Aim. Obesity and insulin resistance are associated with nonalcoholic fatty liver disease (NAFLD). It was recently reported that the ratio between levels of ghrelin and obestatin is also associated with obesity and insulin resistance. We investigated the association between the ghrelin/obestatin ratio and NAFLD.
Methods. This cross-sectional study included 98 subjects (51 NAFLD patients and 47 controls). Anthropometric, metabolic and biochemical variables were measured and serum concentrations of ghrelin and obestatin were determined. Logistic regression analyses (univariate and multivariate) were conducted to determine whether NAFLD was associated with ghrelin and obestatin levels and the ghrelin/obestatin ratio.
Results. We studied 51 NAFLD cases and 47 controls. Men comprised 82% of cases and 61% of controls. The mean ages of the groups differed significantly. Body mass index (P < 0.001), waist circumference (P < 0.001) and WHR (P < 0.001) were significantly greater in the NAFLD group than in the control group. The NAFLD group had higher mean fasting glucose level (P = 0.001), HOMA-IR index (P < 0.001) and triglyceride level (P < 0.001) than the controls. Ghrelin and obestatin concentrations were classed according to tertiles. Multivariate analysis revealed a negative correlation between ghrelin and obestatin levels and an overweight status, obesity and metabolic syndrome. Ghrelin and obestatin were evaluated in multivariate logistic regression analysis, they had a protective effect against hepatic steatosis after controlling for potential confounders.
Conclusion. Serum ghrelin and obestatin concentrations are correlated with a low risk of developing NAFLD. However, ghrelin/obestatin ratio was not correlated with NAFLD.
Author contributions
Ylse Gutierrez-Grobe, Israel Villalobos-Blasquez and Karla Sánchez-Lara performed the research and the contributed equally to this work; Antonio R. Villa did the statistical analysis, Guadalupe Ponciano-Rodríguez and Martha H. Ramos supervised the analytical analysis. Norberto Chavez-Tapia wrote, correct and criticize some parts of the manuscript. Misael Uribe and Nahum Méndez-Sánchez designed the research and wrote the paper.
Supportive foundations
Supported by National Council of Science and Technology of Mexico and Medica Sur Clinic and Foundation
IntroductionNonalcoholic fatty liver disease (NAFLD) is a chronic disease that is attracting interest worldwide because of its increasing incidence and its association with the obesity epidemic. NAFLD may progress to nonalcoholic steatohepatitis, liver cirrhosis and hepatocellular carcinoma.1,2
Ghrelin is a peptide hormone that is produced by the stomach and other organs, including the liver and gallbladder.1 Also induces satiety by binding to its hypothalamic receptor, GHS1-a, and its serum level decreases after a meal and increases during fasting.3 Obese individuals and patients with NAFLD exhibit hypoghrelinemia,4,5 which is associated with insulin resistance (IR).6,7 The effects of ghrelin on hepatic fatty acid metabolism have been observed in experimental models.8 After intravenous administration to rats, ghrelin induces an increase in hepatic triglyceride content by activating acetyl-CoA carboxylase and fatty acid synthase and by downregulating CPT-I.
Obestatin is a recently discovered peptide that is encoded by the preproghrelin gene. Obestatin was originally thought to be a ligand of GPR39, an orphan receptor belonging to the ghrelin receptor family,9 but recent studies were unable to confirm this finding.10,11 Obestatin was also thought to display effects opposite to those of ghrelin.9,12 However, recent studies do not support a role for obestatin in the regulation of food intake, body weight, energy expenditure or growth hormone secretion,13 and other physiological activities has been described as inhibition of thirst, memory, regulation of sleep, cell proliferation and survival of pancreatic β cells.14
Considering the similarities among ghrelin and obestatin the ratio of these hormones has been used to analyze the imbalance of the obestatin and ghrelin system.15 The association between levels of obestatin and ghrelin and NAFLD has not been investigated.
Current research in this field is focused on the ghrelin/obestatin ratio in obesity and related metabolic disorders. Obese subjects exhibit higher preprandial plasma ghrelin/obestatin ratios than healthy individuals with normal body weight.16 It is suggested that the balance between levels of ghrelin and obestatin may play a role in the regulation of energy and lipid homeostasis.
The aim of this study was investigate the association between the ghrelin/obestatin ratio and NAFLD.
Material and MethodsStudy populationThis cross-sectional study was conducted at the check-up unit of the diagnostic clinic of the Medica Sur Clinic and Foundation (University Hospital). This hospital provides care mainly for middle-and high-income individuals from Mexico City and the surrounding metropolitan areas. Our sample population was selected from a series of consecutive asymptomatic subjects who were referred to the check-up unit by their employers as an annual requirement of employment, not because of symptomatic disease. Ninety-eight subjects agreed to participate in the study, 51 of whom were found to have NAFLD (9 women and 42 men) and 47 controls (18 women and 29 men). The study was approved by the Human Subjects Committee of the Medica Sur Clinic and Foundation, and conforms to the ethical guidelines of the 1983 Declaration of Helsinki. Written, informed consent was obtained from all participants before entry into the study.
Real-time ultrasonographic studies were performed while the subjects were fasting. A 3.5 MHz transducer was used to obtain the following images: sagittal view of the right lobe of the liver and right kidney, transverse view of the left lateral segment of the liver and spleen, transverse view of the liver and pancreas and any focal areas of altered echotexture (Elegra; Siemens Medical Systems, Mountain Grove, CA). The severity of echogenicity was graded as follows:17 grade 0, normal echogenicity; grade 1, a slight, diffuse increase in fine echoes in liver parenchyma with normal visualization of the diaphragm and intrahepatic vessel borders; grade 2, a moderate, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and the diaphragm; and grade 3, a marked increase in fine echoes with poor or no visualization of the intrahepatic vessel borders, diaphragm and posterior right lobe of the liver. Sonographic patterns were classed as:
- •
0. Homogeneous, normal.
- •
1. Hyperechoic nodules.
- •
2. Multiple, confluent hyperechoic lesions.
- •
3. Hypoechoic skip nodules.
- •
4. Irregular hyperechoic and hypoechoic areas.
- •
5. Diffuse involvement.
Participants completed a food frequency questionnaire in which commonly used portions were defined. The questionnaire included questions on the brands of multivitamin and individual vitamin supplements used and the frequency with which they were consumed. Daily intakes of energy, protein, carbohydrate, total fat, saturated fat, polyunsaturated fat, monounsaturated fat, vitamins, minerals and antioxidants were determined using SNUT software, a program developed by the Public Health National Institute, Mexico City,18 which is appropriate for the Mexican population.
Physical examinationThe body weight of participants in light clothing and without shoes was measured to the nearest 0.10 kg. Height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Overweight was defined as a BMI of 25.0–29.9 kg/m2 and obesity was defined as a BMI >30 kg/m2. Waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower border of the rib cage and the iliac crest. Hip circumference was measured to the nearest 0.1 cm at the widest point between the hip and buttock. Body fat percentage was measured using bioelectrical impedance (Omron body fat analyzer, model HBF-306INT).
Analytical proceduresInsulin concentrations were measured using an immunoenzymometric assay (MEIA; Abbott Diagnostics) that has inter-and intra-assay coefficients of variation of less than 3%. Plasma glucose concentrations were measured in duplicate in the fasting state using an automated analyzer. The coefficient of variation for a single determination was 1.5%. Total cholesterol, high density lipoprotein cholesterol (HDL-C) and triglyceride concentrations were measured using enzymatic colorimetric methods (CHOL, HDL-C plus [second generation] and TG assays, respectively; Roche Diagnostics Co., Indianapolis, IN). Low density lipoprotein cholesterol (LDL-C) concentrations were calculated using the Friedewald formula.19 Assessment of IR was made using the homeostasis model assessment (HOMA-IR) originally described by Matthews, et al.20 HOMA-IR = fasting insulin level (U/L) fasting glucose level (mmol/L) / 22.5. Serum ghrelin and obestatin concentrations were determined in fast of 8-12 hours using radioimmunoassay kits (Linco Research, St. Charles, MO). The intra-and inter-assay coefficients of variation are both less than 5%.
Statistical analysisThe mean ± SD was used to describe distributions of continuous variables when comparing NAFLD cases with controls. The nonparametric Mann–Whitney U-test was used to compare these variables. Ghrelin and obestatin distributions were compared between NAFLD cases and controls according to tertiles. χ2 testing for linear trend was used to test for dose–response relationships. Logistic regression analysis was conducted to test ghrelin and obestatin distributions according to tertiles as a main effect in determining the probability of hepatic steatosis while controlling for potential confounders. Odds ratios (ORs) were derived for the exponential of the regression coefficient and 95% CI were calculated. All statistical analyses were carried out using SPSS/PC v16.0 software (LEAD Technologies, Chicago, IL).
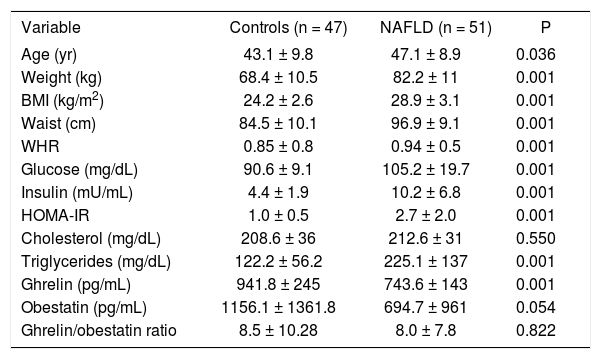
ResultsWe studied 51 NAFLD cases and 47 controls. Men comprised 82% of NAFLD cases and 61% of controls. There were statistically significant differences between NAFLD cases and controls in age, weight, BMI, waist circumference, waist/hip ratio (WHR), IR, and concentrations of glucose, insulin, triglycerides and ghrelin (Table 1). The mean ages of the groups differed slightly but significantly (P < 0.036). BMI (P < 0.001), waist circumference (P < 0.001) and WHR (P < 0.001) were significantly greater in the NAFLD group than in the control group. The NAFLD group had significantly higher mean fasting glucose level (P = 0.001), HOMA-IR index (P < 0.001) and triglyceride level (P < 0.001) than the controls. Compared with the controls, subjects with NAFLD were older, had greater body weight, BMI, central obesity and IR, and had lower serum ghrelin (Figure 1) and obestatin levels.
Clinical, anthropometric, metabolic, and biochemical characteristics of nonalcoholic fatty liver disease cases and controls (mean ± SD).
| Variable | Controls (n = 47) | NAFLD (n = 51) | P |
|---|---|---|---|
| Age (yr) | 43.1 ± 9.8 | 47.1 ± 8.9 | 0.036 |
| Weight (kg) | 68.4 ± 10.5 | 82.2 ± 11 | 0.001 |
| BMI (kg/m2) | 24.2 ± 2.6 | 28.9 ± 3.1 | 0.001 |
| Waist (cm) | 84.5 ± 10.1 | 96.9 ± 9.1 | 0.001 |
| WHR | 0.85 ± 0.8 | 0.94 ± 0.5 | 0.001 |
| Glucose (mg/dL) | 90.6 ± 9.1 | 105.2 ± 19.7 | 0.001 |
| Insulin (mU/mL) | 4.4 ± 1.9 | 10.2 ± 6.8 | 0.001 |
| HOMA-IR | 1.0 ± 0.5 | 2.7 ± 2.0 | 0.001 |
| Cholesterol (mg/dL) | 208.6 ± 36 | 212.6 ± 31 | 0.550 |
| Triglycerides (mg/dL) | 122.2 ± 56.2 | 225.1 ± 137 | 0.001 |
| Ghrelin (pg/mL) | 941.8 ± 245 | 743.6 ± 143 | 0.001 |
| Obestatin (pg/mL) | 1156.1 ± 1361.8 | 694.7 ± 961 | 0.054 |
| Ghrelin/obestatin ratio | 8.5 ± 10.28 | 8.0 ± 7.8 | 0.822 |
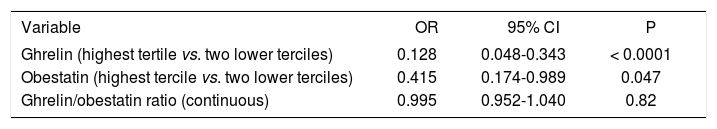
Ghrelin and obestatin concentrations were classed according to tertiles (Figure 2). Multivariate analysis revealed a negative correlation between ghrelin and obestatin levels and an overweight status, obesity and metabolic syndrome. When ghrelin and obestatin were evaluated as main effects in multivariate logistic regression analysis, they had a protective effect against hepatic steatosis after controlling for potential confounders (Table 2). This protective effect increased as the tertile increased. The ghrelin/obestatin ratio did not have any protective effect against hepatic steatosis in patients with NAFLD.
Associations between level of ghrelin and obestatin and nonalcoholic fatty liver disease according to multivariate logistic regression analysis.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Ghrelin (highest tertile vs. two lower terciles) | 0.128 | 0.048-0.343 | < 0.0001 |
| Obestatin (highest tercile vs. two lower terciles) | 0.415 | 0.174-0.989 | 0.047 |
| Ghrelin/obestatin ratio (continuous) | 0.995 | 0.952-1.040 | 0.82 |
The presence of NAFLD is associated with several metabolic disturbances as insulin resistance, low serum adiponectin levels, high leptin levels and other hormones. Most of these changes has been described initially in obese subjects, but also has been observed in NAFLD as independent association 5. This could suggest a direct effect on liver physiology or an indirect effect mediated by the metabolic syndrome.
In this study, we described a negative association between ghrelin and obestatin levels and the presence of NAFLD, but the ghrelin/obestatin ratio was not associated with NAFLD.
Obese subjects had lower serum ghrelin concentrations than controls, and there was a significant negative association between serum ghrelin levels and metabolic syndrome, HOMA-IR and NAFLD, which confirms our previous suggestion that ghrelin plays an important role in the pathogenesis of NAFLD.1 Information on in vitro studies also suggests the importance of ghrelin on liver physiology, particularly on glucose and insulin liver metabolism21 and reduction of liver inflammatory response induced by endotoxemia,22 another potential player in NAFLD23 this anti-inflammatory effect has been explored also in animal models.24
The other hormone disturbance described in this study was the lower levels of obestatin in subjects with NAFLD, this is particularly interesting due to the controversies about the high and low serum levels of obestatin reported in obese subjects.11-13 Obestatin is a peptide that has been suggested to play an important role in energy metabolism, and some reports have shown that low serum concentrations of obestatin are positively correlated with levels of total cholesterol and triglycerides, but not with anthropometric and other metabolic parameters, their role in the regulation of metabolism is still under debate.15,25 We found that low levels of obestatin are associated with overweight status, metabolic syndrome and NAFLD, a similar pattern as those observed on ghrelin levels, and this inverse relationship was also described in human obesity.26 However the lack of association between NAFLD status and ghrelin/obestatin ratio, suggests a dissimilar effect among these two hormones. This observation is different to previous reports on obese women in which the ghrelin/obestatin ratio is inversely associated with insulin resistance and anthropometrical markers of obesity.15 This could be due differences among subjects with obesity and NAFLD.
The findings of this study are only related with image-based NAFLD, and the lack of liver biopsy could be a limitation, but per se NAFLD has been described as a state of metabolic disturbance and risk factor for other non-hepatic diseases.27,28
In summary, we found that high levels of obestatin and ghrelin, but not the ghrelin/obestatin ratio, are correlated with a low risk of developing NAFLD. The ghrelin/obestatin ratio is decreased in patients who have features of metabolic syndrome. Obestatin plays an important role in energy metabolism, and further research is warranted.