Alcoholic liver disease (ALD) covers a wide spectrum of pathology ranging from fatty liver disease to acute steatohepatitis to cirrhosis and/or hepatocellular carcinoma. Alcoholic foamy degeneration (AFD) is an uncommon, potentially life-threatening condition that is part of the spectrum of ALD. It is characterized by extensive microvesicular steatosis in the perivenular areas. Since the first description in 1983, few case reports have been described. Here, we report 2 cases of AFD in patients with a previous history of chronic alcohol abuse and histological diagnosis of AFD with typical clinical, biochemical and histological features. In both cases we provide data on the hepatic hemodynamic status, and in one of them we report liver elastography results, which are features that have not been described previously. In both cases there was rapid resolution of biochemical and clinical abnormalities after complete abstinence, which is the mainstay of treatment for AFD.
Among the variety of liver abnormalities that can be found in patients with an excessive alcohol intake, there is an uncommon condition termed alcoholic foamy degeneration (AFD), characterized by a noninflammatory microvesicular fatty accumulation, particularly of centrilobular distribution.1 AFD may be present in those patients with chronic alcohol abuse after a recent increase in the amount of alcohol consumption. The gold standard for diagnosis is liver biopsy as the histological features are ty-pical.1 The clinical spectrum ranges from asymptomatic patients to an acute liver decompensation and its prognosis is good in the wide majority of cases with alcohol abstinence.2 The laboratory findings are also variable and the most common feature is a high triglyceride and cholesterol concentration. Although there are cases described with a marked elevation of gamma glutamyl transpeptidase (GGT) serum levels the most features are a moderate elevation of serum bilirubin, GGT and amino-transferases.3 Here, we report two cases of patients with a history of chronic alcohol intake presenting an acute impairment of liver function and histologi-cal findings characteristic of AFD.
Case Report # 1A 32 year old male with a history of tobacco smoking and several years of alcohol abuse was admitted with a 20 day history of malaise, asthenia, anorexia and nausea. He acknowledged a recent increase in his usual alcohol consumption up to 220 g per day after a 6 month period of abstinence. He denied the use of other toxics or drugs except for ven-lafaxine for the treatment of a depressive syndrome. The patient specifically denied the use of acetaminophen. Physical examination revealed jaundice and painless hepatomegaly. There were no clinical signs of advanced cirrhosis, ascites or hepatic encephalopathy.
Blood work at admission is listed in table 1. Serological tests ruled out hepatitis B or C virus infection. An abdominal ultrasound showed an enlarged liver with smooth borders and increased echogenicity without liver focal lesions, ascites, portal or hepatic vein thrombosis or other abnormalities.
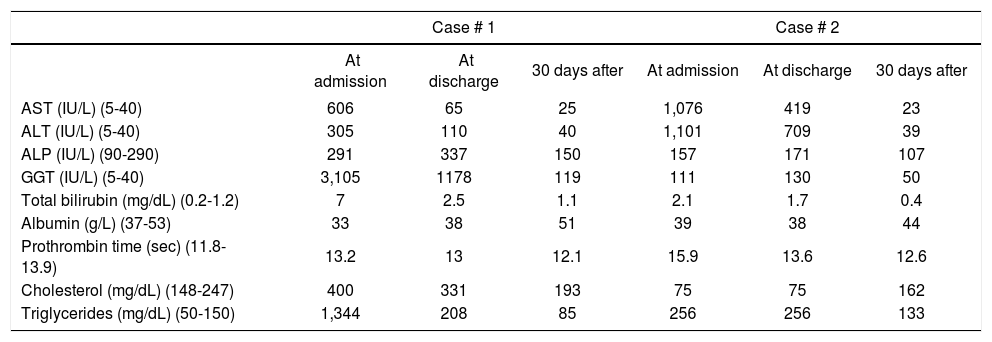
Biochemical characteristics at admission, discharge and after 30 days of hospitalization.
| Case # 1 | Case # 2 | |||||
|---|---|---|---|---|---|---|
| At admission | At discharge | 30 days after | At admission | At discharge | 30 days after | |
| AST (IU/L) (5-40) | 606 | 65 | 25 | 1,076 | 419 | 23 |
| ALT (IU/L) (5-40) | 305 | 110 | 40 | 1,101 | 709 | 39 |
| ALP (IU/L) (90-290) | 291 | 337 | 150 | 157 | 171 | 107 |
| GGT (IU/L) (5-40) | 3,105 | 1178 | 119 | 111 | 130 | 50 |
| Total bilirubin (mg/dL) (0.2-1.2) | 7 | 2.5 | 1.1 | 2.1 | 1.7 | 0.4 |
| Albumin (g/L) (37-53) | 33 | 38 | 51 | 39 | 38 | 44 |
| Prothrombin time (sec) (11.8-13.9) | 13.2 | 13 | 12.1 | 15.9 | 13.6 | 12.6 |
| Cholesterol (mg/dL) (148-247) | 400 | 331 | 193 | 75 | 75 | 162 |
| Triglycerides (mg/dL) (50-150) | 1,344 | 208 | 85 | 256 | 256 | 133 |
AST: aspartate aminotransferase. ALT: alanine aminotransferase. ALP: alkaline phosphatase. GGT: gamma-glutamyl transpeptidase. Laboratory normal values in brackets.
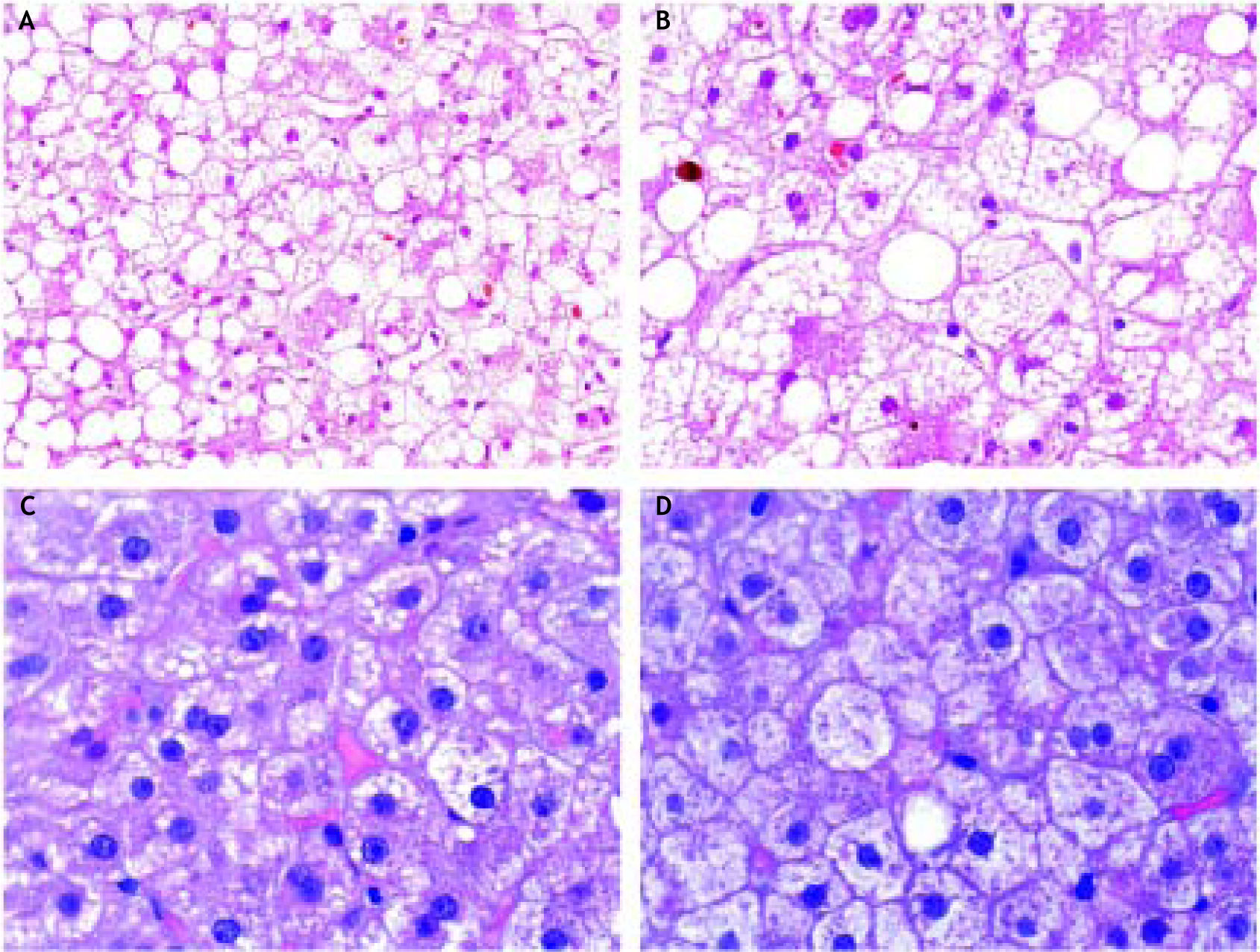
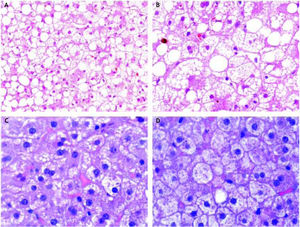
A liver hemodynamic study and a transjugular liver biopsy were performed in order to obtain portal pressure and to conduct a histological study to establish a definite diagnosis. The hepatic venous pressure gradient (HVPG) was 4 mmHg. The histological examination showed a normal hepatic architecture and a microvacuolar steatosis affecting all the lobules, with occasional macrovacuoles. Ca-nalicular cholestasis was found as well. Portal spaces were normal and there was no inflammatory infiltrate (Figure 1). These findings, together with the clinical setting, were considered diagnostic of AFD of the liver.
The patient remained abstinent from alcohol during hospitalization, under treatment with clometia-zole for his alcohol abuse disorder. Consequently there was a marked improvement in liver chemistries (Table 1). The patient was discharged and follow-up one month later all liver tests were normal, except for a mild elevation of GGT, of 119 IU/l.
Case Report # 2A 34 year old male with a history of chronic alcohol abuse and tobacco smoking was admitted to the hospital due to a 3 days history of severe abdominal pain, nausea, and vomiting. The patient had been on a binge of alcohol (at least of 100 g per day for the last week). He admitted having consumed no more than 2 g of acetaminophen for his abdominal pain, denying the intake of any other medication or illicit drug. There were no signs of frank jaundice, ascites or gastrointestinal bleeding. At admission, he was alert and conscious, afebrile and hemodynamically stable. The physical examination revealed diffuse abdominal pain without signs of acute peritonitis, hepatomegaly or splenomegaly. There were no signs of hepatic encephalopathy. Laboratory data at admission is shown in table 1. The blood alcohol content was 1.67 g/L. In the serological exam, positive IgG for hepatitis A, and negative anticore and surface IgG and IgM for hepatitis B virus, and also negative IgG and RNA of hepatitis C virus were found. An ultrasound examination showed a normal sized and structured liver, without focal lesions, normal bile ducts and portal circulation, and absence of splenomegaly. It ruled out other causes of abdominal pain as well. The elastography study found a 4.9 kPa liver stiffness (IQ range 0.6, success rate 100%). A hepatic hemodynamic study showed a HVPG of 5 mmHg. A transjugular biopsy showed diffuse cyto-plasmic microvesicules with small areas of macro-vesicules and megamitochondria. The liver architecture was preserved without evidence of fi-brosis, signs of alcoholic hepatitis (AH) or tissue necrosis. Mild grade necroinflammatory foci with some ballooning degeneration were observed (Figure 1). All these findings were diagnostic of AFD.
Alcoholic liver disease with typical alcoholic foamy degeneration changes. Case report # 1 (A and B). Mixed component of stea-tosis. Although ma-covesicular steatosis is present, large patches of hepatocytes with fat microvesicules filling and expanding the cytoplasm are present. Some bile canalicular thrombi can also be observed. Hematoxylin and eosin stain (H&E). Case report # 2 (C). Extensive areas of microvesicular steatosis could be seen. H&E. D. Areas of hepatocellular ballooning with focal presence of giant mitochondria were observed. H&E.
The abdominal pain responded to analgesic treatment with low doses of acetaminophen. Under complete alcohol abstinence, the patient improved and liver tests normalized. One month after discharge he was abstinent, asymptomatic and laboratory data were normal (Table 1).
DiscussionExcessive alcohol ingestion may induce liver abnormalities, such as simple fatty infiltration, inflammation, fibrosis deposition, AH and cirrhosis with hepatocarcinoma.4 Fatty deposition in alcoholic liver disease is usually characterized by a single large vacuolar fat droplet in hepatocytes.5 In contrast, other type of alcoholic liver injury characterized by a histological pattern of microvesicular fatty infiltration and degeneration has been reported previously.1,2 AFD is characterized by microvesicu-lar fatty deposition with centrilobular distribution, presence of perivenular hepatocyte megamitochon-dria without cytoplasmatic bile pigment deposition and intact lobular architecture.6 The prevalence of AFD is highly variable, ranging from 0.8 to 14%.1,7 In Spain, a previous study from our Liver Unit reported a prevalence of 2.3% among chronic alcoholics.2
The pathogenesis of AFD is not completely understood and other factors apart from alcohol consumption probably contribute to its occurrence.
Neither the amount of alcohol intake nor the duration of alcohol abuse has been related with the development of AFD.3,8 Other factors thus should be taken into account, such as individual genetic susceptibility, nutritional status, impaired intestinal absorption of some trace elements and vitamins.
From a clinical standpoint, AFD is usually characterized by a mild to moderate transient elevation of serum hepatic aminotransferases and bilirubin levels. Leukocytosis is absent in most cases and no serum markers for acute viral hepatitis are identifia-ble.2 Patients with AFD may have an elevated GGT and ALP levels.3 Even though elevated GGT and ALP lack specificity, they are sensitive markers for hepatobiliary disease. In fact, a GGT to ALP ratio greater than 1.4 was reported to have a diagnostic efficiency of 78% for alcoholic liver disease.9 Although patients with AFD have an elevated ALP and GGT, they do not have extrahepatic obstruction that explains the cholestatic picture.3
We describe 2 cases of AFD with some different features. In the first one, we describe the case of a chronic alcoholic with a more typical clinical and biochemical presentation of AFD. The patient had asthenia, abdominal pain and hepatomegaly with a 2:1 ratio of AST to ALT, as typically seen in ALD, moderate hy-perbilirubinemia and increased serum lipid concentrations. Interestingly, this patient also showed markedly high GGT serum levels, a feature that has been described.3 In contrast to chronic alcoholics with liver disease, where the prevalence of hypertri-gliceridemia ranges from 18 to 27%, most patients with AFD have raised serum lipid concentrations. Hyperlipidemia in patients with AFD could be related to alterations in the functional enzymatic activity1,2 (e.g. lecithin cholesterol acyltransferase10 and hepatic lipase11) found in hepatocytes with fatty microvesi-cles. On the other hand, the extensive disruption of intracellular organelles such as the endoplasmic reti-culum, Golgi apparatus, and mitochondria in the he-patocytes has been proposed as the mechanism for elevated GGT in these patients.3
In the second clinical case a more atypical presentation is described. The most relevant clinical difference was the absence of hepatomegaly. Interestingly, liver biochemistry showed markedly high aminotransferase levels with a 1:1 AST/ALT ratio, mild hyperbilirubinemia and normal GGT, cholesterol and tryglicerides. Other causes of acute hepatitis were ruled out. The presence of ballooning degeneration on the liver biopsy could explain, at least in part, the elevated aminotransferase levels of this patient. Ballooning degeneration is a form of liver parenchymal cell death and it is generally regarded to be a form of apoptosis.12 However, some foci of necroinflammation were also seen on histology. This cell necrosis could also be proposed to be the cause of elevated serum aminotransferases. Regarding the presence of necroinflammatory injury in AFD patients, this is a feature also described in the previous series from our unit, where around half of the patients presented neutrophilic exudation and/or hepatocellular necrosis.2
The liver hemodynamic status of patients with AFD has not been described previously. Both cases had a normal hepatic venous pressure gradient and no evidence of macroscopic intrahepatic shunts. This is interesting given that most chronic alcoholics with acute decompensations or with an acute episode of AH have an HVPG which can be markedly eleva-ted.13 Normal HVPG among patients with AFD probably reflects the scarce deposition of perivenular and pericellular fibrosis, a characteristic feature in patients with AH.4,14 In addition, the presence of he-patocellular necrosis has also been associated with increased HVPG in patients with AH.15 The precise mechanism by which hepatocellular necrosis could raise portal pressure is not fully understood. It might be due to an increase in hepatocyte volume or associated with massive hepatocellular necrosis, which collapses the sinusoidal frame.16,17 Liver stiffness is another parameter that has not been reported previously. Here we describe the transient elastography study of our second patient, which showed a normal result. This might be, again, due to the scarce fiber deposition described in the liver biopsy in comparison with that seen in AH, or because of the absence of other phenomena that are known to increase liver stiffness, such as necroinflammation, extrahepatic cholestasis or congestion.18-20 In both patients abstinence of alcohol intake was rapidly followed by a biochemical and clinical improvement and they were discharged without any symptoms or significant biochemical abnormalities.
In summary, we report 2 clinical cases of AFD, a relatively rare condition characterized by extensive microvesicular steatosis. Interestingly, we describe hemodynamic and elastography characteristics, both normal, which had not been reported previously. This entity should be suspected in patients with ALD with an acute deterioration of liver function or decompensation in whom liver chemistries are markedly impaired. Since serum aminotransferase levels tend to be markedly elevated, higher than those usually found in ALD, special effort should be made in order to rule out other causes of liver disease, such as toxics or viruses. A conclusive diagnosis of AFD can only be established by histological examination of the liver. Therefore, a liver biopsy, either percutaneous or transjugular, if significant coagulo-pathy is present, should be performed in order to confirm the diagnosis of this disease, which can be easily managed with abstinence from alcohol.
Potential Conflict of InterestNothing to report.
Grant SupportThis work was supported by grants from Fondo de Investigación Sanitaria (FIS PI080237 and FIS PS09/01164 to RB and JC). JA has a grant from Fundación Banco Bilbao Vizcaya Argentaria (FBBVA). JA and JM are enrolled in the Master on Research in Liver Diseases of the Universitat de Barcelona. CIBERehd is funded by the Instituto de Salud Carlos III.