The interleukin-33/interleukin-13 pathway is involved in the immunopathology of liver fibrosis and recently characterized group 2 innate lymphoid cells (ILC2) were identified as profibrotic immune cells in the liver of mouse models. Our aim was to elucidate whether ILC2 might be present in human liver tissue and whether ILC2 contribute to liver fibrosis.
Materials and methodsTo identify ILC2 in liver tissue and blood, we purified mononuclear immune cells from needle biopsies, cirrhotic explant specimen, and paired peripheral blood samples. Cell suspensions were incubated with specific markers for ILC2 and analyzed by flow cytometry. The CD69 marker was included to assess the activation level of ILC2. In addition, we determined the IL-33 plasma level.
ResultsResults were correlated with the METAVIR fibrotic score of patients enrolled in this study. We detected ILC2 in a higher percentage of CD45+ cells in liver tissue than in paired peripheral blood. The number of ILC2 was significantly increased in fibrotic tissue, but only slightly increased in paired peripheral blood. A higher percentage of CD69+ ILC2 was observed in fibrotic tissue, and this increase correlates positively with aggravation of liver fibrosis measured by fibrotic METAVIR score. A higher level of plasma IL-33 was only detected in samples obtained from cirrhotic patients.
ConclusionOur study indicates that ILC2 are present in the human liver and are activated in tissue contributing to the immunopathology of human liver fibrosis, independently of the etiology; which might be a potential new therapeutic target.
Hepatic fibrogenesis is an important mechanism for wound healing and tissue repair in liver injury. Uncontrolled and perpetual fibrogenesis accompanied by chronic inflammatory responses leads to progressive fibrosis through the worsening of the disease into cirrhosis and hepatocellular carcinoma, and eventually into the complete loss of the liver function [1,2]. Causes of chronic hepatic fibrosis include steatohepatitis (alcoholic (ASH) and non-alcoholic (NASH)), chronic viral infections (e.g. hepatitis C virus (HCV)), and autoimmune diseases (e.g. autoimmune hepatitis (AIH), and primary biliary cholangitis (PBC)). The mechanism of hepatic fibrosis is known to occur through the transdifferentiation of quiescent hepatic stellate cells (HSC) into myofibroblasts, producing large amounts of extracellular matrix proteins [1]. This transdifferentiation of HSC into fibrogenic myofibroblasts is maintained by the cellular crosstalk between diverse pro-inflammatory immune cells and liver resident cells.
One of these inflammatory pathways involved in liver fibrosis is the interleukin-33 (IL-33)/IL-13 axis. IL-33 (a member of the IL-1 superfamily) is categorized as an “alarmin” [3,4], detected in activated HSC [5] in humans and thought to be released after tissue injury-induced necrosis. IL-33 responding cells produce IL-13, an important effector cytokine in the development of fibrosis, this maintains the profibrogenic transdifferentiation of HSC [6,7]. The primary source of fibrogenic IL-13 are CD4+ T helper 2 cells (Th2) and the recently identified group 2 innate lymphoid cells (ILC2) [8,9]. ILC2 are characterized by the lack of antigen-specific B and T cell receptors and other common hematopoietic cell markers for myeloid or dendritic cells [10,11].
So far, ILC2 have been identified as fundamental immune players in the human pathology of atopic dermatitis [12,13], airway diseases [14], and pulmonary fibrosis [15]. However, special attention has been given to the findings in mice showing that ILC2 play a fundamental role in liver fibrosis [16]. By in vivo depletion of ILC2 in the CCL4-induced fibrosis mouse model, the authors could demonstrate that collagen deposits were significantly reduced [16]. In line with these findings, Neumann et al. [17] showed that ILC2 were activated by IL-33 and aggravated the tissue injury in their Con A-induced hepatitis mouse model. We wanted to elucidate whether ILC2 are resident in the human liver and whether ILC2 contribute to liver fibrosis. In this study, we have evaluated the cell number and activation state of both tissue and blood ILC2 in patients with different grade of fibrosis and etiology, as well as from a healthy control group to understand the role of ILC2 in the immunopathology of liver disease.
2Materials and methods2.1PatientsBetween January 2014 and December 2016, liver transplant patients and patients with various liver diseases (who underwent a liver biopsy as part of their diagnostic evaluation or follow up) and healthy volunteers were enrolled in this study. Both female and male patients were included with an age range from 18 to 60 years (Table 1). Patients with human immunodeficiency virus (HIV) infection and hepatocellular carcinoma were excluded due to the immunomodulatory activity of tumor cells. Liver tissue samples were obtained at the time of liver transplantation from healthy donors (n=6) and liver explants (n=11), liver biopsies (n=11) were also obtained from patients with liver disease during follow up. The liver biopsies were divided in two parts, one part for the histological examination and the other part for cell isolation and flow cytometry. All samples were classified at pathology department depending on the severity of fibrosis based on the METAVIR score in fibrotic samples (fibrosis: F1 (mild) and F2 (moderate)) and cirrhotic samples (cirrhosis: F3 and F4). As a healthy control (healthy), we used biopsies obtained from the liver donor during liver transplantation classified as F0 by the METAVIR score. Peripheral blood samples were obtained from patients with liver disease, with liver cirrhosis undergoing transplantation, and healthy volunteers. Our program adheres to the ethical principles of the Istanbul Declaration as well as the document of the Pontifical Academy of Sciences on organ trafficking and transplant tourism. Ethical permission for the study was obtained from the Ethics Committee Board at Favaloro Hospital (DDI(1264)3214), and written informed consent was obtained from each subject included in the study.
Demographic and clinical characteristics of the study groups.
| Characteristics | Healthy | Fibrosis | Cirrhosis |
|---|---|---|---|
| METAVIR score | F0 | F1;2 | F3;4 |
| Number of patients | 17 | 9 | 12 |
| Age (years) | 47±3 | 46±5 | 58±2 |
| Gender | |||
| Male | 6 | 6 | 7 |
| Female | 11 | 3 | 5 |
| Etiology | |||
| NAFLD (non-alcoholic fatty liver disease) | 1/9 | ||
| Steatohepatitis | |||
| NASH (non-alcoholic steatohepatitis) | 4/9 | 1/12 | |
| ASH (alcoholic steatohepatitis) | 4/12 | ||
| Viral hepatitis | |||
| HCV (hepatitis C virus) | 1/9 | 3/12 | |
| Autoimmune diseases | |||
| PBH (primary biliary hepatitis) | 2/9 | 2/12 | |
| PSC (primary sclerosing cholangitis) | 1/12 | ||
| AIH (autoimmune hepatitis) | 1/9 | 1/12 |
Samples of liver tissue were collected in PBS with pH 7.0 containing 0.5mg/mL collagenase (type IV, 312U/mg, Sigma-Aldrich, St. Louis, USA) and then thoroughly dissected using scissors. The sample solution was incubated for 15min at 37°C (enzymatic digestion), and then centrifuged at 30×g for 1min to remove cell clumps and not dissociated tissue. The supernatant was centrifuged at 300×g for 3min, then the pellet was incubated with ACK lysing buffer for red blood cell lysis for another 3min at room temperature. Then, the cells were passed through a 70μm cell strainer and washed with PBS. Finally, the cells were concentrated by centrifugation and resuspended in the final volume to be stained with the antibody cocktail.
Peripheral blood mononuclear cells (PBMC) were isolated freshly from heparinized blood by standard Ficoll-Hypaque (GE Healthcare, USA) density gradient centrifugation. Then, the cells were harvested from the interphase, passed through a 70μm cell strainer, and washed twice with PBS.
2.3Flow cytometry analysisCells were stained with a combination of the following monoclonal antibodies: PE anti-CD127 (clone: hIL-7R; BD Bioscience, New Jersey, USA), FITC anti-CD69 (FN50; BD Bioscience), PerCPeCy5.5 anti-CD294 (BM16; BD Bioscience), APC anti-CD45 (H130; BD Bioscience), APCH7 anti-CD3 (SK7; BD Bioscience), APCCy7 anti-CD19 (BDIB19; Biolegend, San Diego, USA), PECy7 anti-CD117 (104D2; Biolegend). Six-color analysis was performed by FACSCanto II (Becton Dickinson FACS System, Oxford, UK), and the data were analyzed by Flow Jo software (Tree Star, Ashland, USA).
2.4Activation of mononuclear cells in vitroPeripheral blood mononuclear cells were isolated from fresh heparinized blood by standard Ficoll-Hypaque (GE Healthcare) density gradient centrifugation. Then, the cells harvested from the interphase were passed through a 70μm cell strainer and washed twice with PBS. Finally, the cells were concentrated by centrifugation in the final concentration to be stimulated. PBMC (2×106 cells in 200μl) were then stimulated with IL-7 (100ng/mL; Peprotech, NJ, USA); IL-33 (100ng/mL; Biolegend); the synthetic TLR2 ligand Pam3Cys (Novabiochem, Cambridge, UK) at 1μg/mL; or with the TLR4 ligand LPS (1μg/mL; Sigma-Aldrich). Samples were incubated at 37°C and 5% CO2 overnight in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, USA) with 10% FBS (PAA, Pasching, Austria) and an antibiotic-antimitotic cocktail (Thermo Fisher Scientific). Then, cells were centrifuged at 300×g for 5min and stained for flow cytometry analysis.
2.5Detection of IL-33 serum levelFor the determination of human IL-33 concentration in plasma, specific ELISA Kit from Abcam was used according to the manufacturer's instructions (ab119547-IL-33; Abcam, Cambridge, UK).
2.6StatisticsData analysis was performed with GraphPad Prism version 5 software (San Diego, USA). Multiple comparisons were made among the groups using the Kruskal–Wallis with Dunn's post-test. For the in vitro cell analysis, Student's t test was used. Correlations between variables were evaluated with the Spearman rank correlation test. For all tests, a two-sided P value <0.05 was considered significant.
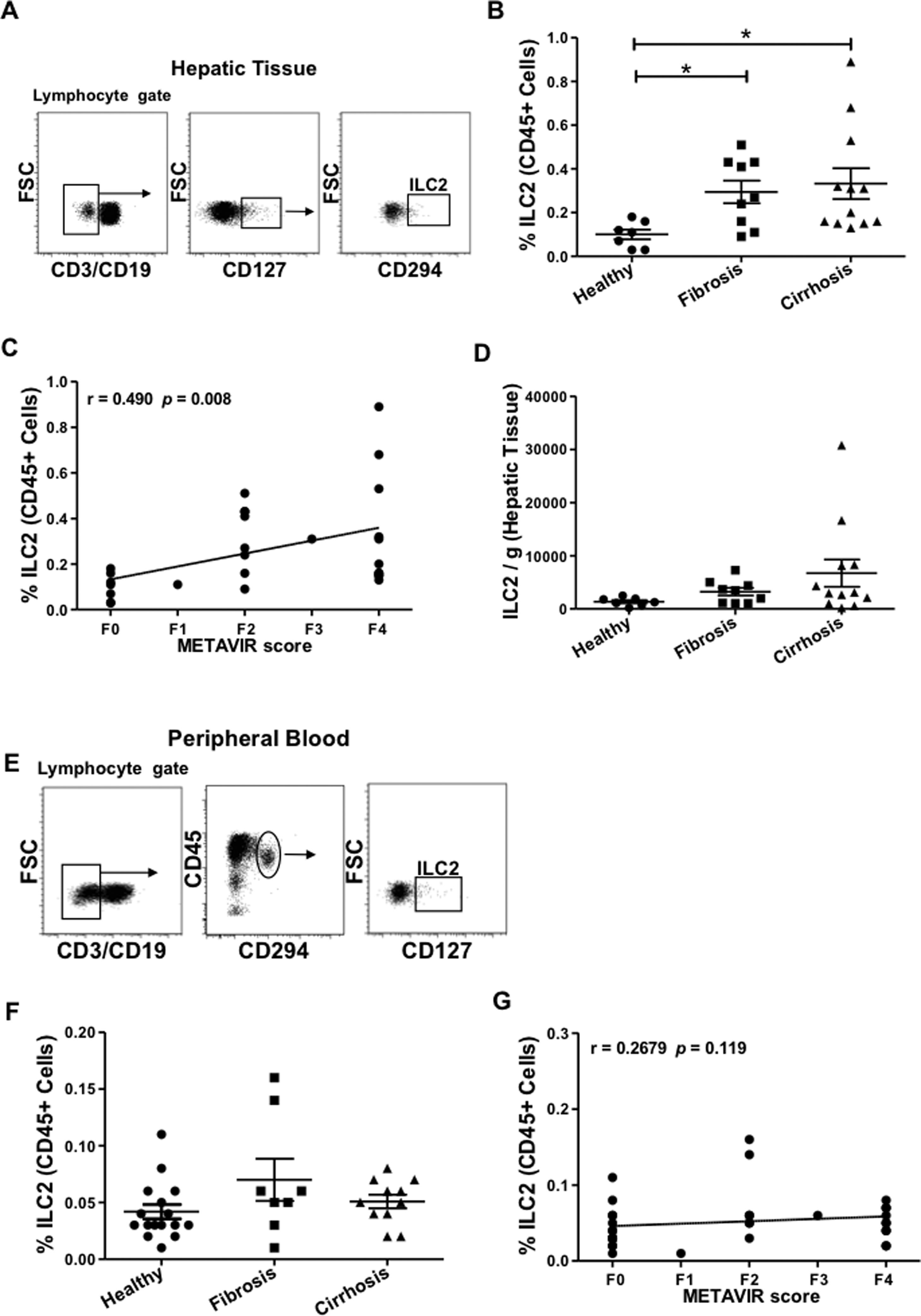
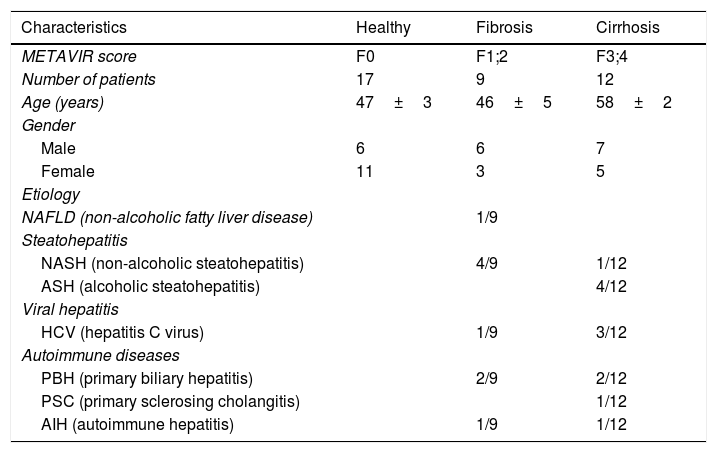
3Results3.1Type 2 innate lymphoid cells are present in hepatic tissueTo determine the role of ILC2 in liver fibrosis, we first evaluated the feasibility of detecting ILC2 in hepatic tissue (hepatic ILC2). So far in non-lymphoid human organs, ILC2 has been found in the intestine [18], the skin [12], and the lung [9]. We obtained hepatic tissue from biopsies (either healthy or fibrotic) or samples from liver explants and performed cell analysis by flow cytometry. To identify ILC2 within the lymphocyte gate (CD45+ mononuclear cells with lymphoid morphology), we used the CD3 and CD19 markers to exclude B and T cells. Then, CD127 positive cells were gated and the specific marker prostaglandin D2 receptor (CRTH2, CD294) identified subsequently ILC2 (Fig. 1A). We used the same antibody cocktail to identify ILC2 in the peripheral blood (peripheral ILC2) (Fig. 1E). We detected ILC2 in all hepatic tissue samples within a range of 0.03–0.89% of CD45+ lymphoid cells (Fig. 1B). This range was considerably higher than the percentage of peripheral blood ILC2 (0.001–0.16% of CD45+ lymphoid cells) (Fig. 1F). To elucidate whether ILC2 might contribute to the fibrogenic process, we compared the healthy samples with fibrotic and cirrhotic samples. The percentage of hepatic ILC2 for the healthy group was 0.1±0.02%, the fibrotic group increased to 0.29±0.05% and the cirrhotic group to 0.33±0.07% (Fig. 1B). When we correlated the percentages of hepatic ILC2 with their individual METAVIR fibrotic scores (F0–F4), we found a positive correlation (Fig. 1C). The absolute cell number of ILC2 per gram of hepatic tissue increases with worsening fibrosis. However, differences between the three groups were not statistically significant (Fig. 1D). Also, we investigated whether the ILC2 number of the paired peripheral blood samples correlates with the fibrosis stage of the liver. On the contrary to the hepatic ILC2, cell percentages of peripheral ILC2 did not show significant differences between the three groups or in the correlation with the METAVIR score (Fig. 1F and G). Our results show that ILC2 are present in human liver tissue, and can be detected in small liver biopsies, although in a low percentage. The correlation between ILC2 frequency and METAVIR score also suggests that ILC2 contribute to the development of liver fibrosis and progressive liver disease.
Detection of innate lymphoid cells group 2 in human hepatic tissue and peripheral blood. Representative plots of the gating strategy for the identification of ILC2 (CD45+ lymphocyte gate, CD3−, CD19−, CD127+, CD294+) by flow cytometry in (A) hepatic tissue or (E) peripheral blood. (B) Comparison of the percentages of ILC2 isolated from hepatic tissue of healthy individuals (n=7) with histologically confirmed METAVIR score F0, patients with fibrosis (n=9, F1–F2), and with cirrhosis (n=12, F3–F4). (C) A positive correlation between percentages of ILC2 and METAVIR scores is shown. (D) Absolute numbers of ILC2 per gram of hepatic tissue were not significantly different between the three groups. (F) Percentages of ILC2 from peripheral blood of healthy (n=16), fibrotic (n=8), and cirrhotic (n=11) samples were compared and no significant difference was detected. (G) No correlation between percentages of peripheral blood ILC2 and METAVIR score was found. Each data point represents an individual patient and the values are shown as mean±SEM. The groups are compared using Kruskal–Wallis test with Dunn's post-test. Correlation analysis is performed by Spearman rank correlation test (r and P values are indicated in the figure). *P<0.05. ILC2, innate lymphoid cells group 2; FSC, forward scatter; SEM, standard error of the mean.
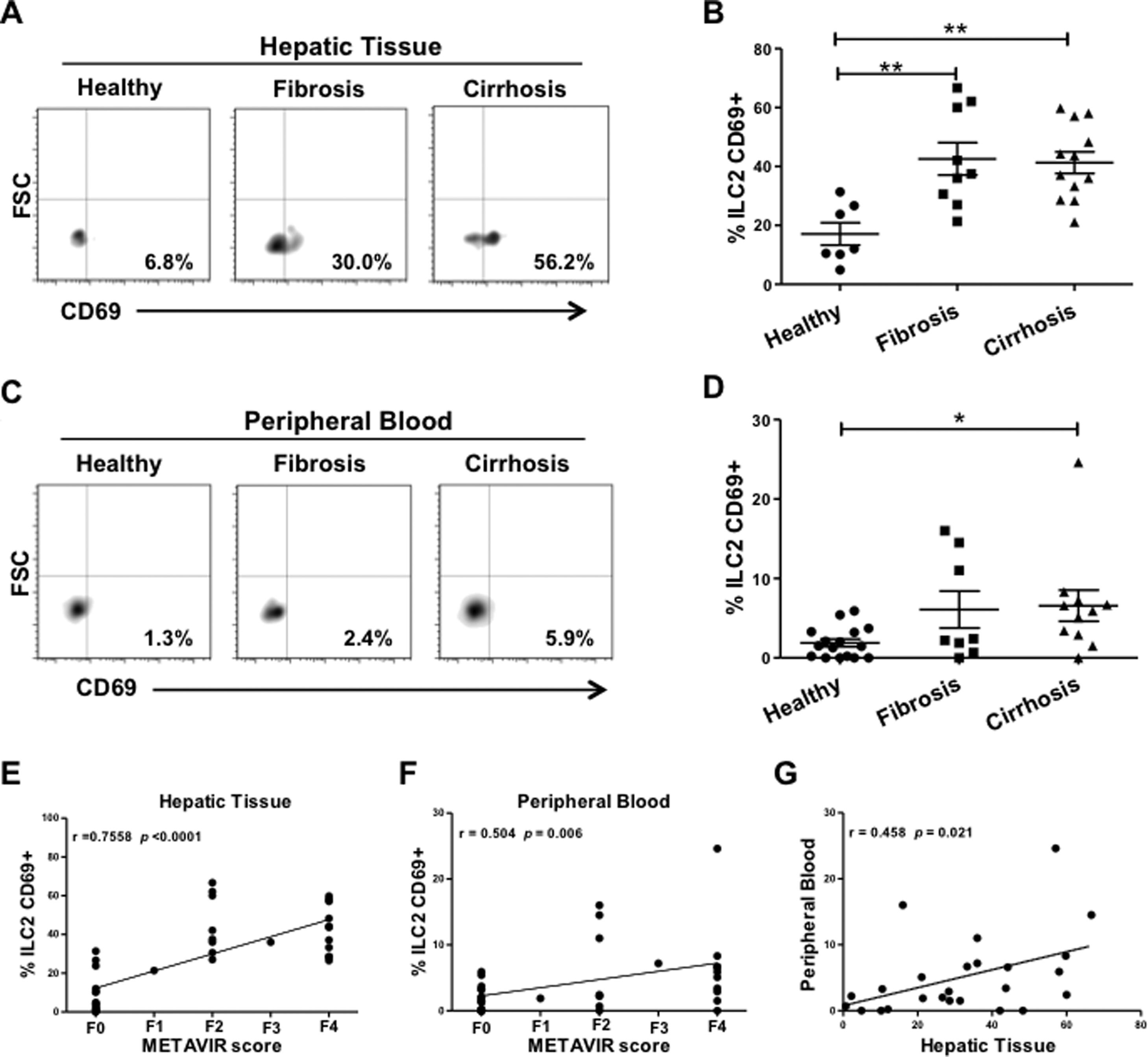
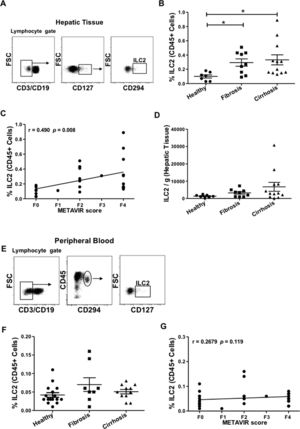
Moreover, we wanted to elucidate whether ILC2 were activated in the fibrotic liver and in the peripheral blood of the patients enrolled in this study. We included the activation marker CD69 in the antibody cocktail for the specific ILC2 detection by flow cytometry (Fig. 2A and C). We observed that ILC2 were significantly more activated in fibrotic and cirrhotic tissue than in healthy tissue (Fig. 2B). Strikingly, the percentage of activated hepatic ILC2 strongly correlated with the METAVIR score (Fig. 2E). On the contrary, the results in peripheral blood were not so conclusive. Although percentages of activated ILC2 in the blood of fibrosis or cirrhosis patients were increased, only the cirrhotic group expressed a significant difference as compared to the healthy group (Fig. 2D). The range of the activated ILC2 level was much higher in the fibrotic and cirrhotic group of hepatic tissue than in the paired samples of peripheral blood (21.1–66.7% vs. 0–24.6%, respectively), supporting our hypothesis that ILC2 are activated locally in the liver (Fig. 2B and D). The level of activated ILC2 in peripheral blood was moderately correlated with the METAVIR score (Fig. 2F). A slight correlation of the activation status of ILC2 between paired samples was observed (Fig. 2G).
Elevated level of activated ILC2 in liver fibrosis. (A) Representative plots of the gating strategy for the identification of activated ILC2 with the commonly used activation marker CD69 (CD45+, CD3−, CD19−, CD127+, CD294+, CD69+). (B) Percentages of activated ILC2 of hepatic tissue samples from healthy individuals (n=7) with a METAVIR score F0; fibrotic patients (n=9) with F1–F2; and cirrhotic patients (n=12) with F3–F4. (C) Representative plots of activated ILC2 from peripheral blood. (D) Percentages of activated ILC2 from peripheral blood of healthy individuals (n=16); patients with fibrosis (n=8); patients with cirrhosis (n=11). (E) A strong correlation between activation status of ILC2 and fibrotic METAVIR score in hepatic tissue is shown, whereas (F) this correlation is weaker in peripheral blood. (G) The activation level of ILC2 correlates between the paired samples of hepatic tissue and peripheral blood. Each data point represents a patient and the values are shown as mean±SEM. The groups were compared using Kruskal–Wallis test with Dunn's post-test. Correlation analyses were performed by Spearman rank correlation test. *P<0.05, **P<0.01.
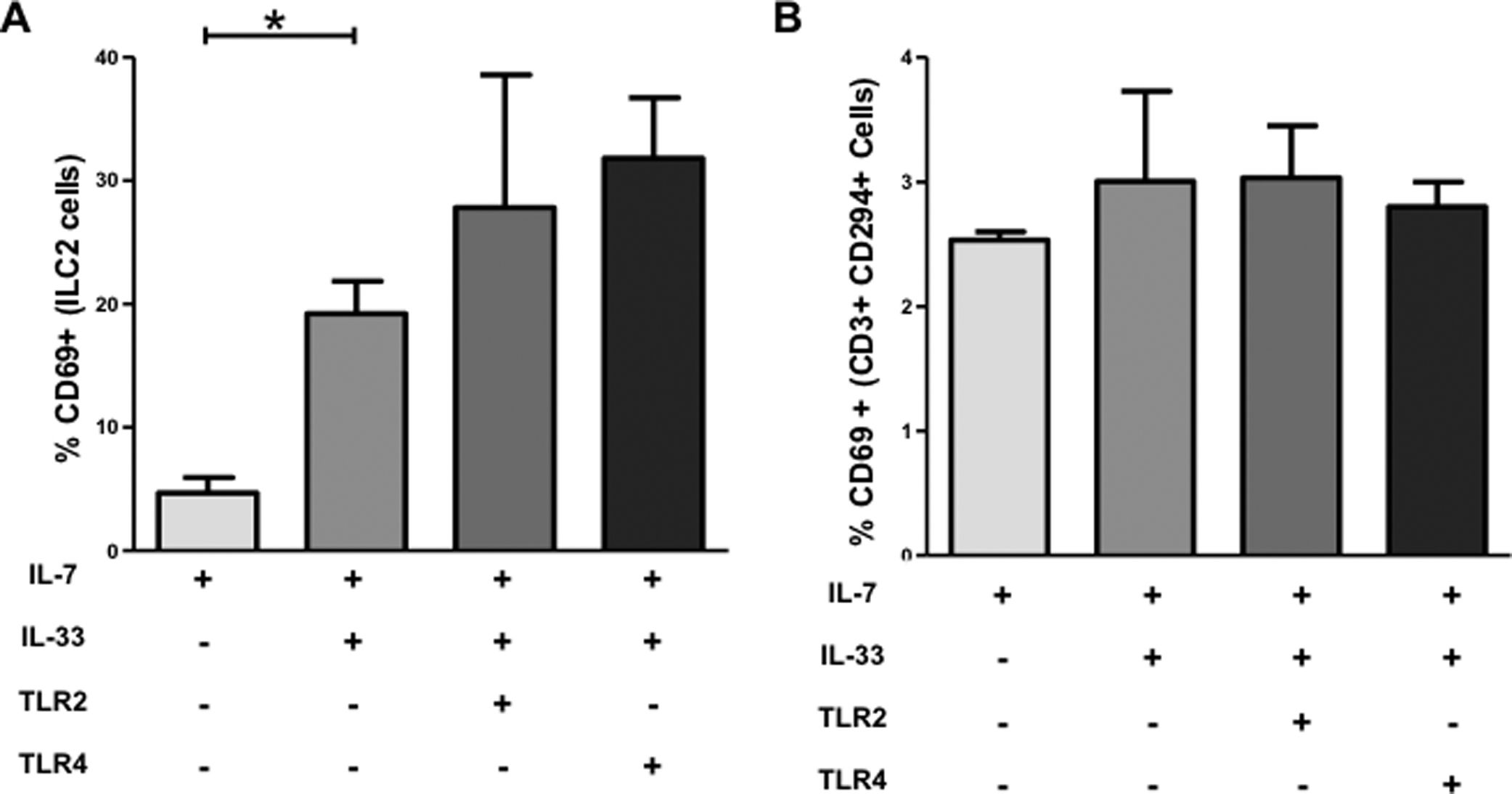
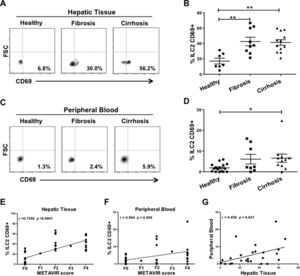
To confirm that IL-33 induces CD69 expression on ILC2, we performed an in vitro experiment with freshly isolated peripheral blood cells. After 24h in vitro stimulation, we found that the mean percentage of CD69+ ILC2 was increased 4-fold in the IL-33 group compared to the control group without IL-33 (4.7±1.2 (SEM) vs. 19.2±2.6%) (Fig. 3A). In addition, when we included TLR2 stimulus PamC3 and the TLR4 stimulus LPS in the IL-7 and IL-33 cocktail, we observed that both stimuli could not significantly further increase the percentage of CD69+ ILC2. These different stimuli did not induce upregulation of CD69 on CD3+ CD69+ CD294+ Th2 cells under the same conditions (Fig. 3B). These results show that hepatic ILC2 are activated locally in fibrotic tissue and the frequency of activated ILC2 increases with progression of liver fibrosis, supporting our hypothesis that ILC2 actively participate in the pathophysiology of the fibrotic process in the liver.
ILC2 express elevated level of CD69 after IL-33 stimulation in vitro. Isolated peripheral blood mononuclear cells from freshly blood of healthy volunteers were stimulated with IL-7, IL-33, the synthetic TLR2 ligand Pam3Cys, or with the TLR4 ligand LPS. (A) Levels of the activation marker CD69 were measured by flow cytometry and results expressed as mean percentages of CD69+ ILC2. (B) Mean of percentages of activated CD3+ CD294+ Th2 cells exhibit no statistical difference between the study groups. The values are shown as mean±SEM and the groups are compared using unpaired Student's t test. *P<0.05. IL, interleukin; TLR, Toll-like receptor. Data are shown of three independent experiments, except for the LPS group with two experiments.
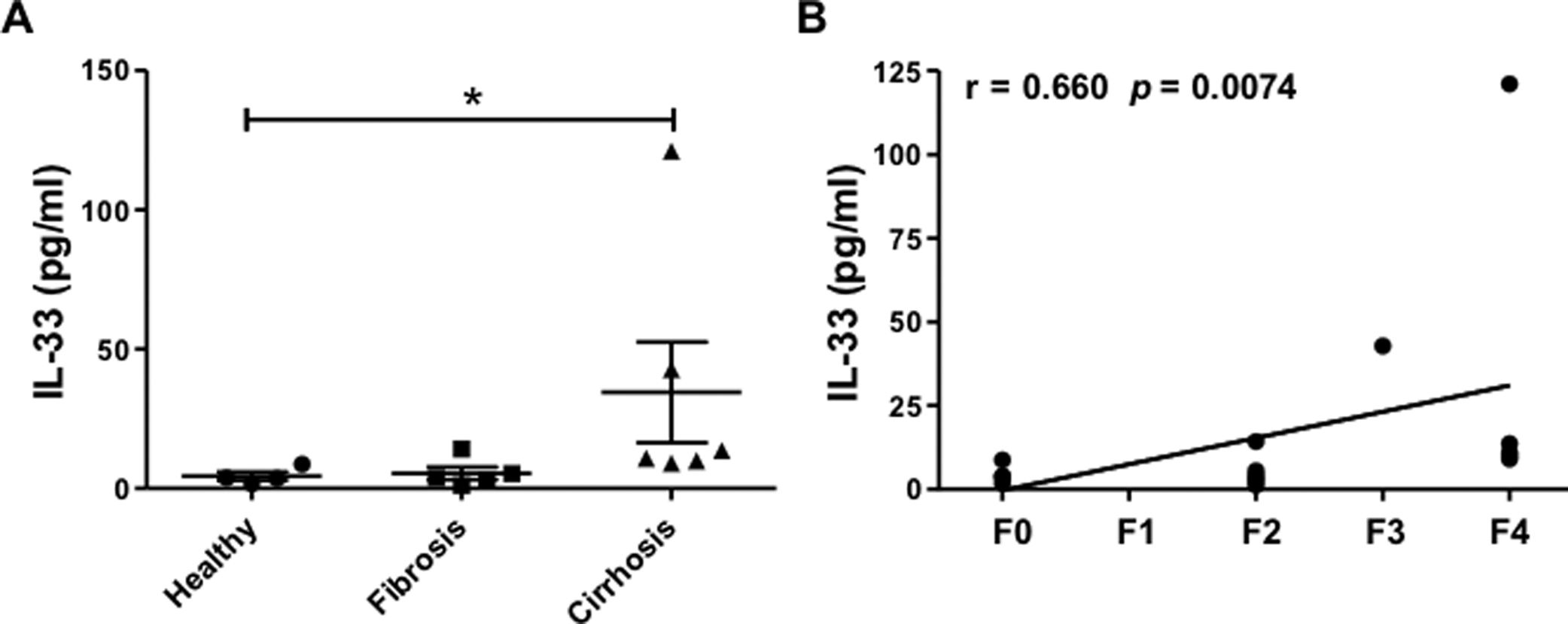
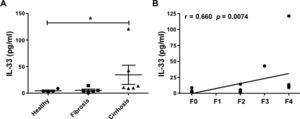
IL-33 is the main cytokine activating ILC2. IL-33, located in different parenchymal cells, is released locally into the tissue microenvironment after cell damage and most probably is active only locally. However, IL-33 might reach peripheral blood and be detectable in serum. We therefore measured plasma level of IL-33 and correlated the amounts with the METAVIR score. A significant increase of plasma IL-33 was observed only in cirrhotic patients with METAVIR 4 (Fig. 4A) and interestingly, the IL-33 level positively correlates with the METAVIR score (Fig. 4B).
Increased plasma levels of interleukin-33 in cirrhotic patients. (A) Levels of IL-33 (pg/mL) in plasma from healthy individuals (n=4), fibrotic (n=5; METAVIR F1–F2) or cirrhotic (n=6; F3–F4) patients estimated by ELISA. (B) Correlation between IL-33 plasma level (pg/mL) and fibrosis METAVIR score. Each symbol represents a patient; lines indicate mean values and SEM. Data assessed using Kruskal–Wallis with Dunn's post-test and Spearman rank correlation test. *P<0.05.
The IL-33/IL-13 axis is a dominant immune pathway in the induction and development of fibrosis. It has been recently found that ILC2 play a key role in this pathological process in mice [16,19]. In this study, we show that ILC2 are present in the human liver, and moreover that importantly hepatic ILC2 contribute to the immunopathology of liver fibrosis. During the submission phase of our manuscript, two articles about human ILC2 were recently published, and the results are in line with our findings [20,21].
However, there are three main differences between our article and theirs. First, we used consequently healthy liver tissue from transplant donors, whereas Forkel et al. [20] and Jeffery et al. [21] received liver tissue from tumor resection surgeries. We excluded this type of tissue due to the immunomodulatory effect of tumor cells in the affected organ. Second, we compared peripheral blood and tissue biopsy ILC2s pairwise from the same patients giving us more accurate results. Third, we correlated our data with the METAVIR score, whereas Forkel et al. [20] used the Batts/Ludwig classification and Jeffery et al. [21] the MELD score. This comparison clearly shows that all three scoring techniques may be applied to correlate ILC cell number or activation status with fibrosis severity.
The presence of ILC2 in human hepatic tissue was detected with specific markers by flow cytometry. We show in our study that the range of hepatic ILC2 frequency (0.03–0.89%) within the CD45+ mononuclear cells is similar to the range in other non-lymphoid human tissues such as skin 0.03–1.3% [12] and the lungs (0.02–0.08%) [18]. The frequency is higher than in the peripheral blood of paired samples (0.001–0.16%) evidencing the important role of ILC2 in the liver. The comparison between the percentages of hepatic ILC2 and the paired peripheral ILC2 with the METAVIR fibrotic score revealed that the aggravation of fibrosis correlates with increase of ILC2 in the tissue, whereas no significant correlation has been observed for peripheral ILC2. This finding is in line with the results obtained in psoriasis and atopic dermatitis patients, where a significant increase of ILC2 counts has only been detected in tissue but not in peripheral blood [12,22]. However, when we divided our results according to the etiology, we found that the autoimmune disease and the HCV group had a slightly higher frequency of peripheral ILC2 compared to the steatohepatitis group regardless of the METAVIR score (data not shown). This is consistent with a previous study in patients with autoimmune systemic sclerosis [22].
This finding that ILC2 contribute to the pathology of liver fibrosis is further supported by the results of our analysis of the ILC2 activation level. We included CD69 (a widely used surface marker for hematopoietic cell activation) in our flow cytometry analysis and observed a significantly higher percentage of hepatic CD69+ ILC2 in both the fibrosis and cirrhosis group compared to the healthy control group. The increase of CD69+ ILC2 frequency in liver tissue correlated with the METAVIR score. On the contrary, peripheral ILC2 did not reach the same high CD69 level. However, we detected a slightly positive correlation of the peripheral activated ILC2 with the METAVIR score. The striking difference of the CD69 level on ILC2 in the paired tissue and blood samples confirms that the purified ILC2 from the tissue are intrahepatic and not circulating cells and that these cells are activated locally in the tissue. This result is in line with the findings in HCV patients showing that a significantly higher CD69+ NK cells was detected in liver tissue than in peripheral blood [23].
Interestingly, the authors of another study pointed out that CD69 in combination with the chemokine receptor CXCR6 are markers to distinguish tissue-resident NK cells from circulating NK cells [24,25]. We showed in vitro that CD69 is upregulated on peripheral ILC2 after IL-33 stimulation confirming CD69 as an ILC2 activation marker. The in vitro upregulation of CD69 was specific for the IL-33, as IL-23 did not induce CD69 expression in ILC2 under the same conditions (unpublished data). Several studies have elucidated that alarmin IL-33 in combination with IL-2 or IL-7 induces the profibrotic cytokines IL-5 and IL-13 in human peripheral ILC2, demonstrating the direct effect of IL-33 on these cells [18,26–28]. Together with our results these findings support the notion that human ILC2 might be key mediator cells in pathological liver fibrosis.
McHedlidze et al. [16] showed that profibrotic IL-33 was highly increased in the sera of cirrhosis patients. However, the authors did not provide additional patient information. Another study reported an elevated IL-33 serum level in patients with acute and chronic liver failure [29] and chronic infection of HBV [30] and HCV [31]. Our data are in line showing a higher level of plasma IL-33 only in cirrhotic patients whereas in fibrotic patients the IL-33 level was similar to the healthy control. Consequently, we conclude that IL-33 is not an early indicator of tissue damage in fibrotic patients.
Due to the low number of ILC2, it was challenging to obtain enough cells from half of a fresh needle biopsy when performing an accurate flow cytometry analysis. Although we reduced the steps of the cell purification protocol to a minimum, we could only mark one sample per patient (paired with the blood sample) for a six-colored flow cytometry analysis. Therefore, we recommend using a flow cytometer with more than six colors for further characterizing hepatic ILC2 from needle biopsies. On the other hand, it was difficult to homogenize explanted cirrhotic tissue with a high grade of fibrosis for cell purification. We were not able to homogenize enough fibrotic tissue for single cell sorting of hepatic ILC2 for further functional studies. Another limitation of our study is that only a relatively small number of patients per etiological group could be included and consequently a larger study population needs to be included.
Although ILC2 is a rare cell population, the striking results in various mouse models, observations in infections and tissue pathologies together with our results in the liver, highlight the importance of ILC2 as a fundamental immunomodulator in human diseases. Although further studies need to be performed to obtain more knowledge of the ILC2 biology, our data support the role of ILC2 in liver fibrosis and point to ILC2 as a possible target for new therapeutic options to reverse the life-threatening process of chronic liver fibrosis.
Financial supportThis study was partly supported by grants from Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT (PICT2530) and from the Italian Liver Foundationvia the International granting program of the Regione FVG.
Conflict of interestThe authors have no conflicts of interest to declare.AbbreviationsACK ammonium chloride potassium autoimmune hepatitis alcoholic steatohepatitis carbon tetrachloride prostaglandin D2 receptor forward scatter hepatitis C virus human immunodeficiency virus hepatic stellate cells interferon gamma interleukin group 2 innate lymphoid cells non-alcoholic steatohepatitis natural killer primary biliary cholangitis peripheral blood mononuclear cells primary sclerosing cholangitis standard error of the mean T helper 2 cells Toll-like receptor