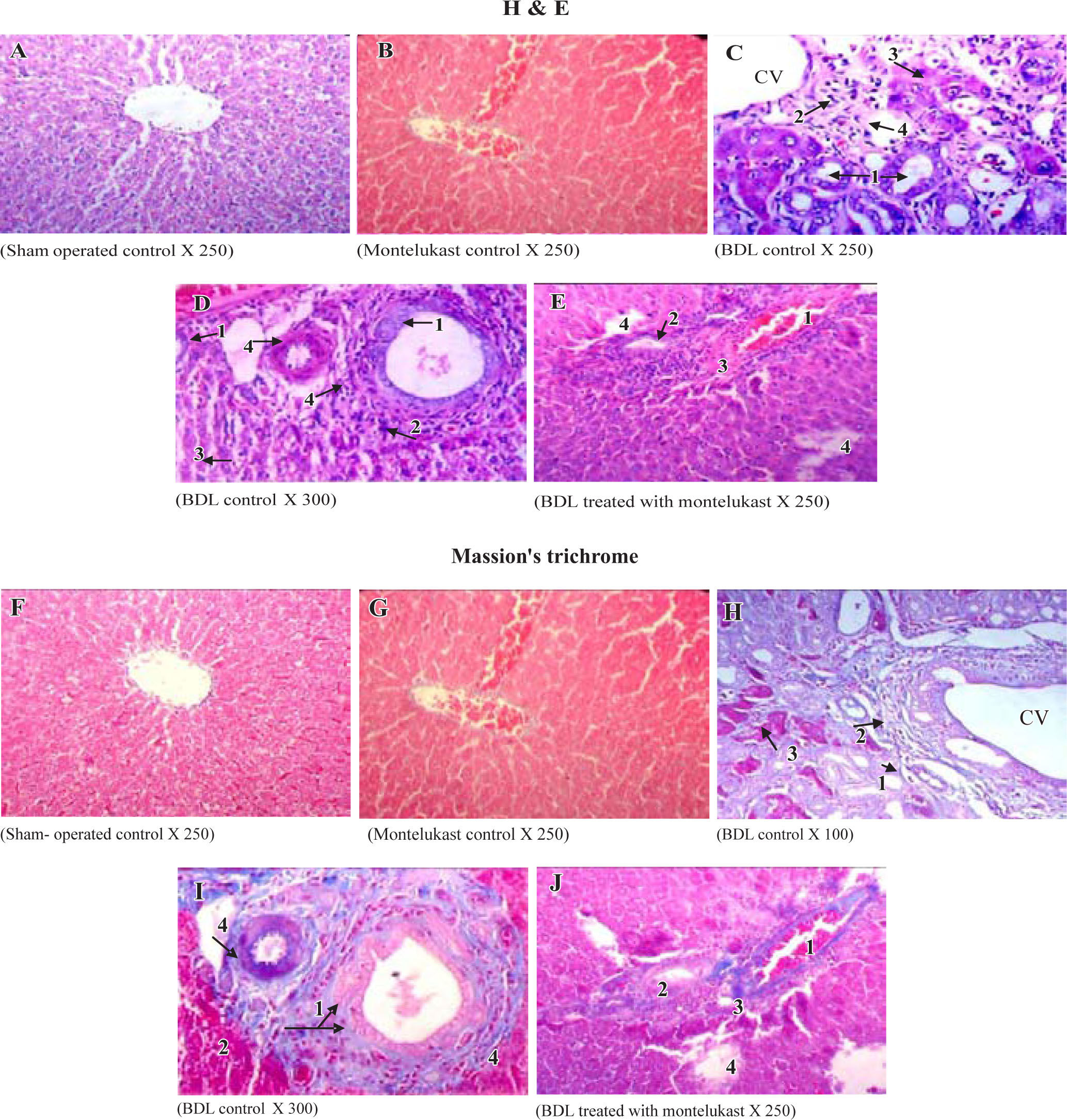
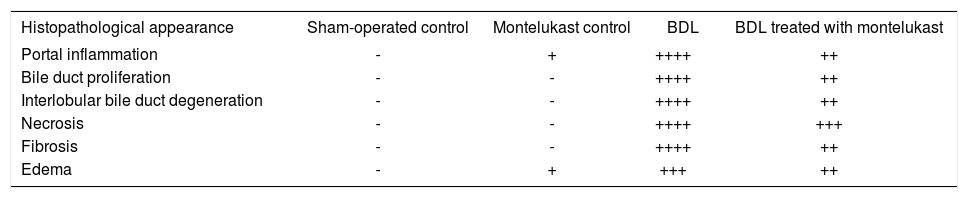
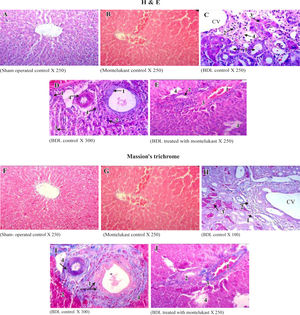
Liver sections from the sham-operated control group rats showed normal hepatocytes, sinusoids and no fibrosis (Figures 3A & 3f). Sham-operated rats treated with montelukast showed mild edema, congestion and nearly normal hepatic cells architecture (Figures 3B & 3G). After BDL, liver of rats revealed extremely severe inflammation, necrosis, fibrosis and ductular proliferation. In addition, an important cellular migration around the portal triad and the central vein (CV) was observed and associated with the relocation of the Kupffer cells (Figures 3C & D). Prolonged biliary obstruction was joined with marked increase in collagen deposition around the portal triad and CV. This was evidenced by the Masson’s trichrome stain that tinges the fibers of the collagen blue. Normal hepatic architecture was also lost, extended necrotic areas and moderate edema were frequently observed with marked ductular proliferation (Figures 3H & I) as compared to sham-operated control group. Mon-telukast treatment significantly changed the profile of collagen fiber deposition in BDL rats. Mild congestion, edema and few bile duct proliferations and degeneration were demonstrated as compared to BDL control. Moderate periportal fibrosis and focal necrosis were also observed (Figures 3E & J. Histopathological changes in liver tissue in the tested groups are illustrated in Table IV.
Histological sections of liver tissue stained with H&E (upper panels, A-B) or Masson’s trichrome (lower panels, F-J). Representative photomicrographs of sham-operated control (A,F) showing normal liver parenchyma with hepatocytes and sinusoids and normal hepatic architecture. sham-operated control treated with montelukast (B,G) showed normal hepatic architecture with mild congestion1 and edema2. BDL rats (C,D,H,I) showed severe bile duct proliferation,1 leukocytes around the portal traid and the CV,2 necrosis of hepatocytes3 and fibrous tissue proliferation.4 BDL rats treated with montelukast (E, J) showed mild congestion,1 few bile duct proliferations with dege-neration,2 mild degree of periportal fibrosis3 and moderate focal necrosis.4
Histopathological changes associated with bile duct ligation and montelukast treatment in rats.
| Histopathological appearance | Sham-operated control | Montelukast control | BDL | BDL treated with montelukast |
|---|---|---|---|---|
| Portal inflammation | - | + | ++++ | ++ |
| Bile duct proliferation | - | - | ++++ | ++ |
| Interlobular bile duct degeneration | - | - | ++++ | ++ |
| Necrosis | - | - | ++++ | +++ |
| Fibrosis | - | - | ++++ | ++ |
| Edema | - | + | +++ | ++ |
Scoring was done as follow: (-) abscent; (+) mild; (++) moderate; (+++) severe, (++++) extremely severe.
Human chronic liver diseases are often characterized by moderate inflammation and necrosis in spite of progression to cirrhosis. The pathogenesis of liver injury during extrahepatic cholestasis induced by bile duct obstruction is poorly understood. Liver dysfunction and cell injury may be contributed.31 In this study, serum levels of liver enzymes (ALT, ALP) and bilirubin were significantly higher in BDL rats than in sham-operated rats. Nishimura et al32 referred to bilirubin as the major harmful factor of harm on the hepatic function during biliary obstruction, but currently the detergent action of bile salt has been emphasized.33 Accumulation of bile salts in the tissue solubilizes membrane phospholipids which in turn can trigger the development of various pathological conditions mediated by cytokines including necrosis and fi-brosis.34 The results of the present study indicated that exacerbation of oxidative injury in liver of BDL rats was more than sham and drug control groups as evidenced by MDA levels. Accordingly, oxidative stress and free radical production may be an important mechanism of chole-static liver injury.35
Fibrosis is an inherent process in liver cirrhosis where cytokines (TGF-β, IL-1β) are involved in triggering and activating of stellate cells (HSCs) to produce collagen.36 Fibrosis was present in BDL rats and it was assessed by two techniques, quantification of collagen and histology with Masson’s trichrome stain. This study demonstrated that BDL induced fibrosis in rats correlated with an increment of TGF-β (important cytokine marker involved in fibrosis) and Hp content of liver tissue. Nuclear factor kappa B (NF-kB) is a nuclear transcriptional activator which plays a central role in the stress response and in-flammation.37 It also plays an important role in liver fi-brosis by regulating the expression of inflammatory response genes and inhibiting apoptosis of HSCs.38 This study reported also increased expression of NF-kB in liver tissue was joined with remarkable increase of liver MDA and TGF-β contents.
Up on liver injury, it is generally accepted that the HSCs play major roles in lesions progression and secretion of extracellular matrix leading to fibrosis develop-ment39. Since collagen is the major component of the extracellular matrix deposited in hepatic fibrosis, most antifibrotic therapies have been directed toward the control of collagen metabolism. Present study evaluated changes in the expression and activity of metalloprotein-ase, MMP-9, and its inhibitor TIMP-1 during liver fibro-sis exacerbations in BDL rats.
Enhanced cysteinyl leukotriene production can increase the tissue damage due to its vasoconstrictive, mu-tagenic, and chemotactic effects, resulting in more mono-nuclear cell recruitment.40 Repetitive tissue damage induces repair processes in liver tissue which are mostly mediated by TGF-β. The latter downregulates the gene encoding for MMP-9, associated with an upregulation of the gene for TIMP.41 Since the action of MMPs is regulated by TIMPs, the MMPs/TIMPs ratio may be considered as evaluating marker for collagenolytic enzyme activity.
VEGF represents an important chemotactic mediator resulting in recruitment of mononuclear cells in damaged tissue. The current findings demonstrated that the inflammatory process occurred during liver cholestasis may involve intrahepatic angiogenesis and up-regula-tion of VEGF. This may be in close relationship to TGF-β expression.42 Experimental data supported the implication of angiogenic factors expressed by hepatocytes as a key event in the development of hepatic fibrosis.43
The failure of bile salt excretion in cholestasis here certainly leads to retention of hydrophobic bile salts within the hepatocytes and causes apoptosis and/or severe fibrosis. This was evidenced by marked increase in collagen content (Hp levels) and its deposition around the portal triad and CV (Masson’s trichrome stain). In ad-dition, extended necrotic areas and edema were frequently observed with marked ductular proliferation. These findings are in agreement with previous studies.1,44
This study evaluates the effects the LT receptor blocker (montelukast) on factors involved in necro-inflammatory lesions and liver fibrogenic events in BDL rats. The current results demonstrated that montelukast effectively reversed fibrosis and liver damage induced by biliary obstruction in rats. It reduced the degree of hepatocellular injury as observed through decreased ALT, ALP activities, liver MDA and morphological aspects of the fibrotic liver. Moreover, the beneficial effect of montelukast on postoperative hepatic cholestasis was shown in this study, it reduced the liver expression of TGF-β and NF-κB by inflammatory cells and increased the expression of MMP-9 by sinusoidal cells. These effects coincided with a significant increase in MMP-9/TIMP-1 ratio suggesting that such treatment favored a collagenolytic activity. These findings are consistent with previous studies showing the potential of 5-LO-derived products to modulate the expression and synthesis of proinflammatory cytokine in monocytes and macrophages45. Blocking the CysLTs receptors are responsible for increased permeability and recruitment of neutrophils and macrophages may be of benefit here. Mon-telukast may also act as an antioxidant not only by blocking the recruitment of neutrophils and macrophages, but also through an interaction with the receptors expressed on the neutrophils and macrophages.46
Furthermore, the LT receptor blocker significantly decreased VEGF expression in agreement with reported studies.47,48 However, the exact explanations for the pathophysiological interactions between VEGF expression and LT production were obscure, we speculated that VEGF expression may be augmented due to endothelial cell damage induced by BDL. In concert with TGF-• •enhanced expression of VEGF resulting in mononuclear cell recruitment into damaged liver tissue, the main source of enhanced LT production.49 The current study demonstrated that LT receptor blocker montelukast was able to down-regulate the expression of VEGF and TGF-• •.
Other mechanisms independent of direct 5-LO inhibition to exert hepatoprotective actions was through activation of peroxisome proliferator-activated receptor-gamma, a transcription factor that mediates anti-inflammatory and antifibrogenic effects in the liver.49
In summary, The current data support the concept that 5-LO pathways have converging functions, not only in the progression of liver inflammation, but also in cell proliferation, angiogenesis and fibrosis in BDL animal model. Our findings suggest that cysteinyl leukotriens antagonist may favor collagenolytic activity by modulating hepatic expression of TGF-β, NF-κB, TIMP-1 and MMPs. These effects may account for the beneficial strategy for prevention of necroinflammatory liver injury and fibrogenesis. This may encourage further experimental and clinical studies to evaluate the efficacy of mon-telukast in the management of hepatic fibrosis and its co-morbid complications.