Objective: Liver adenomatosis (LA) is a rare disease originally defined by Flejou et al. in 1985 from a series of 13 cases. Only 57 cases have been reported in the literature, and all have been documented among Caucasian population. The aim of this study is to review and reappraise the characteristics of this rare liver disease, and to discuss the diagnosis and therapeutic options. Background: LA is defined as the presence of >10 adenomas in an otherwise normal liver parenchyma. Neither female predominance nor a relation with estrogen/progesterone intake has been noted. Natural progression is poorly understood. Methods: We describe the clinical presentation, evolution, radiologic studies, histologic characteristics and therapeutic options in a 3rd generation Mexican woman with LA. We also include an updated review of the literature. Results: The natural history and pathogenesis of LA are unclear. The risk of spontaneous hemorrhage or malignant transformation are a major concern. There is controversy regarding the optimal treatment for this disease; treatment options range from conservative medical therapy to surgical resection and even liver transplantation. Conclusion: LA is a rare disease, more common in women, and its outcome and evolution vary. Most often, conservative surgery is indicated. Liver transplantation is indicated only in highly symptomatic and aggressive forms of the disease.
Hepatocellular adenomas are rare benign tumors of the liver that are solitary in most cases. These tumors have been linked to oral contraceptive use in women and to the longterm use of anabolic corticosteroids in men.1-3 In a few cases, hepatocellular adenomas are a complication of a number of inherited metabolic disturbances such as type I glycogen storage disease.4 Hepatic adenomatosis (HA) can be defined by the presence of multiple (arbitrarily more than 10) hepatocellular adenomas. This entity can be distinguished from solitary adenomas, because it is not associated with oral contraceptive use or steroid medication.5
The natural history and pathogenesis of liver adenomatosis are unclear. The risk of spontaneous hemorrhage or malignant transformation remains controversial.6,7 Hepatic adenomatosis is an exclusion diagnosis when multiple solid hepatic masses are evident. There is controversy regarding the optimal treatment for this disease; treatment options range from conservative medical therapy to surgical resection and even liver transplantation.7,8
Only about 57 cases had been reported prior to the first case series report by Flejou5 et al; and all cases occurred among Caucasian population.7 We report a case of HA in a 27 year-old hispanic woman.9
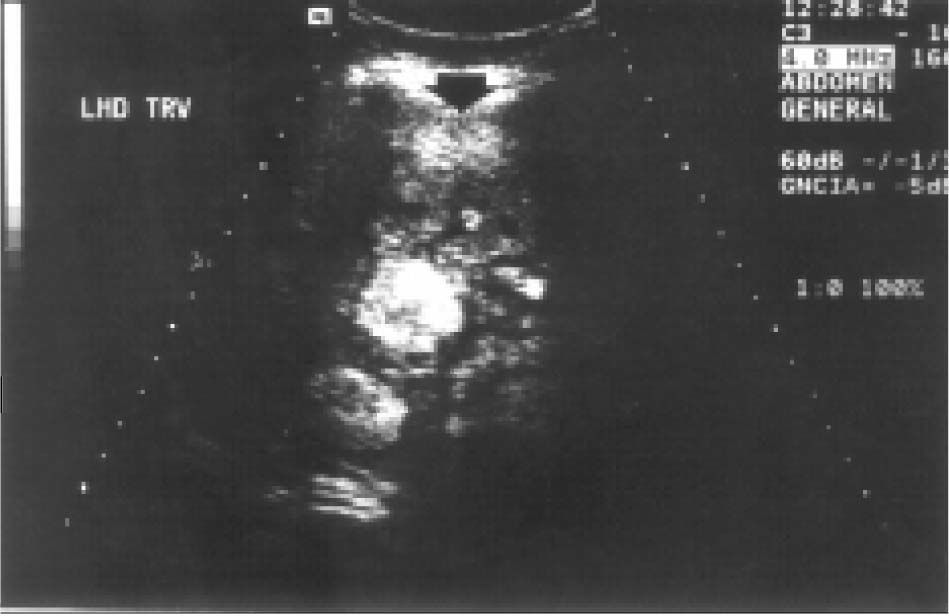
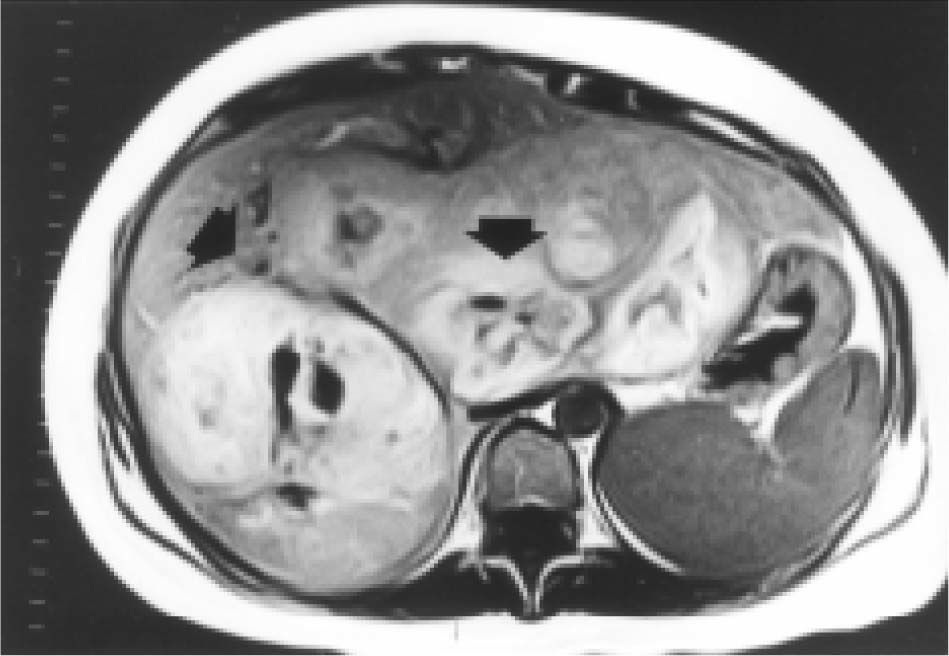
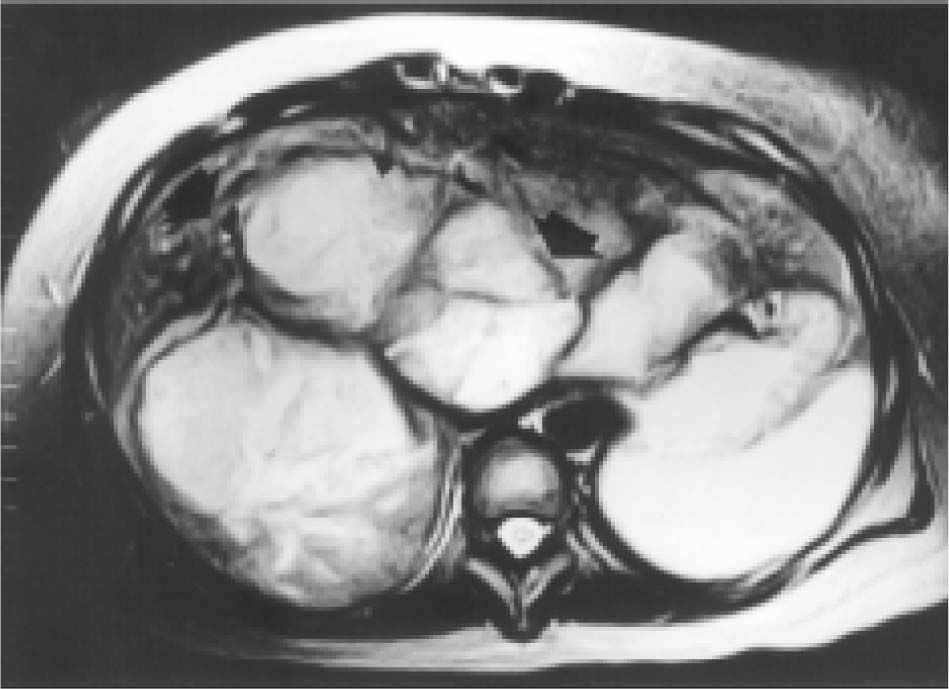
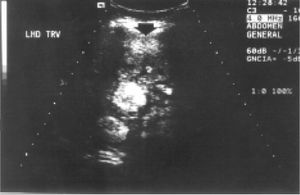
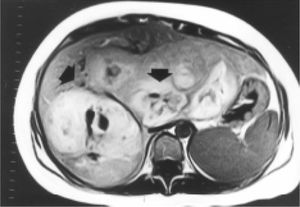
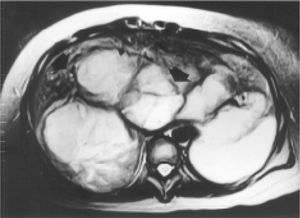
Case reportA 27-year-old woman sought medical attention in a primary care center for right upper abdominal pain lasting for five years. She had never used oral contraceptives or anabolic corticosteroids. After a computed tomography (CT) scan showed a 25 cm mass in the liver, the patient was referred to our institution. Her vital signs were normal and on physical examination the liver was painful and enlarged (measuring 20 cm on the right midclavicular line). The result of liver function test only revealed abnormal serum alkaline phosphatase (754 IU/l [normal 39-131 IU/L]). Serum α-fetoprotein level was 3.8 ng/mL (0.1-10 ng/mL). On the review of the abdominal CT scan from the referring hospital, images before and after intravenous administration of contrast material demonstrated showed evidence of multiple enhanced focal hepatic masses. The lesions were somewhat heterogeneous, but predominantly hypodense. Cystic and calcification foci were also evident. The estimated number of tumors was 10-20, involving multiple hepatic segments without lobe predominance. The largest diameter of the tumors ranged from 0.5 to 15.0 cm. Sonography showed an hyperechoic tumor in the right hepatic lobule measuring 10 cm with a fluid component corresponding to hemorrhage, surrounded by several nodules of different sizes and different behavior patterns (Figure 1). MRI showed at least 10 discrete tumor nodules; in the T1-weigthed images, the tumors were heterogeneously hyperintense; on the T2-weigthed images, most of the masses were heterogeneously hyperintense or homogeneously nearly isointense (Figures 2and3). Laparotomy was performed, and an enlarged liver with its contour deformed by multiple, blunging rounded, yellow nodules measuring 2 to 15 cm was seen.
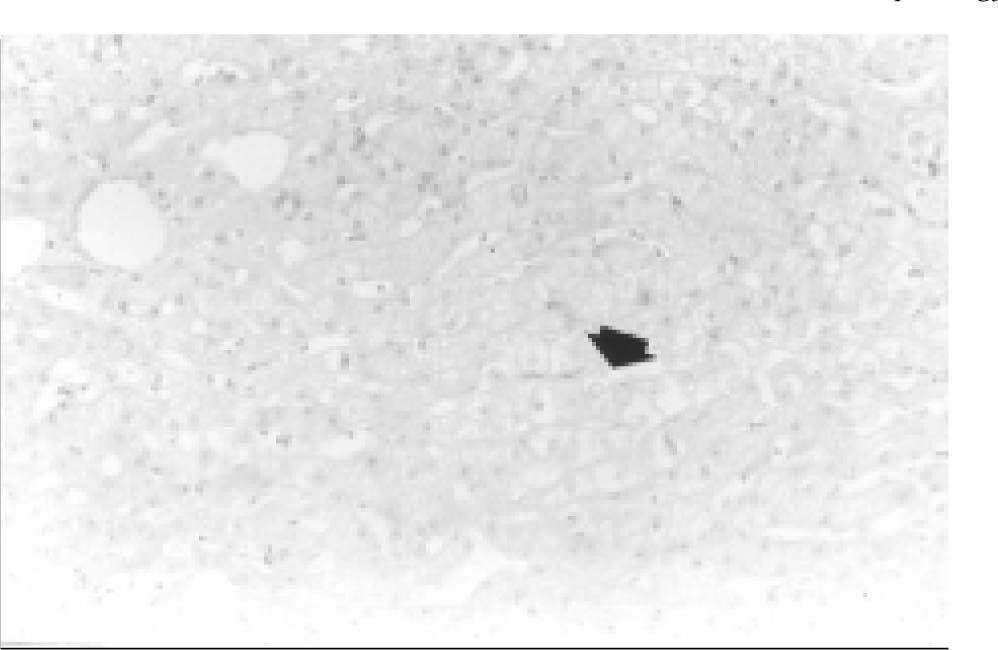
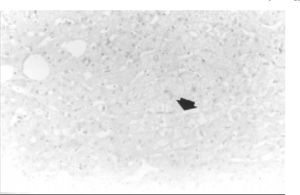
Transoperative core biopsy was performed on one of the lesions to obtain a definitive diagnosis. Surgical excision of the tumors was not possible. Benign liver cells without atypia and parenchyma with bland architecture consistent with hepatic cell adenoma were found at histologic examination; in addition, mild focal steatosis was noted (Figure 4). Currently, the patient is in good condition, but still complains of abdominal pain, and has been considered for orthotopic liver transplantation (OLT) in our institution.
DiscussionIn 1985, Flejou5 et al. described liver adenomatosis as a separate clinical entity which is characterized by the presence of multiple adenomas, lack of correlation with steroid medication and oral contraceptive use, involvement of both men and women, and abnormal increases in serum alkaline phosphatase levels. Some authors distinguished adenomatosis from multiple adenomas associated with glycogen storage disease or anabolic corticosteroids use, and reserved the term “adenomatosis” for patients without history of either condition, as in our case.1,3,10,11
Most steroid-induced adenomas are solitary, or at most, two or three of these lesions manifest in a patient and typically revert after discontinuation of exogenous steroid use.7 These tumors are asymptomatic, unless there is hemorrhage, and liver function tests are always normal.12,13 Hepatic adenomatosis (HA) does not appear to be steroid-dependent, and it does not regress with steroid withdrawal or blockade with tamoxifen or oophorectomy. The lack of estrogen receptors in many lesions suggests that estrogen does not play a dominant role in the pathogenesis of liver adenomatosis.14
The conditions related with the pathogenesis of HA are poorly understood. Recently, Chiche9 et al. suggested an autosomal transmission of this disease. One of the most important hypothesis is related with abnormalities of the hepatic vasculature (congenital or acquired).7,14,15 Focal disturbance of the hepatic blood supply facilitates the hyperplastic development of adenomas. In addition, these lesions are perfused by high-pressure arterial flow, which creates a predisposition to them to hemorraghe.7 Hemorrhage occurred in 40%-60% of the cases. Most cases had intratumoral bleeding, but in a few cases intraperitoneal bleeding can cause hypovolemic shock and death.16,17
Patients with larger lesions, hepatomegaly, abdominal pain, and abnormal liver function are believed to have a higher risk for hemorrhage compared with smaller tumors. 5,14 Malignant progression of adenomas to hepatocellular carcinoma (HCC) has been rarely reported.3,10 Malignant degeneration is difficult to estimate, but it is more common in patients with glycogen storage disease-related adenomas.18
In most cases the diagnosis is made because of complications of adenomas (intraperitoneal bleeding, intratumoral hemorrhage or necrosis producing acute pain), hepatomegaly with chronic pain or, as in our case, as an incidental finding.9,10,17
Multiple adenomas in liver adenomatosis may have a variety of appearances, but the sonography and MRI characteristics are the best clues for diagnosis.6 Because of their vascularity, these tumors may appear isoechoic, hypoechoic or hyperechoic in sonography; the presence of small hyperechoic nodules on ultrasound is the most common finding.17
With the use of contrast-enhanced CT, adenomas are usually isodense or slightly hypodense relative to normal parenchyma. CT has frequently been disappointing because the routine images of the liver are obtained during the venous phase of contrast enhancement, at which time vascular lesions such as adenomas have achieved an equilibrium phase with normal hepatic parenchyma, accounting for the decreased detection rate of these lesions.19,20
In most cases the diagnosis is made because of compli-cations of adenomas (intraperitoneal bleeding, intratumoralhemorrhage or necrosis producing acute pain), hepatome-galy with chronic pain or, as in our case, as an incidentalfinding.9,10,17
MRI is the most useful tool in the diagnosis of hepatic adenomatosis; since it provides information on the nature and the number of lesions, angiomas, steatosis and focal nodular hyperplasia are easily excluded.7,9 Most adenomas have an homogenously increased signal on T1-weigthed images, and a well defined low intensity capsule. On the T2–weighted images, adenomas are heterogeneosuly or slightly hyperintense. On the multiphasic, gadolinium-enhanced MRI studies, most adenomas are predominatly hyperintense on the hepatic arterial phase (HAP) and early portal venous phase (PVP) images, and progressively become more isointense on the later PVP or delayed-phase images.7,9 In conclusion, the combination of sonography and MRI seems to be the most logical diagnostic approach in this setting.
Histology is required to establish the diagnosis. On gross examination adenomas are typically well circumscribed tumors; the minority have a partial or complete fibrous capsule. Microscopically, the lesions consist of sheets of hepatocytes with foamy cytoplasm arranged in cords without bile duct or fibrosis.5,17 Increased steatosis is common and numerous dilated sinusoids composed of thin walled capillaries are also evident.19 These characteristics distinguish hepatic adenomatosis from focal nodular hyperplasia.
Once the diagnosis is made management remains problematic. Estrogen and progesterone therapy should be avoided to prevent complications, such as rupture. Oophorectomy and tamoxifen therapy in an attempt to eliminate endogenous estrogens is not useful.5 Because of the chance of malignant degeneration and hemorrhage, resection of adenomas, or at least of the largest and most vulnerable lesions (subcapsular, exophytic and hemorrhagic lesions), seems to be warranted in many cases; even if the smaller adenomas must remain in place.7,9 Orthotopic liver transplantation (OLT) may be reserved for patients who have progressive signs or symptoms after partial resection, in whom surgical excision may not be possible, or in whom HCC is suspected.
Liver resection, when necessary and possible, is the preferred option because HA is a benign disease that does not cause an impairment of hepatocellular function. OLT remains a difficult decision, although it is sometimes the last option, in the progressive forms of the disease, such as that of our patient.9,10