Orthotopic liver transplantation anastomotic biliary strictures (OLT ABS) are managed with endoscopic biliary stent therapy but the recurrence rate is substantial. Our aims were to retrospectively determine the recurrence rates of OLT ABS after initial successful stent therapy, characterize the management of recurrences and identify associated variables.
Materials and MethodsClinical data from 943 patients receiving non-living donor OLT at our institution from 2005-2012 were reviewed, and 123 OLT ABS patients receiving stent therapy were identified. Features of their endoscopic stent therapy and other pertinent clinical information were evaluated.
ResultsABS recurred in 25.5% of patients (24/94) after an initial successful course of stent therapy. Recurrences were received a second course of endoscopic stent therapy and 67% of patients (16/24) achieved long-term remediation of ABS. Six patients underwent a third course of endoscopic stent therapy with 4 patients achieving remediation. Overall remediation rate among ABS recurrences was 83.3% (20/24). A bivariate comparison demonstrated HCV infection, age, median months of maximal stenting and a lower maximum cumulative stent diameter were risk factors for ABS recurrence. Using a Cox regression model, only HCV status proved to be a risk factor for recurrence.
DiscussionIn conclusion repeat stent therapy achieved high stricture remediation rates. Recurrence after the first or even second course of stenting should not imply failure of endoscopic therapy. A positive HCV status may be associated with higher stricture recurrence rates and this association should be further investigated.
Biliary strictures are a frequent adverse event following orthotopic liver transplantation (OLT) with an incidence of 4% to 13%.1 Strictures are classified as anastomotic or non-anastomotic, with anastomotic biliary strictures (ABS) being more common. Historically, treatment of strictures has involved operative biliary reconstruction. However, this is associated with high morbidity and mortality without eliminating the need for repeat intervention.
Endoscopic stent therapy with an aggressive approach of increasing the number of stents until eradication of a postoperative biliary stricture was described by Costamagna, et al.2 in 2001. The method entails elective endoscopic retrograde cholangiopancreatography (ERCP) for stent exchanges at 3-month intervals; in which the objective is to dilate and increase the stent number or diameter until complete resolution of the stricture is fluoroscopically visualized. This also involves a longer duration of stent-ing, as the mean duration of treatment in the series describing this technique was 12.1 months.2 During ERCP, maneuvers typically performed include balloon dilation and placement of one or multiple temporary biliary stents. This has become a reliable alternative to surgical or percutaneous approaches, with long-term success rates reported to be 64% to 100%1-9 and endoscopic stent therapy has evolved into first line therapy for OLT ABS. However, the risk of stricture recurrence is substantial, with the largest study to date reporting a rate of 18%.4
Specific guidelines for recurrent OLT ABS are lacking, mainly due to the relatively small number of subjects or limited follow-up periods in the published literature. Therefore, some centers pursue operative revision for recurrent OLT ABS after initial successful endoscopic therapy, whereas other institutions repeat another course of endoscopic treatment with varying protocols and degrees of success. The purpose of this study was to determine the rate of success of endoscopic therapy for OLT ABS after a single course of endoscopic therapy, identify the rate of recurrent OLT ABS after initial successful remediation with endoscopic therapy and describe the management of such recurrences. We also sought to identify variables associated with ABS recurrence.
Materials and MethodsThis was a single-center study conducted at the Hospital of the University of Pennsylvania. All the authors were physicians or students at this institution during this study. A clinical data warehouse was queried for ICD9 diagnosis codes for liver transplantation (LT) and procedure codes for ERCP from 2005-2012 were used to identify patients. A secondary search of the endoscopy reporting software was also conducted to ensure that all eligible patients were included in the analysis. Data regarding reason for OLT, operative factors, technical ERCP procedural factors, duration of stenting and pertinent clinical and laboratory data were collected. Patients under the age of 18 years or those who had not yet completed endoscopic stent therapy were excluded. Patients with non-anastomotic biliary strictures, live donor liver transplants, and split grafts were excluded due to the different natural history and outcomes of these strictures and our effort to create a uniform cohort.
Per institutional protocol, all patients with suspected OLT ABS were offered ERCP as the first line of therapy in an effort to avoid biliary revision surgery. Suspicion for OLT ABS was based cumulatively on clinical presentation, liver function tests, and when appropriate, cross-sectional imaging studies and our institutional protocol did not include specific laboratory cut-off values or obligatory imaging. Six individuals performed all ERCPs. Endoscopic stent therapy was performed in a uniform approach. During the initial ERCP, 1 or 2 plastic stents were placed with dilation performed at the discretion of the endoscopist. Fully – covered metal biliary stents have not been routinely utilized for OLT ABS at our institution. Unless clinical suspicion for cholangitis, stent migration or obstruction requires an earlier procedure, repeat ERCP is electively performed every 3 months for stent exchange. During each procedure attempts were made to dilate the stricture with placement of an increasing number or diameter of stents, and achieve a maximum cumulative stent diameter. Generally the aim was for 12 months of stenting. However, the decision to remove and complete stent therapy before 12 months was determined if cholangiography confirmed that the ABS has been remediated with free flow of contrast distal to the anastomosis or a lack of resistance with balloon sweep of the biliary tree and based on a patient's improved liver associated tests. Patients with clinical suspicion of ABS recurrence typically proceeded to repeat endoscopic therapy with stenting following a similar protocol of serial endoscopic therapy with increasing number of stents to achieve remediation. Intravenous antibiotics, typically a fluoroquinolo-ne, were routinely administered before every procedure as prophylaxis for cholangitis. If there was a drug allergy or potential interaction with other medications, an alternative antibiotic was selected. Ursodeoxycholic acid was not administered.
Outcome measurement and assessment of a recurrent ABS was clinically determined. Endoscopic stent therapy was considered successful if there was no development of a stricture or worsening liver associated enzymes suggesting a cholestatic pattern of injury within 1 month of completion of therapy with all stents removed. Primary outcome measure was stricture recurrence, defined as a recurrent ABS identified 1 month or longer after completion of endoscopic stent therapy and initial successful remediation. An ABS identified within 1 month after successful completion of stent therapy, or inability to resolve the stricture, was defined as primary failure. Additional demographic and procedural factors were analyzed. Management of recurrent ABS (i.e. repeat endoscopic stenting versus operative revision) was further reviewed. Bivariate comparison and a Cox regression model were used to analyze for any factors associated with time-to-stricture recurrence. Statistical analysis was performed with the software program Stata 13.0 (College Station, Texas). Wilcoxon signed-rank tests were used to determine p-values. Institutional IRB approval was obtained.
ResultsExcluding living donor transplants, a total of 943 ortho-topic liver transplantations occurred over the study period with an average of 135 transplants per year. Routine biliary reconstruction for deceased donor transplantation was duct-to-duct with no T-tube drainage. One hundred twenty three individuals were identified as undergoing en-doscopic therapy for an OLT ABS, or a mean of 18 patients every year in the defined study period. As such the inferred rate of ABS was 13%, but the exact incidence of OLT ABS could not be calculated as we could not account for individuals who underwent transplantation before the study period or those who received OLT during the study period but were diagnosed with an ABS afterwards. The 123 individuals underwent a total of 757 ERCPs. There were no reports of death, perforation, cholangitis or other complications directly related to the procedure.
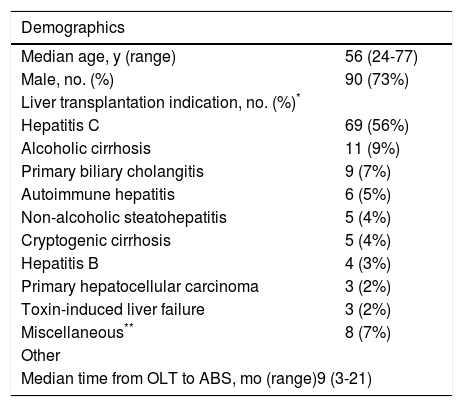
The median onset of ABS after OLT was 9 months (interquartile range [IQR] 3-21 months) and 90 patients (73%) were male. Thirty three individuals (26.8%) developed early-onset ABS defined as within 3 months from the date of transplantation. Indications for liver transplantation are listed in Table 1. Similar to other published series, hepatitis C cirrhosis was the leading indication for OLT (56%) followed by alcoholic cirrhosis (9%) and primary biliary cirrhosis (7%). Primary hepatocellular carcinoma was in the indication for transplantation in 2% of patients though a total of 11% of patients were ultimately diagnosed with hepatocellular carcinoma at the time of transplantation.
Characteristics of 123 patients who received stenting for OLT ABS.
| Demographics | |
|---|---|
| Median age, y (range) | 56 (24-77) |
| Male, no. (%) | 90 (73%) |
| Liver transplantation indication, no. (%)* | |
| Hepatitis C | 69 (56%) |
| Alcoholic cirrhosis | 11 (9%) |
| Primary biliary cholangitis | 9 (7%) |
| Autoimmune hepatitis | 6 (5%) |
| Non-alcoholic steatohepatitis | 5 (4%) |
| Cryptogenic cirrhosis | 5 (4%) |
| Hepatitis B | 4 (3%) |
| Primary hepatocellular carcinoma | 3 (2%) |
| Toxin-induced liver failure | 3 (2%) |
| Miscellaneous** | 8 (7%) |
| Other | |
| Median time from OLT to ABS, mo (range)9 (3-21) | |
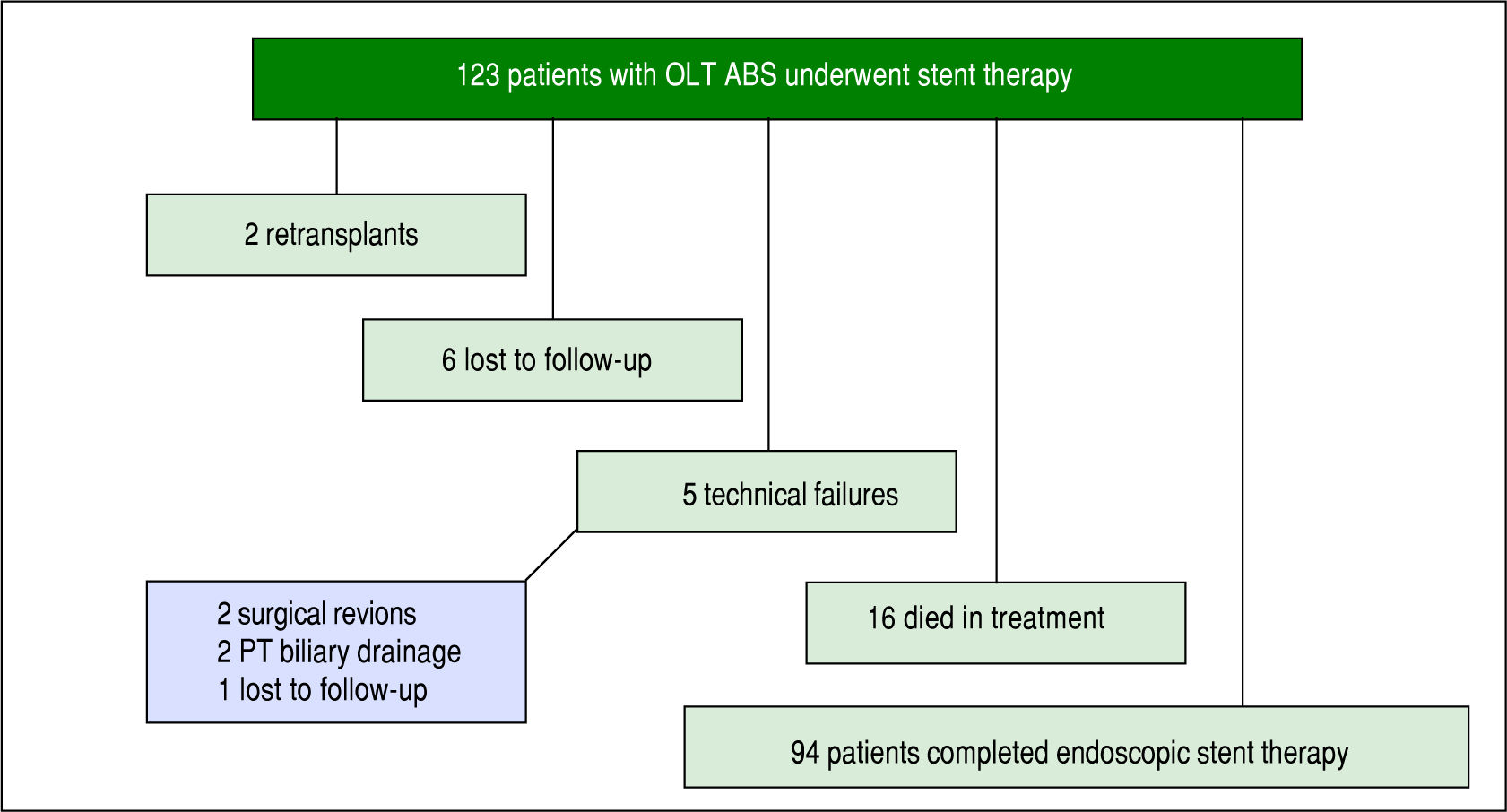
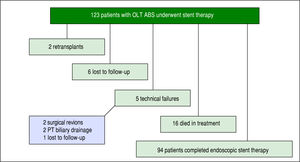
Of the 123 patients, 94 patients completed a course of endoscopic therapy for ABS. Sixteen individuals died during the treatment period due to sepsis (6), liver malignancy (2), unknown causes (2), liver failure from hepatic artery thrombosis (1), liver failure from chronic graft rejection (1), liver failure from recurrent HCV (1), post-transplant lymphoproliferative disorder (1), renal failure (1) and cardiac failure (1). None of the patients who died from sepsis had cholangitis. Six patients were lost to follow-up or received care at another institution with records unavailable for review and 2 patients were re-transplanted due to graft failure. Five patients had unsuccessful ERCP due to technical failure, in which cannulation of the bile duct was achieved and an ABS identified, but could not be traversed with a guidewire. Of these failures 2 went to surgical revision, 2 went to percutaneous transhepatic (PT) biliary drainage and 1 was lost to follow-up (Figure 1).
Of the 94 individuals who completed a course of endo-scopic therapy, long-term success was achieved in 74.5% (70/94) without stricture recurrence. The median number of ERCPs performed per patient was 4 (IQR 3-5) and median months of stenting was 7.3-12 Median follow-up was 21 months (IQR 10-38 months), maximum cumulative stent diameter was 20 French (IQR 10-30 Fr) and duration of maximum stenting was 3 months (IQR 3-6 months). 53.2% (50/94) received balloon dilation during endoscopic therapy. Among the 70 individuals with long-term endo-scopic remediation, 50% (35/70) had received balloon dilation during ERCP.
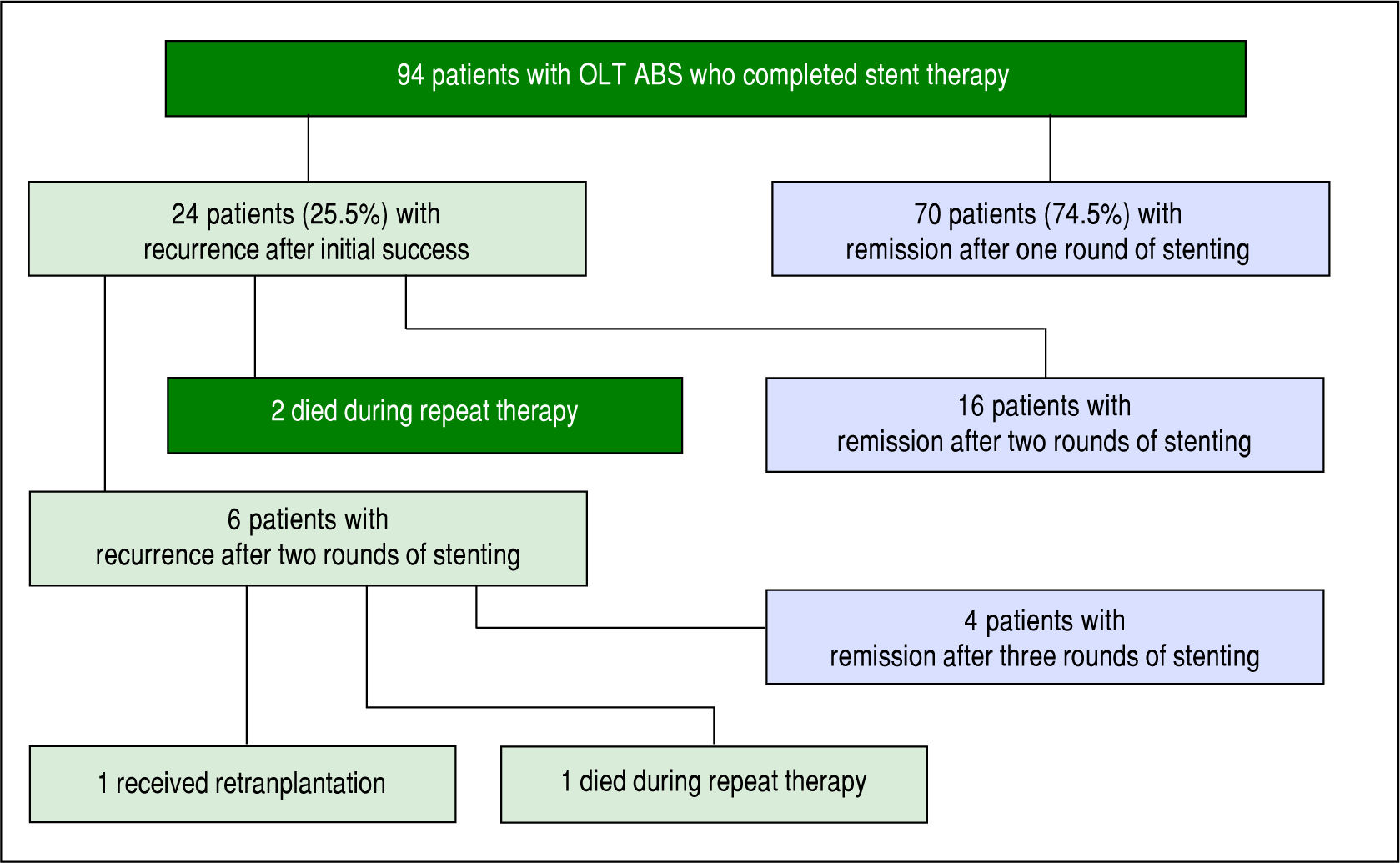
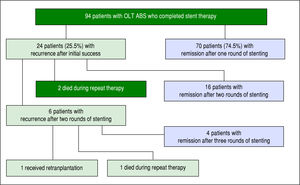
ABS recurred in 25.5% of individuals (24/94) after completion of a course of endoscopic therapy with initial success. In the initial course of stent therapy 62.5% (15/24) received balloon dilation. The median duration of remission before recurrence was 4 months (IQR 2-8 months). All 24 patients were referred for a second course of endo-scopic stent therapy, 22 patients completed the second course while 2 died during treatment due to liver failure from recurrent HCV, and 16 patients (67%) had successful long-term remediation of the ABS afterwards. The median number of ERCPs in the second round was 4 (IQR 3-4). The median maximum cumulative stent diameter was larger in the second round compared to the initial round (20 French [IQR 13.5-20 Fr] vs. 13 Fr [IQR 10-20 Fr] but not statistically significant (p-value = 0.14). The duration of maximal stenting was longer in the second round compared to the initial round (5 months [IQR 3-7.5 months] vs. 3 months [IQR 2.8-3.3] and statistically significant (p-value = 0.03).
All 6 patients who developed another recurrence after two full courses of endoscopic stent therapy underwent a third round of endoscopic stenting. Four patients achieved ABS remission, 1 patient died during stent therapy (sepsis) and 1 patient was re-transplanted for graft failure (Figure 2). Median number of ERCPs in the third round was 2.5 (IQR 2-4.8), median maximum cumulative stent diameter was 18.5 Fr (IQR 10-20 Fr), and median duration of maximal stenting was 3 months (IQR 2-12.3).
Among the 24 recurrences there were 11 patients that did not receive balloon dilation during their first course of stent therapy. Two of the patients did not survive beyond completion of a second round of endoscopic stent therapy while among the remaining 9 patients, 6 patients subsequently received balloon dilation in the second round of stent therapy and achieved long-term remediation. Long-term remediation was also achieved in the remaining 3 patients who did not receive balloon dilation in either their first or subsequent rounds of stent therapy.
Overall, 83.3% (20/24) of those individuals with a recurrent ABS were successfully remediated with endoscopic therapy.
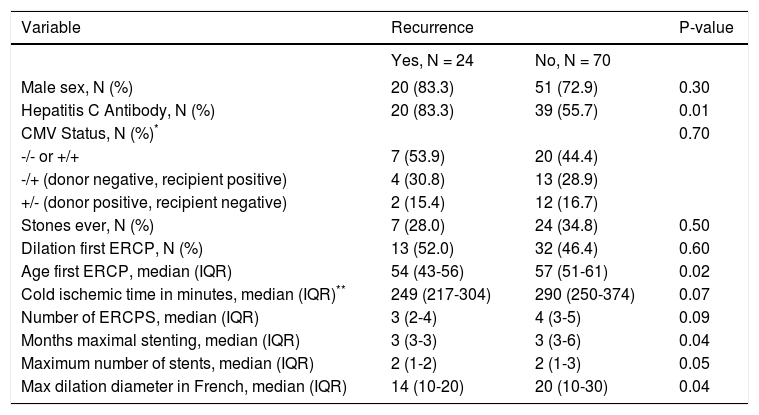
A bivariate comparison and binary outcome of recurrence, independent of time, demonstrated that hepatitis C (HCV) infection, age of first ERCP, median months of maximal stenting and a lower maximum cumulative stent diameter were risk factors for ABS recurrence (Table 2). A total of 59 patients had a positive HCV antibody after transplantation. 53 of these subjects had a liver biopsy confirmed infection, and 3 other patients had detectable serum HCV RNA. In the remaining 3 patients, 1 had both a negative liver biopsy and undetectable serum HCV RNA, 1 patient had an undetectable serum HCV RNA and did not have a liver biopsy, and 1 patient did not have a liver biopsy and a serum HCV RNA was not available.
Bivariate comparisons with binary outcome of recurrence, yes/no.
| Variable | Recurrence | P-value | |
|---|---|---|---|
| Yes, N = 24 | No, N = 70 | ||
| Male sex, N (%) | 20 (83.3) | 51 (72.9) | 0.30 |
| Hepatitis C Antibody, N (%) | 20 (83.3) | 39 (55.7) | 0.01 |
| CMV Status, N (%)* | 0.70 | ||
| -/- or +/+ | 7 (53.9) | 20 (44.4) | |
| -/+ (donor negative, recipient positive) | 4 (30.8) | 13 (28.9) | |
| +/- (donor positive, recipient negative) | 2 (15.4) | 12 (16.7) | |
| Stones ever, N (%) | 7 (28.0) | 24 (34.8) | 0.50 |
| Dilation first ERCP, N (%) | 13 (52.0) | 32 (46.4) | 0.60 |
| Age first ERCP, median (IQR) | 54 (43-56) | 57 (51-61) | 0.02 |
| Cold ischemic time in minutes, median (IQR)** | 249 (217-304) | 290 (250-374) | 0.07 |
| Number of ERCPS, median (IQR) | 3 (2-4) | 4 (3-5) | 0.09 |
| Months maximal stenting, median (IQR) | 3 (3-3) | 3 (3-6) | 0.04 |
| Maximum number of stents, median (IQR) | 2 (1-2) | 2 (1-3) | 0.05 |
| Max dilation diameter in French, median (IQR) | 14 (10-20) | 20 (10-30) | 0.04 |
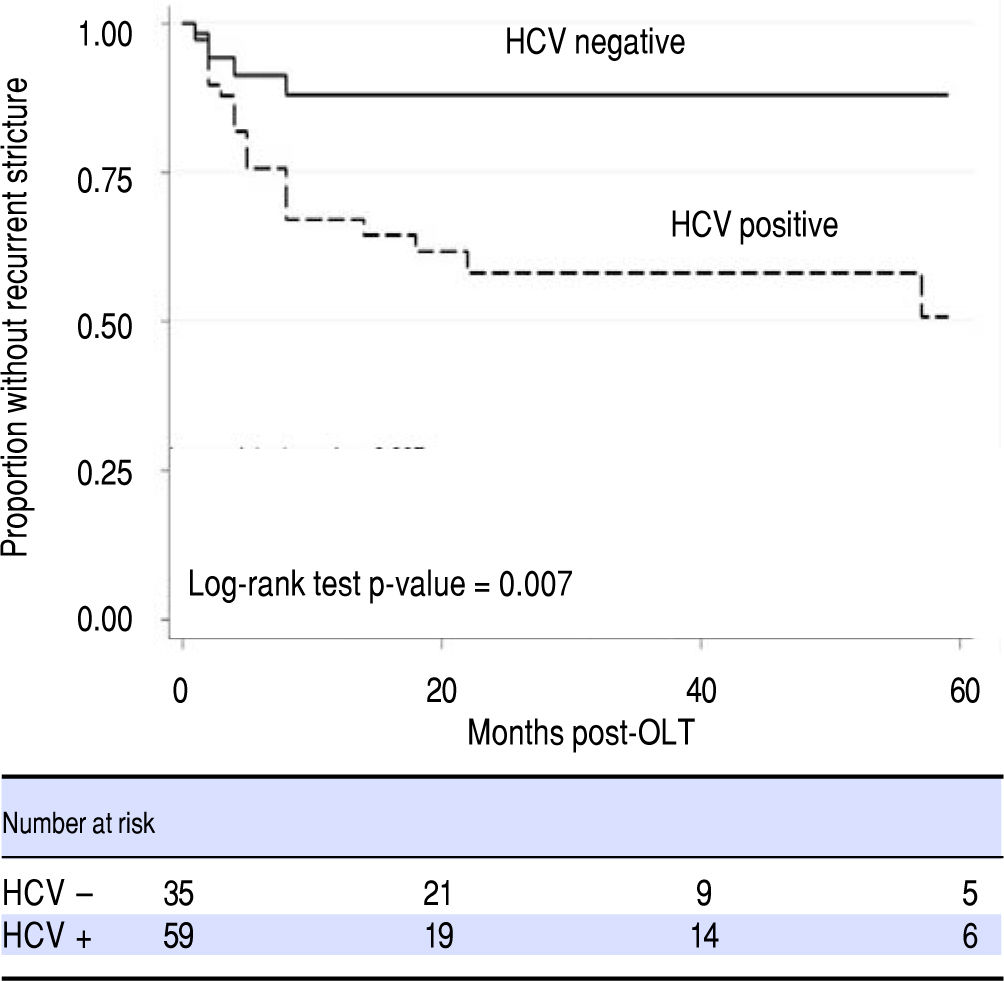
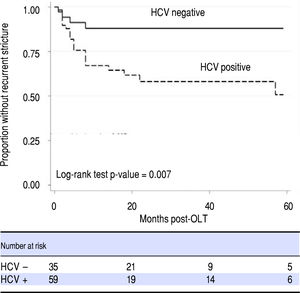
Transplant cold ischemia time and cytomegalovirus (CMV) status (including donor positive, recipient negative and donor negative, recipient positive), and lack of balloon dilation were not associated with higher rates of ABS recurrence. In a Cox regression model that accounted for time to recurrence, only HCV status proved to be a risk factor for ABS recurrence (Figure 3, Table 3). There was no difference in remediation rates between early and late-onset strictures.
Cox regression model for factors associated with recurrence in time-to-event analysis.
| Variable* | Univariable HR | Multivariable HR† | P-value |
|---|---|---|---|
| HCV positivity | 3.81 (1.31-11.1) | 3.62 (1.24-10.60)§ | 0.02 |
| Months maximal stenting | 0.83 (0.68-1.01) | 0.86 (0.70-1.04) | 0.12 |
| Maximum diameter dilation‡ | 0.96 (0.91-1.01) | 0.96 (0.91-1.02) | 0.18 |
Patient sex, donor-recipient CMV status, history of stones on ERCP, need for dilation at first ERCP, and age not include in multivariable models as they were not significant (p > 0.2) in univariable models.
In a final multivariable competing risk model the multivariable sub-hazard ratio for HCV is 3.64 (95% confidence interval: 1.20-10.98, p = 0.02). Number of ERCPs and maximal number of stents included in initial multivariable models but excluded from final model, as they were not significant in multivariable models (p > 0.5) and the need to limit the number of covariates given the limited number of outcomes.
There is a large body of literature describing the technical and clinical success rate of ERCP and stent therapy for OLT ABS. However none describe the management of initial endoscopic failures. By focusing our investigation on recurrent OLT ABS we demonstrate that even with initial endoscopic failure, endoscopic stent therapy remains highly successful. We suspect many OLT ABS patients labeled as endoscopic failures are subsequently sent to surgery for biliary revision when a repeat trial endoscopic stent therapy may have been an effective alternative. Such an alternative requires multiple additional endoscopic procedures though it ultimately spares the patient from co-morbidities and complications that may arise from operative intervention.
In this study an ABS recurrence rate of 25.5% (24/94) was observed after the first round of stent therapy, which is higher than the rate reported in other studies. However, when including patients who received a total of 2 or 3 rounds of stent therapy our remediation rate is 95.7% (90/ 94) in those who completed at least one course of endo-scopic therapy and 73% of all individuals who were identified to have an ABS. Compared to operative biliary revision and the complications it may entail,10 repeating 1 or 2 rounds of stent therapy in our experience is generally the preferred alternative, although this therapy requires multiple sessions over a longer duration. By serially increasing stent number and diameter, the maximum dilation force is utilized at each stent exchange to progressively treat the stricture. When comparing the first course of stent therapy to the later courses in those with ABS recurrence, patients requiring multiple rounds of stent therapy did not have a significantly higher maximum cumulative stent diameter in their later rounds compared to their initial course, but they did have a longer median duration of maximum stent therapy. As such, we believe the main benefit our patients received from multiple courses of stent therapy was prolonged duration of stenting.
In our study use of balloon dilation, number of ER-CPs, maximum size of cumulative stent caliber and duration of maximal stenting during the initial course of stent therapy were not factors for OLT ABS recurrence. There have been multiple studies examining treatment of OLT ABS with variations in endoscopic therapy. A recent study has suggested that balloon dilation of an ABS within 3 months of OLT may be associated with recurrence,11 and an interesting smaller pilot study using dilation with a pa-clitaxel-eluting balloon has achieved high rates of stricture resolution,12 potentially eliminating the need for stenting altogether. Most studies have focused on variations in timing and methodology of endoscopic stent therapy. Several have favored aggressive stenting where as many possible Stents are placed during each ERCP over shorter cumulative treatment durations, therefore minimizing the number of ERCPs and potentially reducing risk for periprocedural complications.3,4,6 In one study ERCPs were performed every 2 weeks if the patient did not demonstrate clinical signs of stricture resolution.6 Other studies have emphasized a longer duration of stenting, with varying degrees of aggressiveness when placing additional Stents.1,5,7 A systematic review by Kao, et al. has suggested that stenting periods of greater than 12 months lead to higher stricture resolution rates,9 and our success with multiple courses of stent therapy appears to support this. The benefits of longer stent duration are believed to be increased time for the anastomosis to remodel around the Stents; therefore minimizing stricture reformation once the stents are removed. However, as we did not identify any endoscopic factors associated with OLT ABS recurrence, it is unclear whether alterations in timing and stent-ing may lead to better long-term remediation. The downside of long-term stenting versus surgical revision is the need for multiple ERCP procedures, which may be a hardship for some patients who live far away and have difficulty traveling.
HCV was observed to be a risk factor for OLT ABS recurrence, though it is unclear if this was a statistical aberration due to a high prevalence of HCV in our subjects or a true clinical association. As elevated liver function enzymes and cholestasis raised suspicion for OLT ABS and were among the reasons our patients were referred for ERCP, this may have created a selection bias towards individuals with HCV infection. Higher rates of HCV infection in OLT ABS recurrence were also reported in a recent study,13 though its competing risk analysis did not identify HCV as a risk factor. HCV has been reported as a risk factor for ABS formation in a study by Fujita, et al.14 hypothesizing that an HCV-specific immune response is generated not just within the hepatic parenchyma but also adjacent sites including the biliary anastomosis; triggering inflammation and stenosis. This mechanism may also be responsible for stricture recurrence. It will be helpful to clarify any association between HCV and OLT ABS recurrence. Further investigation about the effects of HCV in the OLT biliary tract and its role in the pathogenesis of ABS formation may also elucidate a preventative role for HCV antiviral therapy in OLT ABSs. This is particularly important as antiviral therapy shifts away from interferon and ribavirin based regimens, becomes increasingly tolerable to patients with fewer adverse events and may potentially eliminate HCV as a variable for patients in the pre and post-transplant state.15
Several challenges were encountered in our study. Besides the conventional limitations of a retrospective analysis, our data extraction was confined to patients who completed endoscopic Stent therapy. The excluded group of patients who did not complete Stent therapy at our institution represent those who died during the course of treatment or were lost to follow-up, and it was not possible to determine their outcome if they were to complete stent therapy. Using an intention to treat analysis, successful ABS remediation was achieved in 90 out of 123 patients (73%) who were suspected as having an anastomotic biliary stricture. Also, while our study had a relatively robust number of 94 patients completing therapy, the number of recurrences was small and only 2 patients required surgical revision. After the first round of stent therapy there were 24 patients with OLT ABS, a number too small for sub-analysis. Finally, our study only investigated multiple plastic stenting and not fully covered self-expandable metal stents (SEMS). SEMS have garnered considerable interest for OLT ABS remediation, and initial studies of SEMSs as first line therapy report higher stricture resolution rates of 92% to 94%.16,17 Published stricture recurrence rates after SEMS therapy ranges from 8% to 47%18-22 although this includes studies in which SEMSs were deployed as secondary therapy after failure of plastic endo-prosthetics. SEMS migration has been reported in several publications19-24 and one recent study also noted a higher risk of pancreatitis23 but owing to longer patency rates, SEMS are thought to decrease the need for stent exchanges and warrant further study in OLT ABS management.
In conclusion, the incidence of anastomotic biliary strictures following OLT is 13% at our institution. We identified that 25.5% of these individuals has a recurrent OLT ABS stricture after completing an initial round of en-doscopic stent therapy. However, 83% of these recurrences were successfully managed with subsequent cycles of endoscopic therapy. Overall, 95.7% (90/94) of those who completed at least a single course of endoscopic therapy were able to achieve successful stricture remediation. Recurrence after the first or even second course of stenting should not imply failure of endoscopic therapy, as repeating endoscopic stenting can be effective, obviating the need for operative revision. A positive HCV status may be associated with higher stricture recurrence rates and this association should be further investigated. Our data suggest repeating endoscopic stenting may be beneficial for those with recurrent OLT ABS.
Abbreviations- •
ABS: anastomotic biliary stricture.
- •
CMV: cytomegalovirus.
- •
ERCP: endoscopic retrograde cholangiopancreatography.
- •
HCV: hepatitis C.
- •
IQR: interquartile range.
- •
LT: liver transplantation.
- •
OLT: orthotopic liver transplantation.
- •
PT: percutaneous transhepatic.
- •
SEMS: self-expandable metal stent.
This study did not receive any financial support or other funding.
Conflicts of InterestDr. Chandrasekhara, Dr. Jaffe and Dr. Kochman are consultants for Boston Scientific Company. Dr. Kochman is also a consultant for Cook Medical Inc. and Olympus Inc. The rest of the authors do not have conflicts of interest or financial disclosures to declare.