Introduction and aim. The role of hepatitis C virus infection as a risk factor for the development and progression of chronic kidney disease in the general population remains unclear.
Material and methods. A systematic review of the published medical literature was performed to assess whether positive anti-HCV serologic status is associated with higher frequency of chronic kidney disease in the adult general population. We used a random-effects model to generate a summary estimate of the relative risk of chronic kidney disease (defined by lowered glomerular filtration rate or detectable proteinuria) with HCV across the published studies. Meta-regression and stratified analysis were also carried out.
Results. Forty studies were eligible (n = 4,072,867 patients), and separate meta-analyses were conducted according to the outcome. Pooling results of longitudinal studies (n = 15 studies, n = 2,299,134 unique patients) demonstrated an association between positive anti-HCV serologic status and increased incidence of CKD, the summary estimate for adjusted HR with HCV across the surveys, 1.54 (95% CI, 1.26; 1.87) (P < 0.001). Between-study heterogeneity was observed (Q value by Chi-squared [χ2] test 500.3, P < 0.0001). The risk of chronic kidney disease related to HCV, in the subset of surveys from Asia was 1.45 (1.27; 1.65) (P < 0.001) (no heterogeneity). According to our meta-regression, ageing (P < 0.0001) and duration of follow-up (P < 0.0001) increased the risk of chronic kidney disease among HCV-positive subjects. We observed a relationship between anti-HCV positive serologic status and frequency of proteinuria, adjusted effect estimate of proteinuria with HCV among surveys was 1.633 (95% CI, 1,29; 2.05) (P < 0.001) (n = 10 studies; 315,404 unique patients). However, between-studies heterogeneity was noted (P value by Q test < 0.0001).
Conclusion. An association between HCV infection and increased risk of chronic kidney disease in the general population exists. The mechanisms underlying such association are currently under active investigation.
Hepatitis C virus infection is an important cause of liver disease worldwide.1 Recent evidence has been accumulated showing that chronic hepatitis C virus infection plays significant activity in various organs and tissues other than the liver.1 Increasing information exists on the activity of HCV on kidneys and a relationship between chronic hepatitis C virus infection and chronic kidney disease has been mentioned.2 HCV and CKD are major public health issues all over the world; globally, in 2015, an estimated 71 million people were living with chronic HCV infection.3 A novel systematic review reported that the global mean prevalence of CKD in general population was 13.4% in stages 1 to 5 and 10.6% in stages 3 to 5.4
Conventional risk factors for developing chronic renal disease do not fully explain the current frequency of chronic kidney disease in the adult general population of developed world. Various authors have evaluated the impact of HCV on the development of chronic kidney disease in general population;5–7 our meta-analysis of clinical observational studies (n = 9; 1,947,034 unique patients) had demonstrated a relationship between positive anti-HCV serologic status and increased incidence of chronic kidney disease; the summary estimate for adjusted hazard ratio was 1.43 (95% Confidence Interval, 1.23; 1.63, P = 0.0001).6 However, between-studies heterogeneity was noted (P value by Q test < 0.0001) and this precluded more definitive results.
Several biological mechanisms have been advocated to explain the increased risk of CKD in HCV-infected individuals. There is an association between HCV infection and glomerular disease in native kidneys and after solid organ transplant.8 Renal injury in HCV-positive patients can also be given by endothelial dysfunction which is in turn promoted by enhanced oxidative stress, pro-inflammatory cytokines, insulin resistance, or non-alcoholic steato-hep-atitis (NASH).9–11
The recent publication of additional and large studies on this topic has led us to summarize again the scientific evidence on the connection between chronic kidney disease and exposure to HCV infection. We have again reviewed the available evidence on the relationship between HCV infection and the development of chronic kidney disease in the adult general population by performing a systematic review of the literature with a meta-analysis of clinical observational studies.
Material and MethodsThis work is in agreement with the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (Annex 1).12
Search strategy and data extractionEnglish-language citations from the national Library of Medicine’s Medline database from 1989 through December 1, 2017 were reviewed by two authors (F.F., and F.M.D.). The first assay for HCV was manufactured in 1989 and data on HCV status are therefore not available for the time before 1989. Our search was conducted by four Medline databases engines (Embase, Grateful Med, Ovid, and PubMed), and was limited to human studies.
The following algorithm in medical subject heading and in free text words was applied: (“HCV” or “HCV Antibody Positive Serologic Status” or “Hepatitis C” or “Hepatitis C Virus Infection”) and (“CKD” or “Chronic Kidney Disease” or “End-Stage Renal Disease” or “ESRD” or “Glomerulonephritis” or “Low Glomerular Filtration Rate” or “kidney Failure“ or “Kidney Impairment” or “Kidney Insufficiency” or “Renal Failure” or “Renal Impairment” or “Renal Insufficiency”) and (“Interferon” or “IFN” or “pegylated Interferon” or “peg-IFN” or “Ribavirin”) and (“DAAs”) and (“Sustained Virological Response” or “Sustained Viral Response” or “Cure”) and (“Hazard Ratio” or “HR”). We performed an additional search with electronic searches of the Cochrane Library; manual searches of selected specialty journals were done to identify all pertinent literature. We also searched reference lists from qualitative topic reviews and published clinical studies. It was previously demonstrated that a Medline search alone might not be sensitive enough.13 Data on study design, study period, patient characteristics, HCV prevalence, antiviral therapy towards HCV, and kidney disease outcomes were abstracted. Authors of selected papers were contacted to obtain missing data and only data from individuals with known HCV status were included in the meta-analysis. We achieved consensus for all data. We compared studies to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. We pre-specified eligibility and exclusion criteria. Our search was limited to human studies that were published in the English literature.
Inclusion criteriaWe enrolled studies if they met the following inclusion criteria:
- •
They presented original data from cohort and longitudinal studies;
- •
The outcome of interest was clearly defined as frequency of chronic kidney disease, i.e., reduced glomerular filtration rate and/or detectable proteinuria in the adult general population according to anti-HCV serologic status; and
- •
They provided adjusted risk estimates and their confidence intervals. Both case-control studies and cohort studies were considered as eligible for inclusion in the analysis.
If data on the same population were duplicated in more than one study, we included the most recent study in the analysis. Information of HCV serologic status was recorded at the time of enrollment. We enrolled studies were the diagnosis of HCV infection was performed by testing for anti-HCV antibody in serum and/or HCV RNA detection by nucleic acid testing. Surveys based on administrative codes (ICD-9) were also evaluated.
Ineligible studiesWe have excluded studies if they reported inadequate data on the association between chronic kidney disease and anti-HCV positive serologic status (e.g., incomplete information on HCV status or renal outcomes). We have excluded unpublished studies, studies that were only published in abstract form or as interim reports; we have not considered letters and review articles for this systematic review.
Quality assessmentThe quality of the 40 studies was appraised using a scale adapted from the ‘Newcastle/Ottawa Scale (NOS)’.14 The Newcastle-Ottawa scale is a scoring system that assesses every aspect of an observational epidemiologic study from a methodological point of view. When a study included relevant information that could be associated with the NOS, one point was added. Seven items in cross-sectional studies and eight items in cohort and case-control studies that could be related to the NOS were identified. Therefore, cross-sectional studies assigned 8-10, 6-7, 4-5, or 0-3 points (stars) were evaluated as very good, good, satisfactory or unsatisfactory studies, respectively. Similarly, cohort/case-control studies with 7-9, 5-6, 4 and 0-3 points (stars) were identified as very good, good, satisfactory or unsatisfactory, respectively. We carried out subgroup analyses based on those studies provided with very good quality. Data extraction and quality scoring were performed independently by two reviewers (F.F. and F.M. D.) and the results were merged by consensus. The complete protocol for quality scoring is available on-line (Annex 2A).
Outcomes measuresWe made separate meta-analyses according to the outcome. One meta-analysis included longitudinal studies evaluating the incidence of chronic kidney disease, another enrolled cross-sectional studies addressing the prevalence of chronic kidney disease. An additional meta-analysis regarded the frequency of proteinuria (or glomerular disease). Staging of chronic kidney disease was categorized according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) definition, and estimated glomerular filtration rate was calculated using the four-variable MDRD equation.15
The primary end point was to provide adjusted estimates of the risk (and 95% CIs) of incidence (or prevalence) of chronic kidney disease in the adult general population according to anti-HCV serologic status. Multivariate analysis was carried out to evaluate the independent effect of anti-HCV positive status on the frequency of chronic kidney disease after adjustment for potential confounders (covariates) (e.g., age, gender, race/ethnicity, diabetes mellitus, and others). Cox proportional hazard regression analysis and logistic regression analysis were carried out in longitudinal and cross-sectional studies, respectively. An additional end-point was the adjusted estimate of the risk (and 95% CIs) of frequency of proteinuria (or glomerular disease) in the adult general population according to anti-HCV serologic status.
Data synthesis and analysisWe weighted the study-specific log hazard ratios by the inverse of their variance to obtain a pooled effect estimate and its 95% confidence intervals. For each study, we used the estimate of the effect measure that was adjusted for the largest number of confounders. We present both fixed-effects and random-effects pooled estimates but use and report the latter when heterogeneity was present. We used the random-effects approach, as described by DerSimonian and Laird,16 Cochrane Q-test was used for quantifying the heterogeneity.17 The I2 statistic, which is the percentage of total variation across studies due to heterogeneity rather than chance, was also calculated.18 The null hypothesis of this test is the presence of homogeneity (absence of heterogeneity). We explored the origin of heterogeneity by restricting the analysis to subgroups of studies defined by study characteristics such as country of origin, response to antiviral therapy, and others. Heterogeneity was also evaluated by meta-regression in order to look at the effect of potential and continuous covariates on the outcome of interest. Subgroup or stratified analyses and meta-regression were pre-specified. We performed random-effects meta-regression using the method of moments or maximum likelihood approaches where appropriate, a single predictor is allowed in each model (simple meta-regression). Publication bias was assessed by the Egger test for funnel-plot asymmetry. All analyses were done with the statistical package Comprehensive Meta-Analysis (CMA), version 2.0 (Biostat Inc., USA, 2005). The 5% significance level was adopted for α risk. Every estimate was given with its 95% Confidence Intervals.
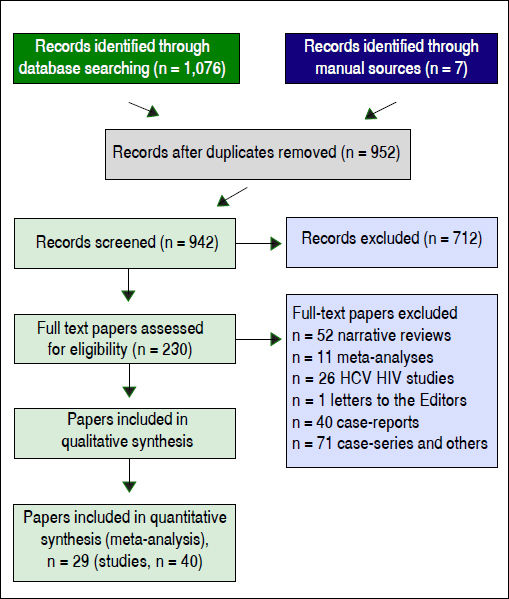
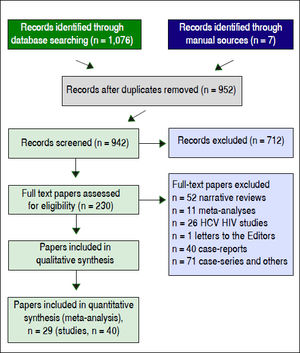
ResultsLiterature reviewAs shown in figure 1, we retrieved 4,533 articles and 230 full-text papers were assessed for eligibility. The list of the 230 full-text papers is reported in the Annex 3. Forty studies met our inclusion criteria and were published in 29 papers (Figure 1) and carried out in 3 continents (n = 4,072,867 patients).19–47 Thus, some studies contributed data on more than one kidney disease outcome, but each cohort was represented once in any meta-analysis. There was a 100% concordance between reviewers with respect to final inclusion and exclusion of studies reviewed based on the predefined inclusion and exclusion criteria.
Information on HCV serological status was collected at the time of enrollment. We included studies where the diagnosis of HCV infection and chronic kidney disease were done by administrative data (ICD-9-CM codes).24–27,30,33,45 In one report the diagnosis of HCV was recorded by historical collection of hepatitis C history (individual interviews).44 Anti-HCV serologic status and occurrence of CKD were detected in the remaining surveys by laboratory tests.19–23,28,29,31,32,34–43
The relationship between HCV infection, as detected by positive HCV RNA in serum, and chronic kidney disease was addressed in three reports only. Two studies evaluated the link between positive HCV RNA status and incidence of ESRD;29,45 one evaluated the prevalence of CKD according to HCV RNA status.32
Patient characteristicsSupplemental tables 1-8 report some salient demographic and clinical characteristics of subjects enrolled in the included studies. The mean age of subject cohorts ranged from 37.6 to 61.9 ± 14 years. The gender distribution ranged from 31.2% to 95.7% male. Eighteen studies were from the US, thirteen were from Taiwan, and three from Europe. There were two reports from Japan, China and Quatar, respectively. The average follow-up ranged between 1.6 ± 0.2 to 16.8 years among longitudinal studies. The quality scores ranged between 4 and 7 (longitudinal studies) (Annex 2B), and 5 and 7 (cross-sectional studies) (data not shown).
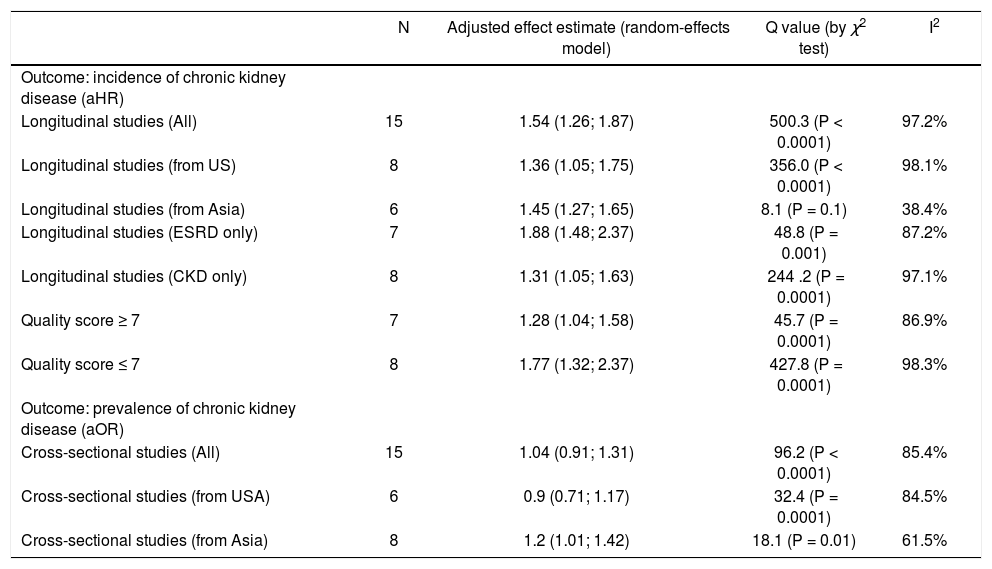
Summary measure for adjusted effect estimate of CKD according to anti-HCV serologic status among various groups of interest.
| N | Adjusted effect estimate (random-effects model) | Q value (by χ2 test) | I2 | |
|---|---|---|---|---|
| Outcome: incidence of chronic kidney disease (aHR) | ||||
| Longitudinal studies (All) | 15 | 1.54 (1.26; 1.87) | 500.3 (P < 0.0001) | 97.2% |
| Longitudinal studies (from US) | 8 | 1.36 (1.05; 1.75) | 356.0 (P < 0.0001) | 98.1% |
| Longitudinal studies (from Asia) | 6 | 1.45 (1.27; 1.65) | 8.1 (P = 0.1) | 38.4% |
| Longitudinal studies (ESRD only) | 7 | 1.88 (1.48; 2.37) | 48.8 (P = 0.001) | 87.2% |
| Longitudinal studies (CKD only) | 8 | 1.31 (1.05; 1.63) | 244 .2 (P = 0.0001) | 97.1% |
| Quality score ≥ 7 | 7 | 1.28 (1.04; 1.58) | 45.7 (P = 0.0001) | 86.9% |
| Quality score ≤ 7 | 8 | 1.77 (1.32; 2.37) | 427.8 (P = 0.0001) | 98.3% |
| Outcome: prevalence of chronic kidney disease (aOR) | ||||
| Cross-sectional studies (All) | 15 | 1.04 (0.91; 1.31) | 96.2 (P < 0.0001) | 85.4% |
| Cross-sectional studies (from USA) | 6 | 0.9 (0.71; 1.17) | 32.4 (P = 0.0001) | 84.5% |
| Cross-sectional studies (from Asia) | 8 | 1.2 (1.01; 1.42) | 18.1 (P = 0.01) | 61.5% |
• Crook, et al.:18 HR adjusted for renal function at baseline, urine protein excretion, blood pressure, gender, race, presence of diabetic nephropathy, age, duration of diabetes, and renin angiotensin system inhibitors at baseline.
• Tsui, et al.:19 HR adjusted for age, gender, race/ethnicity, educational status, smoking status, comorbidities.
• Moe, et al.:20 HR adjusted for age, gender, race, baseline GFR, diabetes, hypertension, AST, HIV.
• Asrani, et al.:21 HR adjusted for age, gender, baseline GFR, comorbidities (cirrhosis, diabetes, hypertension, heart failure, peripheral vascular disease, coronary artery disease, chronic obstructive pulmonary disease, diabetes, HIV), drug abuse, alcohol abuse, depression, diuretics, inhibitors of the renin-angiotensin system.
• Butt, et al.:22 HR adjusted for age, gender, race, baseline eGFR, hypertension, smoking, chronic obstructive pulmonary disease, diabetes, dyslipidemia, anemia, alcohol abuse, drug abuse, ACEi/ARB use, decompensated liver disease.
• Hofmann, et al.:23 HR adjusted for age, gender.
• Su, et al.:24 HR adjusted for gender, age, occupation, urbanization level, CCI.
• Chen, et al.:25 HR adjusted for age, gender, diabetes, hypertension, coronary artery disease, hyperlipidemia, liver cirrhosis, geographic region, urbanization level, enrolee category, number of healthcare visits in 1 year before study entry.
• Chen, et al.:26 HR adjusted for gender, age, diabetes, hypertension, coronary artery disease, hyperlipidemia, cirrhosis, geographic region, urbanization level, enrolee category, number of medical visits in 1 year before study entry.
• Lee, et al.:27 HR adjusted for gender, marital status, educational status, herb use, HBV infection, comorbidity (diabetes mellitus, hypertension, mild liver disease, severe liver disease, cardiovascular disease), body mass index, haemoglobin, platelets, ALT, cholesterol, uric acid, glucose, CKD stage, urine protein/creatinine ratio.
• Molnar, et al.:28 HR adjusted for age, gender, ethnicity, baseline eGFR, comorbidities (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV, depression), body mass index, systolic blood pressure, diastolic blood pressure, socioeconomic parameters (income, marital status, service connection), adherence to medical interventions, medical adherence, number of healthcare encounters during the follow-up, number of prescribed antihypertensive medications and ACEi/ARB usage throughout follow-up.
• Hwang, et al.:29 HR adjusted for age, gender, comorbidity (hypertension, coronary artery disease, hyperlipidemia, gout, liver cirrhosis, HBV).
• Rogal, et al.:30 HR adjusted for age, race, gender, body mass index, diabetes, hypertension, cirrhosis, alcohol abuse or dependence, drug abuse or dependence, ACEi/ARB use at baseline.
• Lai, et al.:31 HR adjusted for age, gender, diabetes, hypertension, baseline chronic kidney disease, serum cholesterol, triglycerides, uric acid, urinary protein excretion.
• Park, et al.:32 HR adjusted for age, gender, cirrhosis, diabetes, comorbidities (hypertension, diabetes, dyslipidemia, alcohol use, drug abuse, chronic obstructive pulmonary disease, heart failure, peripheral vascular disease, cerebrovascular disease, coronary artery disease, hepatitis A, hepatitis B, HIV, cirrhosis, hepatocellular carcinoma), ACEi/ARB, change of comorbidities, change of medication use.
• Tsui, et al.:19 OR adjusted for age, gender, race/ethnicity, educational status, smoking status, diabetes, arterial hypertension.
• Tsui, et al.:33 OR adjusted for age, gender, race/ethnicity, comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, congestive heart failure, coronary artery disease, HIV, substance abuse).
• Dalrymple, et al.:34 OR adjusted for age, gender, race, diabetes, hypertension.
• Ishizaka, et al.:35 OR adjusted for age, gender, systolic blood pressure, HBsAg, and fasting plasma glucose.
• Moe, et al.:20 OR adjusted for age, gender, diabetes, hypertension, AST, HIV status, laboratory values (rheumatoid factor, cryoglobulins).
• Asrani, et al.:21 OR adjusted for age, gender, comorbidities (cirrhosis, diabetes, hypertension, coronary artery disease, chronic obstructive pulmonary disease, peripheral vascular disease, heart failure, and HIV), drug or alcohol abuse, diuretics, inhibitors of the renin-angiotensin system.
• Lee, et al.36 OR adjusted for age, gender, educational status, BMI, albumin level, cholesterol level, uric acid level, hypertension, diabetes mellitus.
• Derbala, et al.:37 OR adjusted for age, gender.
• Aoufi Rabih, et al.:38 OR adjusted for age, gender, diabetes, hypertension, obesity, rheumatic disease.
• Lin, et al.:39 OR adjusted for age, gender, years of education, annual income, medical history (hypertension, diabetes mellitus, cardiovascular disease, stroke, gout, liver disease, urinary tract disease, cancer), health-related behaviors (oral and intravenous analgesic use, cigarette smoking, alcohol drinking, health supplements, Chinese herbs use, betel-nut chewing, Long Dan Xie Gan Tang).
• Li, et al.:40 OR adjusted for age, gender, alcohol drinking status, hypertension, serum creatinine, BMI, waist-to-height ration, fasting glucose, cholesterol, triglycerides, uric acid.
• Zheng, et al.:41 OR adjusted for age, gender, HBV, hypertension, diabetes mellitus, BMI, albumin, high-density cholesterol low-density cholesterol, triglycerides, total cholesterol, uric acid.
• Kurbanova, et al.:42 OR adjusted for age, gender, race, hypertension, diabetes, BMI.
• Su, et al.:43 OR adjusted for gender, age, obesity, income, HBV status, uric acid levels, anaemia, hyperlipidemia, smoking status, alcoholic status, betel nut chewing, exercise habits, groundwater use.
• Lai, et al.:44 OR adjusted for age, male, literate status, cigarette smoking, alcohol consumption, diabetes, hypertension, heart disease, HBsAg status, uric acid levels, serum cholesterol, serum triglycerides.
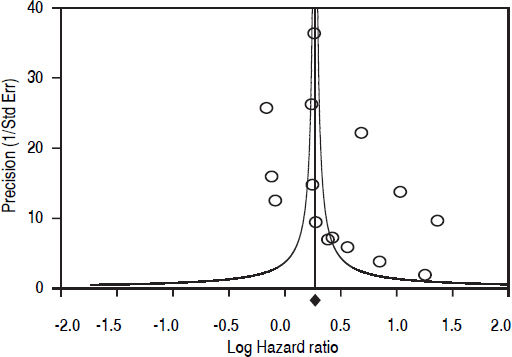
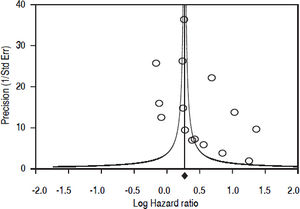
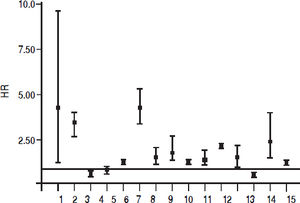
Fifteen longitudinal studies (n = 2,299,134 unique patients; 295,773 HCV-positive and 2,003,361 HCV-negative patients) gave information on the incidence of CKD among HCV-positive subjects.19–33 We found a significant association between positive anti-HCV serologic status and increased incidence of CKD, adjusted HR with HCV across the surveys, 1.54 (95% CI, 1.26; 1.87) (P < 0.001). Test for homogeneity of the aHR across the fifteen studies gave a Q value (by Chi-squared [χ2] test) of 500.3, I2 = 97.2% (P = 0.0001); that is, the homogeneity assumption was rejected (Table 1). The funnel plot concerning the publication bias is reported in figure 2. The Egger test demonstrated no publication bias (P = 0.2). Figure 3 reports the aHR and 95% confidence intervals for each study.
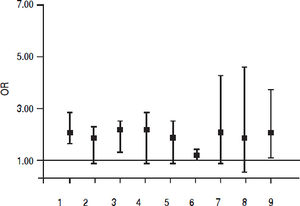
Fifteen studies (n = 865,494 unique patients; 81,054 HCV-positive and 784,175 HCV-negative patients) evaluated the prevalence of CKD in HCV-infected patients.20–22,34–45 We found no association between positive anti-HCV serologic status and increased prevalence of CKD, adjusted OR with HCV across the studies, 1.04 (95% CI, 0.91; 1.31) (P = 0.33). Tests for homogeneity of the aOR across the fifteen studies gave a Q value (by χ2 test) of 96.2 (I2 = 85.4) (P = 0.0001); in other words, the homogeneity assumption was rejected (Table 1). The Egger test demonstrated no publication bias (P = 0.12). Figure 4 reports the aOR and 95% confidence intervals for each study.
aOR and 95% confidence intervals for each study (n = 9 cross-sectional studies; n = 156,297 unique patients) (Outcome: prevalence of proteinuria). aOR of proteinuria associated with HCV (cross-sectional surveys), 1.51 (95% CI, 1,25; 1.82) (P < 0.001). Q value by χ2 test, 15.5 (P = 0.049), I2 = 48.7%.
The adjusted effect estimate of the occurrence of CKD among HCV RNA positive patients was 1.64 (95% CI, 1.32; 2.048) (P = 0.0001). Heterogeneity statistics, Q value (by χ2 test) = 1.23 (P-value = 0.54).
Summary estimate of outcome: Frequency of proteinuriaTen studies (n = 378,769 unique patients; 63,365 HCV-positive and 315,404 HCV-negative patients) evaluated the frequency of proteinuria according to anti-HCV positive serologic status.33,34,36–39,42,43,46,47 We found a significant association between positive anti-HCV serologic status and increased frequency of proteinuria, adjusted risk of proteinuria associated with HCV across the surveys, 1.633 (95% CI, 1,29; 2.05) (P < 0.001). Test for homogeneity of the adjusted risk or proteinuria across the ten studies gave a Q value (by χ2 test) of 37.47 (I2 = 75.9%) (P = 0.0001); that is, the homogeneity assumption was rejected (Table 2). The Egger test demonstrated no publication bias (P = 0.33). Figure 4 reports the aOR and 95% confidence intervals for each cross-sectional study.
Longitudinal studies included in the meta-analysis (outcome: incidence of chronic kidney disease) (I).
| Authors | Crook Ε | Tsui J | Moe S | Asrani S | Butt A |
|---|---|---|---|---|---|
| Reference year | 2005 | 2007 | 2008 | 2010 | 2011 |
| Country | USA | USA | USA | USA | USA |
| Patients, n | 312 | 474,369 | 7,038 | 88,822 | 43,139 |
| Follow-up, years | 1.6± 0.2/2.1 ± 0.1 | 3.6 | 3.46 | 2.1 ±1.05 | 3.1 ± 1.4/3 ± 1.3 |
| Anti-HCV positive patients, n | 26 (8.3%) | 52,874 (11.1%) | 2,243 (31.8%) | 8,063 (9.1%) | 18,002 (41.7%) |
| Age, years | 55.6 ± 2/60.5 ± 0.78 | 52 ± 9/59 ± 13 | 42.2 ± 11 | 48.7 ± 8/43.2 ± 11 | 51.9 ± 7/52.8 + 7 |
| Male, n | 110 (35%) | 447,494 (94.3%) | 3,481 (49.5%) | 37,724 (42.4%) | 41,974 (97.3%) |
| Caucasian, n | 49 (15.7%) | 318,854 (67%) | 3,556 (50.5%) | NA | 24,347 (56%) |
| Diabetes mellitus, n | 312 (100%) | 120,692 (25%) | 1,319 (18.7%) | 9,317 (10.4%) | 10,809 (25%) |
| Outcome | ESRD | ESRD | CKD stages 3-5 | CKD stages 3-5 | CKD stages 3-5 |
| Adjusted HR (95% CI) | 3.49 (1.27; 9.57) | 2.8 (2.43, 3.23) | 0.89 (0.79; 1.015) | 0.92 (0.79; 1.08) | 1.3 (1.23; 1.37) |
Longitudinal studies included in the meta-analysis (outcome: incidence of chronic kidney disease) (II).
| Authors | Hofmann J | Su F | Chen Y | Chen Y | Lee J |
|---|---|---|---|---|---|
| Reference vear | 2011 | 2012 | 2013 | 2014 | 2014 |
| Country | Sweden | Taiwan | Taiwan | Taiwan | Taiwan |
| Patients, n | 222.536 | 37.746 | 15.910 | 47.150 | 4.185 |
| Follow-up, years | 9.3 | 5.58 ± 2.04 | 5.8/5.92 | 7.1/7.43 | 2.2 ± 1.6 |
| Anti-HCV positive patients, n | 25,412 (11.4%) | 6,291 (16.6%) | 3,182 (20%) | 9,430 (20%) | 317 (7.6%) |
| Age, years | 37.6/NA | NA | NA | NA | 61.9 ± 14 |
| Male, n | 69.1 %/N A | 19,074 (50.5%) | 8,095 (50.8%) | 23,365 (49.5%) | 2,447 (58.5%) |
| Caucasian, n | 89.2%/NA | NA | NA | NA | NA |
| Diabetes mellitus, n | 3.7%/NA | NA | 981 (6.2%) | 7,792 (16.5%) | 1,504 (36.2%) |
| Outcome | CKD stages 1-5 | ESRD | CKD stages 1-5 | CKD Stages 1-5 | ESRD |
| Adjusted HR (95% CI) | 3.9 (3.2; 4.8) | 1.53 (1.17; 2.01) | 1.75 (1.25; 2.43) | 1.28 (1.12; 1.46) | 1.32(1.07; 1.62) |
Longitudinal studies included in the meta-analysis (outcome: incidence of chronic kidney disease) (III).
| Authors | Molnar M | Hwang J | Rogal S | Lai Τ | Park Η |
|---|---|---|---|---|---|
| Reference year | 2015 | 2016 | 2016 | 2017 | 2017 |
| Country | USA | Taiwan | USA | Taiwan | USA |
| Patients, n | 1,021,049 | 19,574 | 71,528 | 19,984 | 225,792 |
| Follow-up, years | 8.0 | 12 | 4.9±2/5.9 ± 2.8 | 16.8 | 1.75 |
| Anti-HCV positive patients, n | 100,518 (9.8%) | 9,787 (50%) | 2,589 (3.6%) | 591 (2.9%) | 56,448 (25%) |
| Age, years | 54.5 ± 13 | 55.7 ± 12.1 | 51 (43; 57)/55 (51; 59) | 47.3 ± 10 | NA |
| Male, n | 939,365 (92%) | 10,044 (51.3%) | 68,463 (95.7%) | 9,804 (49.1%) | 137,231 (60.7%) |
| Caucasian, n | 705,537 (69%) | NA | 40,647 (56.8%) | NA | NA |
| Diabetes mellitus, n | 216,933 (21.2%) | 19,574 (100%) | 17,593 (24%) | 1,616 (8.1%) | 36,739 (16.3%) |
| Outcome | ESRD | ESRD | CKD stages 3-5 | ESRD | CKD stages 3-5 |
| Adjusted HR (95% CI) | 1.98 (1.81; 2.16) | 1.47 (1.1; 1.93) | 0.86 (0.79; 0.92) | 2.33 (1.40; 3.89) | 1.27 (1.18; 1.37) |
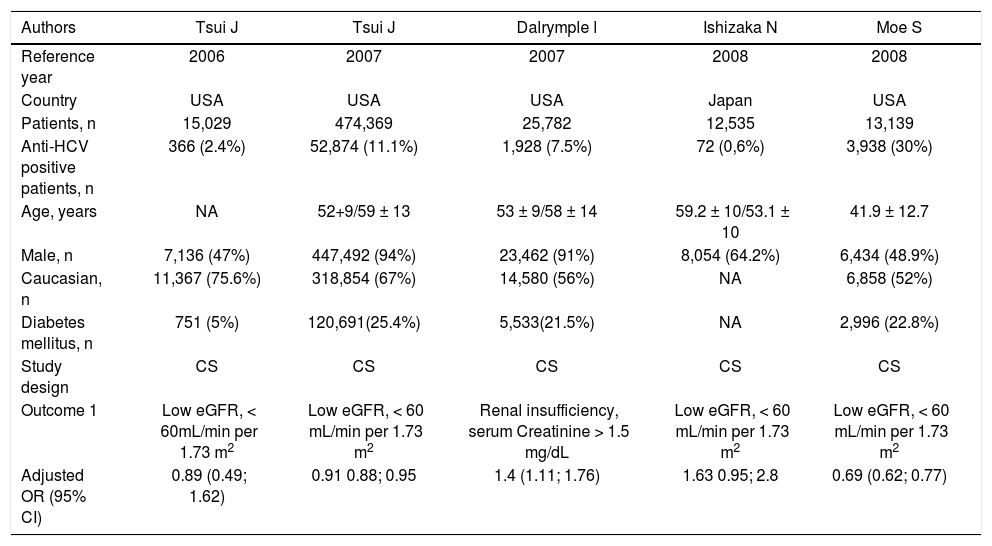
Cross-sectional studies included in the meta-analysis: prevalence of chronic kidney disease (I).
| Authors | Tsui J | Tsui J | Dalrymple l | Ishizaka N | Moe S |
|---|---|---|---|---|---|
| Reference year | 2006 | 2007 | 2007 | 2008 | 2008 |
| Country | USA | USA | USA | Japan | USA |
| Patients, n | 15,029 | 474,369 | 25,782 | 12,535 | 13,139 |
| Anti-HCV positive patients, n | 366 (2.4%) | 52,874 (11.1%) | 1,928 (7.5%) | 72 (0,6%) | 3,938 (30%) |
| Age, years | NA | 52+9/59 ± 13 | 53 ± 9/58 ± 14 | 59.2 ± 10/53.1 ± 10 | 41.9 ± 12.7 |
| Male, n | 7,136 (47%) | 447,492 (94%) | 23,462 (91%) | 8,054 (64.2%) | 6,434 (48.9%) |
| Caucasian, n | 11,367 (75.6%) | 318,854 (67%) | 14,580 (56%) | NA | 6,858 (52%) |
| Diabetes mellitus, n | 751 (5%) | 120,691(25.4%) | 5,533(21.5%) | NA | 2,996 (22.8%) |
| Study design | CS | CS | CS | CS | CS |
| Outcome 1 | Low eGFR, < 60mL/min per 1.73 m2 | Low eGFR, < 60 mL/min per 1.73 m2 | Renal insufficiency, serum Creatinine > 1.5 mg/dL | Low eGFR, < 60 mL/min per 1.73 m2 | Low eGFR, < 60 mL/min per 1.73 m2 |
| Adjusted OR (95% CI) | 0.89 (0.49; 1.62) | 0.91 0.88; 0.95 | 1.4 (1.11; 1.76) | 1.63 0.95; 2.8 | 0.69 (0.62; 0.77) |
Cross-sectional studies included in the meta-analysis: prevalence of chronic kidney disease (II).
| Authors | Asrani S. | Lee J. | Derbala M. | Aoufi Rabih S. | Lin M. |
|---|---|---|---|---|---|
| Reference year | 2010 | 2010 | 2010 | 2012 | 2013 |
| Country | USA | Taiwan | Quatar | Spain | Taiwan |
| Patients, n | 167,569 | 54,966 | 300 | 265 | 3,352 |
| Anti-HCV | 13,384 | 5,189 | 233 | 120 | 187 |
| positive patients, n | (7.9%) | (9.4%) | (77.7%) | (72.7%) | (5.6%) |
| Age, years | 47.8 ±8.6/40.4 ± 11.8 | 60.8 ±11.5 | 46 (41 ; 53) | 56 ± 16.6/55.3 ± 15.7 | 47.5 ± 17.4 |
| Male, n | 75,577 (45%) | 17,168 (31.2%) | 239 (79.7%) | 140 (53%) | 1,629 (48.6%) |
| Caucasian, n | NA | NA | 0 | NA | 0 |
| Diabetes | 11,614 | 5,302 | 138 | 25 | 191 |
| mellitus, n | (6.9%) | (9.6%) | (46%) | (9%) | (5.6%) |
| btudy design | CS | CS | CS | CS | CS |
| Outcome | Low eGFR, <60 mL/min per 1.73m2 | Low eGFR, <60 mL/min per 1.73m2 | Low eGFR, <60 mL/min per 1.73m2 | Low eGFR, <60 mL/min per 1.73m2 | CKD |
| Adjusted OR (95% CI) | 0.90 (0.36; 2.27) | 1.3 (1.2; 1.42) | 1.12 (0.5; 1.5) | 18.3 (2.3; 143) | 0.65 (0.45; 0.94) |
Cross-sectional studies included in the meta-analysis: prevalence of chronic kidney disease (III).
| Authors | Li W. | Zeng Q. | Kurbanova N. | Su S. | Lai T. |
|---|---|---|---|---|---|
| Reference year | 2014 | 2014 | 2015 | 2015 | 2017 |
| Country | Taiwan | China | USA | Taiwan | Taiwan |
| Patients, n | 24,642 | 15,549 | 33,729 | 10,463 | 13,805 |
| Anti-HCV positive patients, n | 1,699 (6.9%) | 94 (0.6%) | 659 (1.9%) | NA | 431 (3.1%) |
| Age, years | 42.9 ± 14.5 | 49.2 + 9.3 | 49.8 | 54 + 15.1 | 47.5 ± 10 |
| Male, n | 12,827 (52.1%) | 10,909 (67.5%) | 16,284 (48%) | 5,218 (49.8%) | 6,601 (47.8%) |
| Caucasian, n | 0 | NA | 16,147 (47.9%) | 0 | 0 |
| Diabetes mellitus, n | NA | 1,508 (9.7%) | 4,143 (12.2%) | 3,182 (30.4%) | 335 (0.02%) |
| Study design | CS | CS | CS | CCS | CS |
| Outcome | CKD | CKD | CKD | CKD | CKD |
| Adjusted OR (95% CI) | 1.24 (1.05; 1.48) | 0.74 (0.18; 3.04) | 0.88 (0.57; 1.37) | 1.22 (0.85; 1.74) | 1.91 (1.27; 2.88) |
Studies included in the meta-analysis: frequency of proteinuria (or glomerular disease) (I).
| Authors | Liangpunsakul S. | Tsui J. | Huang J. | Ishixzaka N. | Derbala M. |
|---|---|---|---|---|---|
| Reference year | 2005 | 2006 | 2006 | 2008 | 2010 |
| Country | USA | USA | Taiwan | Japan | Quatar |
| Patients, n | 13,990 | 15,029 | 9,934 | 12,535 | 300 |
| Anti-HCV positive patients, n | 368 (2.6%) | 366 (2.4%) | 646 (6.5%) | 72 (0.6%) | 233 (77.7%) |
| Age, years | 47.6 ± 19 | NA | 55.2 ± 6 | 53.1 ± 10.6 | 46 (41 ; 53) |
| Male, n | 7,192 (46.9%) | 7,136 (47.5%) | 4,291 (43.1%) | 8,054 1(64.2%) | 239 (79.9%) |
| Caucasian, n | 10,505 (68.5%) | 11,367 (75.6%) | 0 | 0 | NA |
| Diabetes mellitus, n | 1,349 (8.8%) | 751 (5%) | 1,241 (12.5%) | NA | 138 (46%) |
| Study type | Nested case-control | CS | CS | CS | CS |
| Outcome | Urine albumin excretion/ creatinine ratio, > 30 mcg/mg | Spot urine albumin/ creatinine ratio, > 17 mcg/mg | Urine protein > 1+ | Urine albumin excretion ratio (UAER), > 30 mg/g | Albumin Creatinine ratio, > 2.2 mg/mmol |
| Adjusted OR (95% CI) | 1.99 (1.38; 2.85) | 1.38 (0.91; 2.07) | 1.648 (1.246; 2.17) | 1.59 (0.83; 3.02) | 1.4 (0.8; 2.3) |
Studies included in the meta-analysis: frequency of proteinuria (or glomerular disease) (II).
| Authors | Lee J. | Aoufi Rabih S. | Zeng Q. | Kurbanova N. | Park Η. |
|---|---|---|---|---|---|
| Reference year | 2010 | 2012 | 2014 | 2015 | 2017 |
| Country | Taiwan | Spain | China | USA | USA |
| Patients, n | 54,966 | 265 | 15,549 | 33,729 | 222,472 |
| Anti-HCV positive patients, n | 5,189 (9.4%) | 120 (72.7%) | 94 (0.6%) | 659 (1.9%) | 55,618 (25%) |
| Age, years | 60.8 ±11.5 | 56 + 16/ 55.3 ± 15 | 49.2 ± 9.3 | 49.8 ± 18.7 | NA |
| Male, n | 17,168 (31.2%) | 140 (52.8%) | 10,509 (67.5%) | 16,284 (48.2%) | 137,231 (60.7%) |
| Caucasian, n | NA | NA | 0 | 16,147 | NA |
| Diabetes mellitus, n | 5,302 (9.6%) | 25 (9%) | 1,508 (9.7%) | (47.9%) 4,143 (12.2%) | 36,739 (16.3%) |
| Study type | CS | CS | CS | CS | Longitudinal |
| Outcome | Urine protein, > 1 + | Microalbuminuria/ creatinine, > 30 mcg/L | Albumin Excretion ratio, > 30 mg/g | Urine albumin creatinine ratio, > 30 mg/g | MPGN |
| Adjusted effect estimate (95% CI) | 1.14 (1.0; 1.3) | 2.05 (0.98; 4.29) | 1.3 (0.32; 5.32) | 1.95 (1.11; 3.41) | 2.23 (1.84; 2.71) |
| Estimate | OR | OR | OR | OR | HR |
PRISMA 2009 checklist. PRISMA’s items and their application within the paper.
| Section/topic | N | Checklist item on page n | Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 5 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 6-7 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 6-7 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 6-7 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 8-9 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 8-9 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 9-10 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 9-10 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 9-10 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 9-10 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 11 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Tables 1-8 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Tables 1-8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Tables 1-8 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 12-13 Tables 9-10 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 12-13 Tables 9-10 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 13-14 Tables 11-12 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 15 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 16 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 17 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 18 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit:www.prisma-statement.org.
Quality study. Details on the quality study process (longitudinal studies).
| A. NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE (cohort studies) |
|---|
| Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability (maximum 8 items, 9 stars). |
| Selection (Maximum 4 stars) |
| 1) Representativeness of the exposed cohort. |
| a) Truly representative of the average __ (describe) in the community. |
| b) Somewhat representative of the average __ in the community. |
| c) Selected group of users e.g. nurses, volunteers. |
| d) No description of the derivation of the cohort. |
| 2) Selection of the non exposed cohort. |
| a) Drawn from the same community as the exposed cohort. |
| b) Drawn from a different source. |
| c) No description of the derivation of the non exposed cohort. |
| 3) Ascertainment of exposure. |
| a) Secure record (e.g. surgical records). |
| b) Structured interview. |
| c) Written self report. |
| d) No description. |
| 4) Demonstration that outcome of interest was not present at start of study. |
| a) Yes. |
| b) No. |
| Comparability (Maximum 2 stars) |
| 1) Comparability of cohorts on the basis of the design or analysis. |
| a) Study controls for __ (select the most important factor). |
| b) Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor). |
| Outcome (Maximum 3 stars) |
| 1) Assessment of outcome. |
| a) Independent blind assessment. |
| b) Record linkage. |
| c) Self report. |
| d) No description. |
| 2) Was follow-up long enough for outcomes to occur. |
| a) Yes (select an adequate follow up period for outcome of interest). |
| b) No. |
| 3) Adequacy of follow up of cohorts. |
| a) Complete follow up - all subjects accounted for. |
| b) Subjects lost to follow up unlikely to introduce bias - small number lost - > __ % (select an adequate %) follow up, or description provided of those lost). |
| c) Follow up rate < __ % (select an adequate %) and no description of those lost. |
| d) No statement. |
Quality study. Details on the quality study process (longitudinal studies).
| NEWCASTLE-OTTAWA QUALITY ASSESSMENT SCALE 1 (cohort studies) | ||
|---|---|---|
| Crook E, et al. (Diabetes Care, 2005) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2b 3a one star N = 5 stars | Butt A, et al. (Am J Kidney Dis, 2011) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 6 stars | Chen Y, et al. (Kidney Int, 2014) SELECTION 1a one star 2a one star 3a one star COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 7 stars |
| Tsui J, et al. (Arch Intern Med, 2007) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 6 stars | Hofmann J, et al. (Eur J Cancer Prev, 2011) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b 2a one star 3a one star N = 6 stars | Lee J, et al. (PLos One, 2014) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 7 stars |
| Moe S, et al. (Am J Kidney Dis, 2016) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a no star N = 7 stars | Su F, et al. (Am J Kidney Dis, 2012) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 7stars | Molnar M, et al. (Hepatology 2015) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N=6 stars |
| Asrani S, et al. (Clin Gastroenterol Hepatol, 2010) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a no star N = 7 stars | Chen Y, et al. (BMC Nephrol, 2013) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 7 stars | Hwang J, et al. (Medicine 2016) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a OUTCOME 1b 2a one star 3a one star N = 4 stars |
| Rogal S, et al. (Dig Dis Sci 2016) SELECTION 1c 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 6 stars | Lai T, et al. (Hepatology 2017) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2a one star 3a one star N = 7 stars | Park E, et al. (Hepatology 2017) SELECTION 1a one star 2a one star 3a one star 4b COMPARABILITY 1a one star OUTCOME 1b one star 2b 3a one star N= 6 stars |
List of full-text papers assessed for eligibility (sorted by publication year).
| Full text articles assessed for eligibility (n = 230). | |
|---|---|
| 1. | Bonomo L, Casato M, Afeltra A, Caccavo D. Treatment of idiopathic mixed cryoglobulinemia with alpha interferon. Am J Med 1987; 83: 726-30. |
| 2. | Casato M, Lagana B, Antonelli G, Dianzani F, Bonomo L. Long-term results of therapy with interferon-alpha for type II essential mixed cryoglobulinemia. Blood 1991; 78: 3142-7. |
| 3. | Johnson R, Gretch D, Yamabe H, Hart J, Bacchi C, Hartwell P, Couser W, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993; 328: 465-70. |
| 4. | Johnson R, Gretch D, Couser WG, Alpers C, Wilson J, Chung M, Hart J, et al. Hepatitis C virus-associated glomerulonephritis. Effect of alpha-interferon therapy. Kidney Int 1994; 46: 1700-04. |
| 5. | Dammacco F, Sansonno D, Han J, Shymala V, Cornacchiulo V, Iacobelli A, Lauletta G, et al. Natural interferon alpha versus its combination with 6-methylprednisolone in the therapy of type II mixed cryoglobulinemia: a long-term randomized cross-over controlled trial. Blood 1994; 84: 3336-43. |
| 6. | Pucillo LP, Agnello V. Membranoproliferative glomerulonephritis associated with hepatitis B and C viral infections: from virus like particles in the cryoprecipitate to viral localization in paramesangial deposits, problematic investigations prone to artifacts. Curr Opin Nephrol Hypertens 1994; 3: 465-70. |
| 7. | Misiani R, Bellavita P, Fenili D, Vicari O, Marchesi D, Sironi P, Zilio P, et al. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med 1994; 330: 751-6. |
| 8. | Davis C, Gretch D, Perkins J, Harris A, Wener M, Alpers C, Lesniewski R, et al. Hepatitis C-associated glomerular disease in liver transplant recipients. Liver Transplant Surg 1995; 1: 166-75. |
| 9. | Migliaresi S, Tirri G. Interferon in the treatment of mixed cryoglobulinemia. Clin Exp Rheumathol 1995; 13 (Suppl. 13): S175-S180. |
| 10. | Altraif I, Abdulla A, Al Sebayel M, Said R, Al Suhaibani M, Jones A. Hepatitis C associated glomerulonephritis. Am J Nephrol 1995; 15: 407-10. |
| 11. | Yamabe H, Johnson R, Gretch D, Osawa H, Inuma H, Sasaki T, Kaizuka M, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus responsive to interferon-alpha. Am J Kidney Dis 1995; 25: 67-9. |
| 12. | Stehman-Breen C, Willson R, Alpers C, Gretch D, Johnson R. Hepatitis C virus associated glomerulonephritis. Curr Opin Nephrol Hypertens 1995; 4: 287-94. |
| 13. | Roth D. Hepatitis C virus: the nephrologist’s view. Am J Kidney Dis 1995; 25: 3-16. |
| 14. | Komatsuda A, Imai H, Wakui H, Hamai K, Ohtani H, Kodama T, Oyama Y, et al. Clinicopathological analysis and therapy in hepatitis C virus-associated nephropathy. Intern Med 1996; 35: 529-33. |
| 15. | Gilli P, Stabellini N, Storari A, Gualandi G, Guerra G, Ghinelli F. Effect of human leukocyte alpha interferon on cryoglobulinemic membranoproliferative glomerulonephritis associated with hepatitis C virus infection. Nephrol Dial Transplant 1996; 11: 526-8. |
| 16. | Morosetti M, Sciarra G, Meloni C, Palmieri G, Palombo G, Taccone-Gallucci M, Casciani C. Membranoproliferative glomerulonephritis and hepatitis C: effects of interferon-alpha therapy on clinical out come and histological pattern. Nephrol Dial Transplant 1996; 11: 532-4. |
| 17. | Matyus J, Kovacs J, Ujhelyi L, Karpati I, Dalmi L, Kakuk G. Interferon therapy in cryoglobulinemic membranoproliferative glomerulonephritis associated with hepatitis C virus infection. Orv Hetil 1996; 137: 2527-30. |
| 18. | Kendrick E, McVicar J, Kowdley K, Bronner M, Emond M, Alpers C, Gretch D, et al. Renal disease in hepatitis C-positive liver transplant recipients. Transplantation 1997; 63: 1287-93. |
| 19. | Moses P, Krawitz E, Aziz W, Corwin H. Renal failure associated with hepatitis C virus infection. Improvement in renal function after treatment with interferon-alpha. Dig Dis Sci 1997; 42: 443-6. |
| 20. | Sarac E, Bastacky S, Johnson J. Response to high-dose interferon-alpha after failure of standard therapy in MPGN associated with hepatitis C virus infection. Am J Kidney Dis 1997; 30: 113-5. |
| 21. | Casato M, Agnello V, Pucillo L, Knight G, Leoni M, Del Vecchio S, Mazzilli C, et al. Predictors of long-term response to high-dose interferon therapy in type II cryoglobulinemia associated with hepatitis C virus infection. Blood 1997; 90: 3865-73. |
| 22. | Adinolfi L, Utili R, Zampino R, Ragone E, Mormone G, Ruggiero G. Effects of long-term course of alpha-interferon in patients with chronic hepatitis C associated to mixed cryoglobulinemia. Eur J Gastroenterol Hepatol 1997; 29: 343-50. |
| 23. | Mazzaro C, Carniello G, Colle R, Doretto P, Mazzi G, Crovatto M, Santini G, et al. Interferon therapy in HCV-positive mixed cryoglobulinemia: viral and host factors contributing to efficacy of the therapy. Ital J Gastroenterol Hepatol 1997; 29: 343-50. |
| 24. | Pham H, Feray C, Samuel D, Gigou M, Azoulay D, Paradis V, Ducret F, et al. Effects of ribavirin on hepatitis C-associated nephrotic syndrome in four liver transplant recipients. Kidney Int 1998; 54: 1311-9. |
| 25. | Fabrizi F, Pozzi C, Farina M, Dattolo P, Lunghi G, Badalamenti S, Pagano A, et al. Hepatitis C virus infection and acute or chronic glomerulonephritis: an epidemiological and clinical appraisal. Nephrol Dial Transplant 1998; 13: 1991-7. |
| 26. | D’Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int 1998; 54: 650-71. |
| 27. | Stehman-Breen C, Johnson R. Hepatitis C virus-associated glomerulonephritis. Adv Intern Med 1998; 43: 79-97. |
| 28. | Cresta P, Musset L, Cacoub P, Frangeoul L, Vitour D, Poynard T, Opolon P, et al. Response to interferon-alpha treatment and disappearance of cryoglobulinemia in patients infected by hepatitis C virus. Gut 1999; 45: 122-8. |
| 29. | Kiyomoto H, Hitomi H, Hosotani Y, Hashimoto M, Uchida K, Kurokoucji K, Nagai M, et al. The effect of combination therapy with interferon and cryofiltration on mesangial proliferative glomerulonephritis originating from mixed cryoglobulinemia in chronic hepatitis C virus infection. Ther Apher 1999; 3: 329-33. |
| 30. | Misiani R, Bellavita P, Baio P, Caldara R, Ferruzzi S, Rossi S, Tengattini F. Successful treatment of HCV-associated cryoglobulinemic glomerulonephritis with a combination of interferon-alpha and ribavirin. Nephrol Dial Transplant 1999; 14: 1558-60. |
| 31. | Stehman-Breen C, Alpers C, Fleet W, Johnson R. Focal segmental sclerosis among patients infected with HCV. Nephron 1999; 81: 37-40. |
| 32. | Ezaki Y, Tanaka U, Minoshima S, Endou M, Kuwaki K, Arimura Y, Nakabayashi K, et al. Focal segmental glomerulosclerosis associated with type C virus hepatitis and decrement of proteinuria by interferon-alpha therapy. Nippon Jinzo Gakkai Shi 1999; 41: 83-8. |
| 33. | Daghestani L, Pomeroy C. Renal manifestations of hepatitis C infection. Am J Med 1999; 106: 347-54. |
| 34. | Stehman-Breen C, Alpers C, Fleet W, Johnson R. Focal segmental sclerosis among patients infected with HCV. Nephron 1999; 81: 37-40. |
| 35. | Al-Wakeel J, Mitwalli A, Tarif N, Al-Mohaya S, Malik G, Khalil M. Role of interferon-alpha in the treatment of primary glomerulonephritis. Am J Kidney Dis 1999; 33: 1142-6. |
| 36. | Kiyomoto H, Hitomi H, Hosotani Y, Hashimoto M, Uchida K, Kurokouchi K, Nagai M, et al. The effect of combination therapy with interferon and cryofiltration on mesangial proliferative glomerulonephritis originating from mixed cryoglobulinemia in chronic hepatitis C virus infection. Ther Apher 1999; 3: 329-33. |
| 37. | Calleja J, Albillos A, Moreno-Otero R, Rossi I, Cacho G, Domper F, Yebra M, et al. Sustained response to interferonalpha or to interferon-alpha plus ribavirin in hepatitis C virus associated symptomatic mixed cryoglobulinemia. Aliment Pharmacol Ther 1999; 13: 1179-86. |
| 38. | Nishi S, Ueno M, Shomada H, Oosawa Y, Iino N, Iguchi S, Karasawa R, et al. Treatment of membranoproliferative glomerulonephritis associated with hepatitis C virus infection. Nijgata Research Group of Glomerulonephritis and Nephrotic Syndrome. Intern Med 2000; 39: 788-93. |
| 39. | Matsumoto S, Nakjima S, Nakamura K, Etani Y, Hirai H, Shimizu N, Yokoyama H, et al. Interferon treatment on glomerulonephritis associated with hepatitis C virus. Pediatr Nephrol 2000; 15: 271-3. |
| 40. | Mazzaro C, Panarello G, Carniello S, Faelli A, Mazzi G, Crovatto M, Baracetti S, et al. Interferon versus steroids in patients with hepatitis C virus-associated cryoglobulinaemic glomerulonephritis. Dig Liver Dis 2000; 32: 708-15. |
| 41. | Laganovic M, Jelakovich B, Kuzmanic D, Ascukanec-Spoliar M, Roncevic T, Cuzic S, Ostojic R. Complete remission of cryoglobulinemic glomerulonephritis (HCV-positive) after high dose interferon therapy. Wien Klin Wochenschr 2000; 112: 596-600. |
| 42. | Soma J, Saito T, Taguma Y, Chiba S, Sato H, Sugimura K, Ogawa S, et al. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol 2000; 11: 690-9. |
| 43. | Yamabe H. How do we treat patients with hepatitis C virus associated glomerulonephritis? Intern Med 2000; 39: 525-6. |
| 44. | Jefferson J, Johnson R. Treatment of hepatitis C associated glomerular disease. Semin Nephrol 2000; 20: 286-92. |
| 45. | Sinico R, Fornasieri A, D’Amico G. Renal manifestations associated with hepatitis C virus. Ann Med Interne 2000; 151: 41-5. |
| 46. | Fabrizi F, Martin P, Ponticelli C. Hepatitis C virus infection and renal transplantation. Am J Kidney Dis 2001; 38: 919-34. |
| 47. | Motta M, Malaguarnera M, Restuccia N, Romano M, Vinci E, Pistone G. Focal segmental glomerulosclerosis and hepatitis C virus: a case report. Panminerva Med 2001; 43: 49-52. |
| 48. | Suzuki T, Yonemura K, Miyaji T, Suzuki H, Takahira R, Fujigaki Y, Fujimoto T, et al. Progressive renal failure and blindness due to retinal haemorrhage after interferon therapy for hepatitis C virus-associated membrano-proliferative glomerulonephritis. Intern Med 2001; 40: 708-12. |
| 49. | Philipneri M, Bastani B. Kidney disease in patients with chronic hepatitis C. Curr Gastroenterol Rep 2001; 3: 79-83. |
| 50. | Mehta S, Levey J, Bonkovsky H. Extrahepatic manifestations of infection with hepatitis C virus. Clin Liver Dis 2001; 5: 979-1008. |
| 51. | Naarendorp M, Kallemuchikkal U, Nuovo G, Gorevic P. Long-term efficacy of interferon-alpha for extra-hepatic disease associated with hepatitis C virus infection. J Rheumatol 2001; 28: 2466-73. |
| 52. | Dussol B, Moal V, Daniel L, Pin C, Berland Y. Spontaneous remission of HCV-induced cryoglobulinemic glomerulonephritis. Nephrol Dial Transplant 2001; 16: 156-9. |
| 53. | Garini G, Allegri L, Carnevali L, Catellani W, Manganelli P, Buzio C. Interferon-alpha in combination with ribavirin as initial treatment for hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis. Am J Kidney Dis 2001; 38: E35. |
| 54. | Lhotta K. Beyond hepatorenal syndrome: glomerulonephritis in patients with liver disease. Semin Nephrol 2002; 22: 302-8. |
| 55. | Sabry A, Sobh M, Sheashaa H, Kudesia G, Wild G, Fox S, Wagner B, et al. Effect of combination therapy (ribavirin and interferon) in HCV-related glomerulopathy. Nephrol Dial Transplant 2002; 17: 1924-30. |
| 56. | Perez-Calvo J, Lasierra P, Moros M, Inigo P. Role of ribavirin in membranoproliferative glomerulonephritis associated with hepatitis C virus infection refractory to alpha-interferon. Nephron 2002; 92: 459-62. |
| 57. | Cacoub P. Treatment of extrahepatic manifestations associated with hepatitis C virus infection. Gastroenterol Clin Biol 2002; 26: B210-B219. |
| 58. | Mazzaro C, Colle R, Baracetti S, Nascimbeni F, Zorat F, Pozzato G. Effectiveness of leukocyte interferon in patients affected by HCV positive mixed cryoglobulinemia resistant to recombinant alpha-interferon. Clin Exp Rheumatol 2002; 20: 27-34. |
| 59. | Beddhu S, Bastacky S, Johnson P. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore) 2002; 81: 398-403. |
| 60. | Guarnieri A, Marazzi F, Giorgi M, Canepari G, Pino C, Moggia E, Manca A, et al. Steroids, interferon-alpha and ribavirin treatment of cryoglobulinemic glomerulonephritis concurrent with HCV infection. Giorn It Nefrol 2002; 19: 79-81. |
| 61. | Fabrizi F, Colucci P, Ponticelli C, Locatelli F. Kidney and liver involvement in cryoglobulinemia. Semin Nephrol 2002; 22: 309-318. |
| 62. | Szczech L, Ganger S, van der Host C, Bartlett J, Young M, Cohen M, Anastos K, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int 2002; 61: 195-202. |
| 63. | Bruchfeld A, Lindahl K, Stahle L, Soderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C -associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18: 1573-80. |
| 64. | Lopes E, Valente L, Silva E, Kirsztajn G, Cruz C, Hoofnagle J. Therapy with interferon-alpha plus ribavirin for membranoproliferative glomerulonephritis induced by hepatitis C virus. Braz J Infect Dis 2003; 7: 353-7. |
| 65. | Meyers C, Seeff L, Stehman-Breen C, Hoofnagle J. Hepatitis C and renal disease: an update. Am J Kidney Dis 2003; 42: 631-57. |
| 66. | Rossi P, Bertani T, Baio P, Caldara R, Luliri P, Tengattini F, Bellavita P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int 2003; 63: 2236-41. |
| 67. | Tedaldi E, Baker R, Moorman A, Alzola C, Furher J, McCabe R, Wood K, et al. Influence of co-infection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis 2003; 36: 363-7. |
| 68. | Ghinoi A, Mascia M, Puccini R, Ferri C. Autoimmune and lymphoproliferative HCV-correlated manifestations: example of mixed cryoglobulinaemia (review). G Ital Nefrol 2004; 21: 225-37. |
| 69. | Alric L, Plaisier E, Thebault S, Peron J, Rostaing L, Pourrat J, Ronco P, et al. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43: 617-23. |
| 70. | Szczech L, Gupta S, Habash R, Guasch A, Kalayjian R, Appel R, Fields T, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 2004; 66: 1145-52. |
| 71. | Poordad F, Fabrizi F, Martin P. Hepatitis C infection associated with renal disease and chronic renal failure. Semin Liver Dis 2004; 24 (Suppl. 2): 69-77. |
| 72. | Vassalle C, Masini S, Bianchi F, Zucchelli G. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 2004; 90: 565-6. |
| 73. | Matsumori A. Effect of IFN treatment for extrahepatic manifestations. Nihon Rinsho 2004; 62 (Suppl. 7): 569-73. |
| 74. | Uchiyama -Tanaka Y, Mori Y, Kishimoto N, Nose A, Kijima Y, Nagata T, Umeda Y, et al. Membranous glomerulonephritis associated with hepatitis C virus infection: case report and literature review. Clin Nephrol 2004; 61: 144-50. |
| 75. | Orlent H, Mathot R, Van Bommel E, Vulto A, Schalm S, Brouwer J. Peginterferon and dose-titrated ribavirin for hepatitis C-associated nephrotic membranoproliferative glomerulonephritis type I. Dig Dis Sci 2005; 50: 1804-6. |
| 76. | Garini G, Allegri L, Vaglio A, Buzio C. Hepatitis C virus-related cryoglobulinemia and glomerulonephritis: pathogenesis and therapeutic strategies. Ann Intern Med 2005; 20: 71-80. |
| 77. | Sansonno D, Lauletta G, Montrone M, Grandaliano G, Schena F. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol 2005; 140: 498-506. |
| 78. | Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int 2005; 67: 285-290. |
| 79. | Hu S, Jaber B. Ribavirin monotherapy for hepatitis C virus-associated membranous nephropathy. Clin Nephrol 2005; 63: 41-5. |
| 80. | Crook E, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer survival in diabetic patients. Diabetes Care 2005; 28: 2187-91. |
| 81. | Cua I, Kwan V, Henriquez M, Kench J, George J. Long-term suppressive therapy with pegylated interferon for chronic hepatitis C associated membranoproliferative glomerulonephritis. Gut 2006; 55: 1521-2. |
| 82. | Huang J, Chuang W, Dai C, Ho C, Hwang S, Chen S, Lin Z, et al. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link ? J Int Med 2006, 260: 255-62. |
| 83. | Tsui J, Vittinghoff E, Shlipak M, O’Hare A. Relationship between hepatitis C an chronic kidney disease: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2006; 17: 1168-74. |
| 84. | Kamar N, Rosating L, Alric L. Treatment of hepatitis C-virus-related glomerulonephritis. Kidney Int 2006; 69: 436-9. |
| 85. | Bruchfeld A, Lindahl K, Stahle L, Schvarcz R. Interferon/pegylated interferon and ribavirin in HCV-associated kidney disease with or without cryoglobulinemia. J Hepatol 2006; 44: 432-3. |
| 86. | Saadoun D, Resche-Rigon M, Thibault V, Piette J, Cacoub P. Antiviral therapy for hepatitis C virus-associated mixed cryoglobulinemia vasculitis: a long-term follow-up study. Arthritis Rheum 2006; 54: 3696-76. |
| 87. | Zeman M, Cambell P, Bain V. Hepatitis C eradication and improvement of cryoglobulinemia-associated rash and membranoproliferative glomerulonephritis with interferon and ribavirin after kidney transplantation. Can J Gastroenterol 2006; 20: 427-31. |
| 88. | Kayali Z, Labrecque D, Schmidt W. Treatment of hepatitis C cryoglobulinemia: mission and challenges. Curr Treat Options Gastroenterol 2006; 9: 497-507. |
| 89. | Fabrizi F, Bruchfeld A, Mangano S, Dixit V, Messa P, Martin P. Interferon therapy for HCV-associated glomerulonephritis: meta-analysis of controlled trials. Int J Artif Organs 2007; 30: 212-9. |
| 90. | Fabrizi F, Lunghi G, Ganeshan S, Martin P, Messa P. Hepatitis C virus infection and the dialysis patient. Semin Dial 2007; 20: 416-22. |
| 91. | Garini G, Allegri L, Iannuzzella F, Vaglio A, Buzio C. HCV-related cryoglobulinemic glomerulonephritis: implications of antiviral and immunosuppressive therapies. Acta Biomed 2007; 78: 51-9. |
| 92. | Roccatello D, Fornasieri D, Giachino O, Roccatello D, Rossi D, Beltrame A, Banfi G, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis: implications of antiviral and immunosuppressive therapies. Am J Kidney Dis 2007; 49: 69-82. |
| 93. | Koziolek H, Scheel A, Bramlage C, Groene H, Mueller G, Strutz F. Effective treatment of hepatitis C-associated immune-complex nephritis with cryoprecipitate apheresis and antiviral therapy. Clin Nephrol 2007; 67: 245-9. |
| 94. | Tsui J, Vittinghoff E, Shlipak M, Bertenthal D, Inadomi J, Rodriguez R, O’Hare A. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Int Med 2007; 167: 1271-6. |
| 95. | Dalrymple L, Koepsell T, Sampson J, Louie T, Dominitz J, Young B, Kestenbaum B. Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol 2007; 2: 715-21. |
| 96. | Joshi S, Kuczynski M, Heathcote E. Symptomatic and virological response to antiviral therapy in hepatitis C associated with extrahepatic complications of cryoglobulinemia. Dig Dis Sci 2007; 52: 2410-7. |
| 97. | Montalbano M, Pasulo L, Sonzogni A, Remuzzi G, Colledan M, Strazzabosco M. Treatment with pegylated interferon and ribavirin for hepatitis C virus-associated severe cryoglobulinemia in a liver/kidney transplant recipient. J Clin Gastroenterol 2007; 41: 216-20. |
| 98. | Ahmed M, Wong C, Shawki H, Kapoor N, Pandya B. Rapidly deteriorating renal function with membranoproliferative glomerulonephritis Type 1 associated with hepatitis C treated successfully with steroids and antiviral therapy: a case report and review of literature. Clin Nephrol 2008; 69: 298-301. |
| 99. | Wyatt C, Malvestutto C, Coca S, Klotman P, Parikh C. The impact of hepatitis C virus-co-infection on HIV-related kidney disease: A systematic review and meta-analysis. AIDS 2008; 22: 1789-807. |
| 100. | Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol 2008; 49: 613-624. |
| 101. | Fabrizi F, Lunghi G, Messa P, Martin P. Therapy of hepatitis C virus-associated glomerulonephritis: current approaches. J Nephrol 2008; 21: 813-25. |
| 102. | Abbas G, Hussain S, Shafi T. Effect of antiviral therapy on hepatitis C virus related glomerulopathy. Saudi J Kindey Dis Transpl 2008; 19: 775-80. |
| 103. | Mangia A, Burra P, Ciancio A, Fagiuoli S, Guido M, Picciotto A, Fabrizi F, et al. Hepatitis C infection in patients with chronic kidney disease. Int J Artif Organs 2008; 31: 15-33. |
| 104. | Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Hepatitis C in chronic kidney disease. Kidney International 2008; 73 (Suppl 109): S1-S99. |
| 105. | Ishizaka N, Ishizaka Y, Seki G, Nagai R, Yamakado M, Koike K. Association between hepatitis B/C viral infection, chronic kidney disease and insulin resistance in individuals undergoing general health screening. Hepatology Research 2008; 38: 775-83. |
| 106. | Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, Celebi A, Ilkay E. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 2008; 72: 1960-5. |
| 107. | Iannuzzella F, Garini S. Current therapeutic strategies for HCV-associated cryoglobulinemia. Reumatismo 2008; 60: 163-73. |
| 108. | Moe S, Pampalone A, Ofner S, Rosenman M, Teal E, Hui S. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis 2008; 51: 885-92. |
| 109. | Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis 2008; 3: 25. |
| 110. | Sugiura T, Yamada T, Kimpara Y, Fuijta N, Goto K, Koyama N. Effects of pegylated interferon alpha-2a on hepatitis -C-virus associated glomerulonephritis. Pediatr Nephrol 2009; 24: 199-202. |
| 111. | Laurino S, Borrelli S, Catapano F, Mascia S, D’Angiò P, Calabria M, Grimaldi M, et al. Treatment of HCV-associated cryoglobulinemic glomerulonephritis. G Ital Nefrol 2009; 26: 318-27. |
| 112. | Tsui J, Vittinghoff E, Anastos K, Augenbraun M, Young M, Newicki M, Cohen M, et al. Hepatitis C seropositivity and kidney function decline among women with HIV: data from the Women’s Interagency HIV Study. Am J Kidney Dis 2009; 54: 43-50. |
| 113. | Lo K, Chen C, Lee C. Hepatitis C virus-associated type II mixed cryoglobulinemia vasculitis complicated with membranous proliferative glomerulonephritis. Ren Fail 2009; 31: 149-52. |
| 114. | Charles E, Dustin L. Hepatitis C virus-induced cryoglobulinemia. Kidney Int 2009; 76: 818-24. |
| 115. | Fabrizi F, Messa P, Basile C, Martin P. Hepatic disorders in chronic kidney disease. Nat Rev Nephrol 2010; 6: 395-403. |
| 116. | Asrani S, Buchanani P, Pinsky B, Rocca Rey L, Schnitzler M, Kanwal F. Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol 2010; 8: 79-84. |
| 117. | Lee M, Yang H, Wang C, Jen C, Yeh S, Liu C. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 2010; 41: 2894-900. |
| 118. | Noureddine L, Usman S, Yu Z, Moorthl R, Moe S. Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol 2010; 32: 311-6. |
| 119. | Lee J, Lin M, Yang Y, Lu S, Chen H, Hwang S. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis 2010; 56: 23-31. |
| 120. | Fabrizi F, Martin P, Messa P. Hepatitis B and hepatitis C virus and chronic kidney disease. Acta Gastroenterol Belg 2010; 73: 465-71. |
| 121. | Mima A, Iehara N, Matsubara T, Yamamoto S, Abe H, Nagai K, Matsura M, et al. Successful treatment of membrano-proliferative glomerulonephritis associated with hepatitis B and C virus simultaneous infection patient. Clin Nephrol 2010; 73: 167-9. |
| 122. | Ji F, Li Z, Ge H, Deng H. Successful interferon-alpha treatment in a patient with IgA nephropathy associated with hepatitis C virus infection. Intern Med 2010; 49: 2531-2. |
| 123. | Namba T, Shiba R, Yamamoto T, Hirai Y, Moriwaki T, Matsuda J, Kadoya H, et al. Successful treatment of HCV-related cryoglobulinemic glomerulonephritis with double-filtration plasmapheresis and interferon combination therapy. Clin Exp Nephrol 2010; 75: 374-9. |
| 124. | Derbala M, Shebl F, Rashid A, Amer A, Bener A. Microalbuminuria in hepatitis C-genotype 4: effect of pegylated interferon and ribavirin. World J Gastroenterol 2010; 16: 1226-31. |
| 125. | Mostafa A, Mohamed M, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, et al. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut 2010; 59: 1135-40. |
| 126. | Fischer M, Wyatt C, Gordon K, Giber C, Brown S, Rimland D, Rodriguez-Barradas M, et al. Hepatitis C and the risk of kidney disease and mortality in veterans with HIV. J Acquir Immune Defic Syndr 2010; 53: 222-6. |
| 127. | Arase Y, Suzuki F, Kawamura Y, Suzuki Y, Kobayashi M, Matsumoto N, Akuta N, et al. Development rate of chronic kidney disease in hepatitis C virus patients with advanced fibrosis after interferon therapy. Hepatology Research 2011; 41: 946-54. |
| 128. | Aoufi Rabih S, Garcia Agudo R. Management of HCV infection in chronic kidney disease. Nefrologia 2011; 31: 260-7. |
| 129. | George E, Nadkharni G, Estrella M, Lucas G, Sperati J, Atta M, Fine D. The impact of hepatitis C co-infection on kidney disease related to human immunodeficiency virus. Medicine (Baltimore) 2011; 90: 89-295. |
| 130. | Flandre P, Pugliese P, Cuzin L, Bagnis I, Tack I, Cabiè A, Poizot-Martin I, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol 2011; 6: 1700-7. |
| 131. | Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K. Urinary albumin excretion within the normal range is an independent risk factor for near-term development of kidney disease in HIV-infected patients. Nephrol Dial Transplant 2011; 26: 3923-9. |
| 132. | Hofmann J, Torner A, Chow W, Ye W, Purdue M, Duberg A. Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: a nationwide register-based cohort study in Sweden. Eur J Cancer Prev 2011; 20: 326-30. |
| 133. | Butt A, Wang X, Fried L. HCV and the incidence of CKD. Am J Kidney Dis 2011; 57: 396-402. |
| 134. | Colucci G, Manno C, Grandaliano G, Schena F. Cryoglobulinemic membranoproliferative glomerulonephritis: beyond conventional therapy. Clin Nephrol 2011; 75: 374-9. |
| 135. | Pietrogrande M, De Vita S, Zignego AL, Pioltelli P, Sansonno D, Sollima S, Atzeni F, et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev 2011; 10: 444-54. |
| 136. | Yanagisawa N, Ando M, Ajisawa A, Imamura A, Suganuma A, Tsuchiya K, Nitta K. Clinical characteristics of kidney disease in Japanese HIV-infected patients. Nephron Clin Pract 2011; 118: c291-c295. |
| 137. | Di Biagio A, Rosso R, Vitale F, Cardinale F, Sormani M, Secondo G, Di Stefano L, et al. Risk factors for chronic kidney disease amonh human immunodeficiency virus-infected patients: A European case control study. Clin Nephrol 2011; 75: 518-623. |
| 138. | Feng B, Eknoyan G, Guo ZS, Jadoul M, Rao H, Zhang W, Wei L. Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27: 640-6. |
| 139. | Su F, Su C, Chang S, Chen P, Sung F, Lin C, Yeh C. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kidney Dis 2012; 60: 553-60. |
| 140. | Aoufi Rabih S, Agudo R, Burillo J, Carrillo F, Gonzalez P, Roldan F, Ferrus M, et al. Microalbuminuria and ren.al insufficiency in chronic hepatitis C virus infection. Gastroenterol Hepatol 2012; 35: 309-16. |
| 141. | Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2012; 7: 549-57. |
| 142. | Van der Meer A, Veldt B, Feld J, Wedemeyer H, Dufour J, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584-93. |
| 143. | Satapathy S, Lingisetty C, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int 2012; 6: 369-77. |
| 144. | Fabrizi F, Fogazzi G, Cresseri D, Passerini P, Martin P, Donato M, Rumi G, et al. Antiviral therapy for HCV-associated cryoglobulinemic glomerulonephritis: case report and review of the literature. Kidney Blood Press Res 2012; 35: 687-93. |
| 145. | Peters L, Grint D, Lundgreen J, Rockstroh J, Soriano V, Reiss P, Grzeszczuk A, et al. Hepatitis C virus viremia increased the incidence of chronic kidney disease in HIV-infected patients. AIDS 2012; 26: 1917-26. |
| 146. | Mocroft A, Neuhaus J, Peters L, Ryom L, Bickel M, Grint D, Koirala J, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive antiretroviral-treated adults. PLos One 2012; 7: e40245. |
| 147. | Jotwani V, Li Y, Grunfeld C, Choi A, Shlipak M. Risk factors for ESRD in HIV- infected individuals: traditional and HIV-related factors. Am J Kidney Dis 2012; 59: 628-35. |
| 148. | Kalayian R, Lau B, Mechekano R, Crane H, Rodriguez B, Salata R, Krishnasami Z, et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS 2012; 26: 1907-15. |
| 149. | Donato M, Fabrizi F, Fogazzi G, Cresseri D, Passerini P, Martin P, Messa P. Remission of HCV-associated glomerulonephritis with pegylated interferon and ribavirin therapy after liver transplantation: case report and literature review. Int J Artif Organs 2013; 36: 63-8. |
| 150. | Sperati C. Stabilization of hepatitis C associated collapsing focal segmental glomerulosclerosis with interferon alpha-2a and ribavirin. Clin Nephrol 2013; 80: 231-4. |
| 151. | Shah H, Patel C. Long-term response to peginterferon in hepatitis C virus-associated nephrotic syndrome from focal segmental glomerulosclerosis. Ren Fail 2013; 35: 1182-5. |
| 152. | Tang S, Lai K. Hepatitis C virus-associated glomerulonephritis. Contr Nephrol 2013; 181: 194-206. |
| 153. | Bahirwani R, Barin B, Olthoff K, Stock P, Murphy B, Reddy R, for the Solid Organ Transplantation in HIV: Multi-Site Investigators. Chronic kidney disease after liver transplantation in human immunodeficiency virus /hepatitis C virus co-infected recipients versus human immunodeficiency virus-infected recipients without hepatitis C virus: results from the National Institutes of Health Multi-Site Study. Liver Transplant 2013; 19: 619-28. |
| 154. | Estrella M, Wyatt C, Pearce C, Li M, Shlipak M, Gustafson D, Cohen M, et al. Host APOL1 genotype is independently associated with proteinuria in HIV infection. Kidney Int 2013; 84: 834-40. |
| 155. | Reynes J, Cournil A, Peyriere H, Psomas C, Guiller E, Chatron M, Cristol J, Badiou S. Tubular and glomerular proteinuria in HIV-infected adults with estimated glomerular filtration rate 60 mL/min per 1.72 m2. AIDS 2013; 27: 1295-302. |
| 156. | Fabri M, Ruzic M, Lendak D, Preveden T, Fabri I, Petric V. Extrahepatic manifestations of chronic hepatitis C and their influence on response to treatment with pegylated interferon alfa-2a and ribavirin. Srp Arh Celok Lek 2013; 141: 320-4. |
| 157. | Donato M, Banfi G, Cresseri D, Fogazzi G, Martin P, Messa P, Fabrizi F. Antiviral therapy of symptomatic HCV-mixed cryoglobulinemia after liver transplant: case report and literature review. Int J Artif Organs 2013; 36: 367-72. |
| 158. | Maruyama S, Koda M, Oyake N, Sato H, Fuji Y, Horie Y, Murawaki Y. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol 2013; 58: 11-5. |
| 159. | Chen Y, Chiou W, Hung S, Su Y, Hwang S. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrology 2013; 14: 187. |
| 160. | Lin M, Chiu Y, Lee C, Yu H, Chen H, Wu M, Hwang S. Factors associated with CKD in the elderly and nonelderly population. Clin J Am Soc Nephrol 2013; 8: 33-40. |
| 161. | Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV-associated cryoglobulinemia: meta-analysis of clinical studies. J Med Virol 2013; 85: 1019-27. |
| 162. | Fabrizi D, Dixit V, Messa P. Interferon mono-therapy for symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. Acta Gastro-Enterol Belg 2013, 76: 363-71. |
| 163. | Fabrizi F, Plasisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis 2013; 61: 623-37. |
| 164. | Lucas G, Jing Y, Sulkowski M, Abraham A, Estrella M, Atta M, Fine D, et al. Hepatitis C viremia and the risk of chronic kidney disease in HIV-infected individuals. J Infect Dis 2013; 208: 1240-9. |
| 165. | Gordovskaia N, Kozlovskaia L, Milavanova S, Ignatova T, Korotchaeva I. Hepatitis C virus-related cryoglobulinemic vasculitis with renal involvement: current possibilities of treatment. Ter Arkh 2013; 85: 78-84. |
| 166. | Bickel M, Marben W, Betz C, Khaykin P, Stephan C, Gute P, Haberl A, et al. End-stage renal disease and dialysis in HIV-positive patients: observations form a long-term cohort study with a follow-up of 23 years. HIV Medicine 2013; 14: 127-35. |
| 167. | Cao Y, Gong M, Han Y, Xie J, Li X, Zhang L, Li Y, et al. Prevalence and risk factors for chronic kidney disease among HIV-infected antiretroviral therapy-naïve patients in mainland China: a multicenter cross-sectional study. Nephrology 2013; 18: 307-12. |
| 168. | Mauro E, Gattei V, Mazzaro C. Recombinant human erythropoietic and granular colony stimulating factor in hepatitis C virus related to mixed cryoglobulinaemia associated to membranoproliferative glomerulonephritis type I: a case report description. Infez Med 2014; 22: 337-41. |
| 169. | Ble M, Aguilera V, Rubin A, Vinaixa C, Prieto M, Berenguer M. Improved renal function in liver transplant recipients treated for hepatitis C virus with a sustained virological response and mild chronic kidney disease. Liver Transpl 2014; 20: 25-34. |
| 170. | Fabrizi F, Messa P, Martin P. The unravelled link between chronic kidney disease and hepatitis C infection. New J Science 2014; 180203. |
| 171. | Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol 2014; 20: 7544-54. |
| 172. | Fabrizi F, Messa P, Martin P. Novel evidence on hepatitis C virus-associated glomerular disease. Kidney Int 2014; 86: 466-9. |
| 173. | Fabrizi F, Donato F, Messa P. Hepatitis C virus infection and glomerular disease. Minerva Urol Nefrol 2014; 66: 139-49. |
| 174. | De Nicola S, Aghemo A, Campise M, D’Ambrosio R, Rumi M, Messa P, Colombo M. Telaprevir in a patient with chronic hepatitis C and cryoglobulinemic glomerulonephritis. Antivir Ther 2014; 19: 517-31. |
| 175. | Banerjee T, Scherzer R, Powe N, Steffick D, Shanihnian V, Saran R, Pavkov M, et al. Race and other risk factors for incident proteinuria in a national cohort of HIV-infected veterans. J Acquir Immun Defic Syndr 2014; 67: 145-52. |
| 176. | Petta S, Macaluso F, Craxi A. Cardiovascular diseases and HCV infection: a simple association or more ? Gut 2014; 63: 369-75. |
| 177. | Lee J, Lin M, Chang J, Hung C, Chang J, Chen H, Yu M, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. Plos One 2014; 9: e100790. |
| 178. | Zeng Q, Gong Y, Dong S, Xiang H, Wu Q. Association between exposure to hepatitis B virus and chronic kidney disease in China. Int Med Res 2014; 42: 1178-84. |
| 179. | Li W, Lee Y, Chen I, Wang S, Hsiao C, Loke S. Age and gender differences in the relationship between hepatitis C infection and all stages of chronic kidney disease. J Viral Hepatitis 2014; 21: 706715. |
| 180. | Chen Y, Lin H, Li C, Lee M, Su Y. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 2014, 85: 1200-7. |
| 181. | Hsu Y, Lin J, Ho H, Kao Y, Huang Y, Hsiao N, Wu M, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014; 59: 1293-302. |
| 182. | Humphries K, Darling J, Barritt A. Membranoproliferative glomerulonephritis, type II cryoglobulinemia and triple therapy for hepatitis C: A case series and review of the literature. Dig Dis Sci 2014; 59: 2007-12. |
| 183. | Stine J, Cornella S, Shah N. Treatment of chronic hepatitis C complicated by mixed cryoglobulinemia with new protease inhibitor, sofosbuvir. Ann Rheum Dis 2014; 73: e64. |
| 184. | Hsieh M, Lu P, Kuo M, Lin W, Lin C, Lai C, Tsai J, et al. Prevalence and associated factors with chronic kidney disease in human immunodeficiency -infected patients in Taiwan. J Microbiol Immunol Infect 2015; 48: 256-62. |
| 185. | Makara M, Sulyok M, Csacsovski O, Sulyok Z, Valyu -Nagy I. Successful treatment of HCV-associated cryoglobulinemia with ombitasvir/paritaprevir/ritonavir, dasabuvir, and ribavirin: A case report. J Clin Virol 2015; 72: 66-8. |
| 186. | Ennaifer R, Sabbah M, Hefaiedh R, Romdhane H, Cheikh M, Belhadj N. Antiviral therapy for hepatitis C virus infection, cryoglobulinemic glomerulonephritis, and low-grade malignant lymphoma: a challenge? La Tunisie Medícale 2015; 93: 203-4. |
| 187. | Cornella S, Stine J, Kelly V, Caldwell S, Shah N. Persistence of mixed cryoglobulinemia despite cure of hepatitis C with new oral antiviral therapy including direct-acting antiviral sofosbuvir: A case series. Postgraduate Medicine 2015; 127: 413-7. |
| 188. | Gragnani L, Fognani E, Piluso A, Boldrini B, Urraro T, Fabbrizzi A, Stasi C, et al. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: A prospective, controlled, open-label, cohort study. Hepatology 2015; 61: 1145-53. |
| 189. | Saadoun D, Resche Rigon M, Pol S, Thibault V, Blanc F, Pialoux G, Karras A, et al. PegIFNalpha/ribavirin/protease inhibitor combination in severe hepatitis C virus-associated mixed cryoglobulinemia vasculitis. J Hepatol 2015; 62: 24-30. |
| 190. | Su S, Lin C, Kao S, Wu C, Lu K, Lai C, Yang H, et al. Risk factors and their interaction on chronic kidney disease: a multicentre case control study in Taiwan. BMC Nephrology 2015; 16: 83. |
| 191. | Mazzaro C, Panarello G, Mauri E, Gattei V, Pozzato G. Efficacy and safety of pegylated interferon plus ribavirin for the treatment of hepatitis C virus-positive cryoglobulinemic glomerulonephritis. Dig Dis Sci 2015; 47: 613-6. |
| 192. | Morisue A, Fukuoka K, Goto R, Ota K, Yamashita H, Shinno Y, Yamadori I. Hepatitis C virus-related glomerulonephritis with acute kidney injury requiring haemodialysis that improved with virus removal and eradication using double-filtration plasmapheresis without interferon. CEN Case Rep 2015; 4: 38-42. |
| 193. | Molnar M, Alhourani H, Wall B, Lu L, Streja E, Kalantar-Zadeh K, Kovesdy C. Association of hepatitis C virus infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 2015; 61: 1495-502. |
| 194. | Chen Y, Hwang S, Li C, Wu C, Lin C. A Taiwanese nationwide cohort study shows interferon-based therapy for chronic hepatitis C reduces the risks of chronic kidney disease. Medicine (Baltimore) 2015; 94: e1334. |
| 195. | Fabrizi F, Martin P, Cacoub P, Messa P, Donato F. Treatment of hepatitis C-related kidney disease. Expert Opin Pharmacother 2015; 16: 1815-27. |
| 196. | Negro F, Forton D, Craxi A, Sulkowski M, Feld J, Manns M. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 2015; 149: 1345-60. |
| 197. | Kurbanova N, Qayyum R. Association of hepatitis C virus infection with proteinuria and glomerular filtration rate. Clin Transl Sci 2015; 8: 421-24. |
| 198. | Elshahawi Y, Sany D, Abd Elmohsen W, Tantawi T. Microalbuminuria and pegylated interferon in hepatitis C patients. Saudi J Kidney Dis Transpl 2015; 26: 1183-9. |
| 199. | Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney diseases: a systematic review and meta-analysis. Dig Dis Sci 2015; 60: 3801-13. |
| 200. | Hsu Y, Ho H, Huang Y, Wang H, Wu M, Lin J, Wu C. Association between antiviral treatment and extra-hepatic outcomes in patients with hepatitis C virus infection. Gut 2015; 64: 495-503. |
| 201. | Krajewska M, Rukasz D, Jakuszko K, Augustyniak -Bartosik H, Penar J, Bednarz Z, Klinger M. Hepatitis C-associated glomerulonephritis mimicking systemic lupus erythematosus. Scand J Rheumatol 2015; 44: 343-44 [letter]. |
| 202. | Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta-analytic assessment on the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat 2015; 22: 897-905. |
| 203. | Li M, Wang P, Yang C, Jiang W, Wei X, Mu X, Li X, et al. A systematic review and meta-analysis: Does hepatitis C virus infection predispose to the development of chronic kidney disease? Oncotarget 2017; 8: 10692-702. |
| 204. | Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int 2016; 10: 415-23. |
| 205. | Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C virus increases the risk of kidney disease among HIV -positive patients: Systematic review and meta-analysis. J Med Virol 2016; 88: 487-97. |
| 206. | Van der Meer A, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol 2016; 65: S95-S108. |
| 207. | Rogal S, Yan P, Rimland D, Lo Re V, Al-Rowais H, Fried L, Butt A, et al. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci 2016; 61: 930-6. |
| 208. | Hwang J, Jiang M, Lu Y, Weng S. Impact of HCV infection on diabetes patients for the risk of end-stage renal failure. Medicine 2016; 95: e2431. |
| 209. | Gragnani L, Piluso A, Urraro T, Fabbrizzi A, Fognani E, Petraccia L, Genovesi A, et al. Virological and clinical response to interferon-free regimens in patients with HCV-related mixed cryoglobulinemia: preliminary results of a prospective pilot study. Curr Drug Targets 2016; Feb 8. [Epub ahead of print]. |
| 210. | Sise M, Bloom A, Wisocky J, Lin M, Gustafson J, Lundquist A, Steele D, et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemic with direct-acting antiviral agents. Hepatology 2016; 63: 408-17. |
| 211. | Saadoun D, Thibault V, Si Ahmed S, Alric L, Mallet M, Guillaud C, Izzedine H, et al. Sofosbuvir plus ribavirin for hepatitis C virus -associated cryoglobulinemia vasculitis: VASCUVALDIC study. Ann Rheum Dis 2016; 75: 1. |
| 212. | Leone S, Prosperi M, Costarelli S, Nasta P, Maggiolo F, Di Giambenedetto S, Saracino A, et al. Incidence ad predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV co-infected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis 2016; 35: 1511-20. |
| 213. | Ferri C, Ramos-Casals M, Zignego A, Arcaini P, Roccatello D, Antonelli A, Saadoun D, et al. ISG-EHCV Coauthors. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev 2016; 15: 1145-60. |
| 214. | Petta S, Maida M, Macaluso F, Barbara M, Licata A, Craxi A, Camma C. Hepatitis C virus infection is associated with increased cardiovascular mortality: A meta-analysis of observational studies. Gastroenterology 2016; 150: 145-55. |
| 215. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016; 150: 1599-608. |
| 216. | Berenguer J, Rodriguez-Castellano E, Carrero A, Von Wichmann M, Montero M, Galindo M, Mallolas J, et al. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 2017; 66: 344-56. |
| 217. | Mahale P, Engels A, Li R, Torres H, Hwang L, Brown E, Kramer J. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut 2017; doi: 10.1136/gutjnl-2017-313983. |
| 218. | Sise M, Backman E, Ortiz G, Hundemer G, Ufere N, Chute D, Brancale J, et al. Effect of sofosbuvir -based hepatitis C virus therapy on kidney function in patients with chronic kidney disease. Clin J Am Soc Nephrol 2017; 12: 1615-23. |
| 219. | Johnson R, Shimada M. Contemporary management of hepatitis C in patients with chronic kidney disease. Clin J Am Soc Nephrol 2017; 12: 1563-65. |
| 220. | Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 2017; 152: 142-56. |
| 221. | Kupin W. Viral-associated glomerulonephritis: Hepatitis C and HIV. Clin J Am Soc Nephrol 2017; 12: 337-1342. |
| 222. | Lai T, Lee M, Yang H, You S, Lu S, Wang L, Yuan Y, et al. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease.Kidney Int 2017; http://dx.doi.org/10.1016/j.kint.2017.03.02. |
| 223. | Lai T, Lee M, Yang W, You S, Lu S, Wang L, Yuan Y, et al. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology 2017; 66: 784-93. |
| 224. | Park H, Chen C, Wang W, Henry L, Cook R, Nelson D. Chronic hepatitis C increases the risk of chronic kidney disease while effective treatment decreases the incidence of CKD. Hepatology 2017 Sep 5. doi: 10.1002/hep.29505 [Epub ahead of print]. |
| 225. | Petta S. Hepatitis C virus and cardiovascular: A review. J Advanced Res 2017; 8: 161-80. |
| 226. | Lin M, Chen T, Lin W, Liu C, Hsieh Y, Chiu W, Chang C, et al. Add-on neurological benefits of antiviral therapy in HCV patients with chronic kidney disease- a nationwide cohort study. BMC Gastroenterology 2017; 17: 99. |
| 227. | Obata F, Murakami T, Miyagi J, Ueda S, Inagaki T, Minato M, Ono H, et al. A case of rapid amelioration of hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis treated by interferon-free directly acting antivirals for HCV in the absence of immunosuppressant. CEN Case Rep 2017; 6: 55-60. |
| 228. | Martin P, Fabrizi F. Benefit of direct-acting antiviral therapy for cyroglobulinemia due to hepatitis C infection. Am J Gastroenterol 2017; 112: 1309-10. |
| 229. | Fabrizi F, Donato F, Messa P. Hepatitis C and its metabolic consequences in kidney disease. Ann Hepatol 2017; 16: 851-61. |
| 230. | Rossi C, Raboud J, Walmsley S, Cooper C, Antoniou T, Burchell A, Hull M, et al. Hepatitis C co-infection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect Dis 2017; 17: 246. |
The papers that appear in bold are those that were included in the meta-analysis.
Summary measure for adjusted effect estimate (outcome: frequency of proteinuria or glomerular disease) according to antiHCV serologic status among various groups of interest.
| N | Adjusted effect estimate (random-effects model) | Q value (by χ2 test) | I2 | |
|---|---|---|---|---|
| Outcome: proteinuria (frequency) | ||||
| All studies | 10 | 1.63 (1.29; 2.05) | 37.4 (P = 0.000) | 75.9% |
| US studies | 4 | 1.95 (1.58; 2.39) | 4.3 (P = 0.22) | 30.6% |
| Asian studies | 5 | 1.33 (1.09; 1.62) | 6.1 (P = 0.18) | 34.88% |
| Studies based on dipstick analysis | 2 | 1.33 (0.93; 1.90) | 5.35 (P = 0.02) | 81% |
| Studies based on spot urine albumin/creatinine ratio | 7 | 1.66 (1.36; 2.01) | 3.1 (P = 0.7) | 0.0 |
| Cross-sectional studies Outcome: proteinuria (prevalence) aOR | 8 | 1.41 (1.18; 1.67) | 10.3 (P = 0.1) | 32.2% |
| Cross-sectional and nested case-control studies Outcome: proteimuria (prevalence) aOR | 9 | 1.51 (1.25; 1.82) | 15.5 (P = 0.049) | 48.7% |
• Liangpunsakul, et al.:45 OR adjusted for age, race, hypertension, gender, body mass index.
• Tsui, et al.:33 OR adjusted for age, gender, race/ethnicity, educational status, smoking status, diabetes, hypertension.
• Huang, et al.:46 OR adjusted for diabetes, hypertension, BMI, age, triglycerides, gender, ALT, total cholesterol, triglycerides, HBsAg status.
• Ishizaka, et al.:35 OR adjusted for age, gender, systolic blood pressure, fasting plasma glucose, ALT, HBsAg status.
• Lee, et al.:36 OR adjusted for age, gender, educational status, BMI, hemoglobin level, albumin level, cholesterol level, uric acid level, hypertension, diabetes mellitus.
• Derbala, et al.:37 OR adjusted for diabetes, age, gender, cryoglobulinemia, creatinine.
• Aoufi Rabih, et al.:38 OR adjusted age, gender, hypertension, diabetes, obesity, rheumatic disease.
• Zeng, et al.:41 OR adjusted for age, gender, BMI, albumin, hypertension, diabetes, total cholesterol, triglycerides, low-density lipoprotein cholesterol.
• Kurbanova, et al.:42 OR adjusted for age, gender, race, hypertension, diabetes, BMI.
• Park, et al.:32 HR adjusted for age, gender, calendar year, comorbidities (hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, heart failure, peripheral vascular disease, cerebrovascular disease, coronary artery disease, HIV, HAV, HBV, cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, alcohol abuse, drug abuse).
As shown in tables 1 and 2, our stratified analysis showed some substantial differences in pooled aHR across various subgroups. There was a significant association between anti-HCV positive serologic status and prevalence of chronic kidney disease among studies coming from Asia, 1.2 (1.01; 1.42) (P < 0.01) (Table 1); heterogeneity persisted, Q value (by χ2 test) of 32.4 (P = 0.0001). Tables 1 and 2 report that the homogeneity assumption was rejected in numerous subsets.
Tables 3 and 4 report the impact of continuous variables on the aHR (incidence of CKD) and proteinuria frequency among anti-HCV positive patients (meta-regression analysis). As reported in table 3, meta-regression demonstrated a positive impact of ageing (P < 0.0001) and duration of follow-up (P < 0.0001) on the adjusted HR of incidence of CKD among HCV-positive patients.
Meta-regression: Impact of continuous variables on aHR (n = 15 studies, n = 2,299,134 unique patients) (incidence of CKD).
| Regression coefficient | Standard error | 95% CI | Z value | P value | |
|---|---|---|---|---|---|
| Reference year | -0.030 | 0.033 | -0.09; 0.03 | -0.90 | 0.36 |
| Size | 0.00 | 0.000 | -0.00; +0.00 | 1.50 | 0.13 |
| Age | 0.034 | 0.003 | 0.026; 0.04 | 8.70 | 0.000 |
| Follow-up | 0.041 | 0.006 | 0.031; 0.055 | 6.96 | 0.000 |
| Diabetics | 0.003 | 0.003 | -0.004; 0.010 | 0.803 | 0.421 |
| Males | 0.002 | 0.004 | -0.007; 0.01 | 0.48 | 0.62 |
| HIV | -0.04 | 0.04 | -0.131; 0.039 | -1.06 | 0.286 |
| HBsAg | 0.034 | 0.017 | 0.00; 0.06 | 2.01 | 0.06 |
| HCV rate | -0.004 | 0.007 | -0.019; 0.009 | -0.66 | 0.50 |
| Hypertension | 0.00 | 0.05 | -0.01; 0.01 | 0.066 | 0.99 |
Meta-regression: Impact of continuous variables on adjusted effect estimate (n = 10 studies, n = 315,404 unique patients) (frequency of proteinuria).
| Regression coefficient | Standard error | 95% CI | Z value | P value | |
|---|---|---|---|---|---|
| Reference year | 0.02 | 0.02 | -0.017; 0.06 | 1.10 | 0.26 |
| Size | 0.000 | 0.000 | -0.000; 0.000 | 1.43 | 0.151 |
| Age | -0.034 | 0.010 | -0.054; -0.013 | -3.25 | 0.001 |
| Diabetics | -0.001 | 0.008 | -0.018; 0.016 | -0.117 | 0.90 |
| Males | 0.008 | 0.005 | -0.002; 0.019 | 1.5 | 0.132 |
| HBsAg | -0.03 | 0.01 | -0.067; -0.005 | -2.27 | 0.022 |
| HCV | 0.0006 | 0.003 | -0.006; 0.008 | 0.18 | 0.85 |
| Hypertension | 0.067 | 0.014 | 0.038; 0.09 | 4.50 | 0.001 |
There is growing evidence in the medical literature suggesting that HCV infection is not a liver-focused disease but a systemic illness giving several extra-hepatic (including renal) manifestations. This meta-analysis (n = 40 studies; 4,072,867 patients) includes a number of reports almost double compared to the previous one and confirms the higher risk of chronic kidney disease among HCV-in-fected patients, aHR, 1.43 (95% CI, 1.23; 1.63) (P = 0.0001). This result has been observed in longitudinal studies (n = 15) provided with large size and appropriate follow-ups. Also, a higher rate of proteinuria among HCV-infected patients was recorded, adjusted effect estimate of proteinuria with HCV among surveys was 1.633 (95% CI, 1,29; 2.05) (P < 0.001) (n = 10 studies; 315,404 unique patients). The findings reported here are in keeping with other pieces of evidence; cohort studies carried out among individuals with biopsy-proven glomerular disease48 or diabetic nephropathy49 and patients with HCV-HIV co-in-fection50 suggested a consistent link between anti-HCV positive serologic status and incidence or progression of chronic kidney disease. Antiviral therapy towards HCV has been able to slow down the progression of chronic kidney disease in HCV-infected populations. In a retrospective observational cohort of patients with stage 3 CKD, regression models reported that sustained viral response was associated with a 9.3 (95% CI, 0.44 to 18) mL/ min per 1.73 m2 increase in eGFR during the 6-month post-treatment follow-up period.51 IFN-based therapies improved renal survival among diabetics,52 liver transplant recipients,53 and HCV-HIV infected patients.54
The relationship between anti-HCV positive status and prevalence of chronic kidney disease was not significant in many comparisons. The lack of an appropriate follow-up could explain the discrepancy between longitudinal and cross-sectional studies. According to our meta-regression analysis, the impact of HCV on the incidence of CKD was more prominent in those longitudinal studies provided with longer follow-ups and aged populations. The stratified analysis showed a consistent association between anti-HCV positive serologic status and prevalence of chronic kidney disease in the subset of Asian studies (1.2, 95% CI, 1.01; 1.42) (P < 0.001). This conferred robustness to the current meta-analysis even if significant between-study heterogeneity persisted in several comparisons. According to our meta-regression, the impact of HCV on the incidence of CKD was more evident in those longitudinal studies provided with longer follow-ups and aged populations.
The findings from the current meta-analysis present several limitations. First, many reports show retrospective and cohort design- from a theoretical point of view, a randomized controlled trial with placebo gives the best evidence on the efficacy of an intervention. However, a large sample and a long follow-up are needed to perform a RCT in this setting as the frequency of events is low; also, the current availability of safe and effective drugs (DAAs) for the treatment of HCV makes the randomisation to placebo not ethically acceptable. Secondly, multivariate analysis was performed in all the studies retrieved in the current meta-analysis but residual confounding (confounding remaining after adjustment) cannot be excluded as full information was not given on various confounders. As an example, data on life style, illicit drug abuse, and family history were often missed. Thirdly, our stratified analysis and meta-regression was not able to capture the sources of the great heterogeneity we have observed. The high heterogeneity suggests that all the studies included in the analysis are not functionally identical and this precluded the adoption of a fixed-effects model. Fourth, the relationship between HCV infection, as detected by positive HCV RNA in serum, and chronic kidney disease was addressed in a few surveys. Moreover, individual data from each study (‘meta-analysis at patient level’) were not available; thus, it was impossible to perform our own adjustments even if the studies included in this meta-analysis adjusted for numerous factors (‘covariates’) that could prove to be potential confounders. Finally, as with all meta-analyses, this study has the potential limitation of publication bias as negative or non-significant studies are less likely to be published (“file-drawer effect”).55 One approach to address this topic is to gather data from as many sources as possible. On the other hand, we have not included trials published as abstracts; information presented in abstract format is often without high quality and can give greater treatment effect.
We need more studies based on nucleic acid tests (instead of serological assays) to evaluate the link between HCV infection and occurrence of CKD. CKD appears more frequently in viraemic patients than HCV negative individuals. As reported above, this information was not available in most studies; however, the absence of statistical heterogeneity confers reliability to our results.
The results of the current meta-analyses support the notion that the kidneys appear an important target of the extra-hepatic activity of HCV, and various mechanisms of renal disease among patients with chronic HCV have been described. In the context of membrano-proliferative glomerular disease, HCV gives glomerular damage through activation and deposition of cryoglobulins. Also, HCV may cause tubulo-interstitial damage via a direct cytopathic effect or antibody immune complex. Additionally, non-immunological pathways (i.e., oxidative stress, pro-inflammatory cytokines, and others) help the development of renal disease by vascular injury.56 Some investigators addressed the influence of HCV eradication on extra-hepatic outcomes. In a prospective French study, 668 cirrhotic patients (50.5%) underwent antiviral treatment for HCV and achieved SVR; they had a lower risk of cardiovascular events (HR, 0.42; 95% CI, 0.25-0.69; P = 0.001) and bacterial infections (HR, 0.44; 95% CI, 0.29-0.68; P < 0.001).57
This meta-analysis of observational studies shows a link between anti-HCV positive serologic status and greater frequency of low eGFR and/or abnormal proteinuria in the adult general population. We need more studies in order to identify the pathophysiological mechanisms underlying such association and to deepen the sources of the heterogeneity identified. In the meantime, early initiation of antiviral therapy for HCV is encouraged to improve kidney survival regardless staging of liver disease.
Abbreviations- •
ACEi: angiotensin-converting enzyme inhibitor.
- •
AH: arterial hypertension.
- •
aHR: adjusted hazard ratio.
- •
ALT: alanine aminotransferase.
- •
aOR: adjusted odds ratio.
- •
ARB: angiotensin II receptor blocker.
- •
aRR: adjusted relative risk.
- •
AST: aspartate aminotransferase.
- •
CI: confidence intervals.
- •
CKD: chronic kidney disease.
- •
CNI: calcineurin inhibitors.
- •
CRF: chronic renal failure.
- •
CV: cardiovascular.
- •
DAAs: direct-acting antiviral agents.
- •
DM: diabetes mellitus.
- •
eGFR: estimated glomerular filtration rate.
- •
EOT: end of treatment.
- •
ESRD: end-stage renal disease.
- •
GN: glomerulonephritis.
- •
HCV: hepatitis C virus.
- •
HD: haemodialysis.
- •
HIV: human immunodeficiency virus.
- •
ICD-9-CM: International Classification of Diseases: Ninth Revision: Clinical Modification.
- •
IFN: interferon.
- •
ITT: intention-to-treat analysis.
- •
MC: mixed cryoglobulinemia.
- •
MDRD: modification of diet in renal disease.
- •
NA: not available.
- •
NOS: Newcastle/Ottawa Scale.
- •
NSAID: non-steroidal anti-inflammatory drug.
- •
PRISMA: Preferred Reporting Items for Systemic Reviews and Meta-Analyses statement.
- •
RBV: ribavirin.
- •
SVR: sustained virological response.
No sources of funding were used for the preparation of this manuscript.
Conflict of Interest StatementFabrizio Fabrizi: consultant or advisor to AbbVie, Merck & Co; Maria Francesca Donato: speaker bureau Abbvie, Gilead, MSD.