Introduction. In patients with chronic hepatitis C it is still debated whether previous exposure to the hepatitis B virus, diagnosed from the presence of the anti-HBc antibody, is linked to a greater risk of severe hepatitis. The aim of the study was to evaluate whether the presence of anti-HBc antibodies is associated with cirrhosis in patients with HBsAg-negative chronic hepatitis C.
Material and methods. Two hundred twenty-two consecutive HBsAg-negative patients with HCV-related chronic hepatitis were enrolled at their first liver biopsy. Ishak’s scoring system was used to grade necroinflammation and fibrosis and the patients with stage 5 or 6 were considered as having histological cirrhosis.
Results. Patients with histological cirrhosis had a higher mean age, AST, ALT, a lower platelet count and prothrombin activity compared to those with milder fibrosis. The presence of anti-HBc was identified in 21 (63.6%) of the 33 patients with fibrosis score 5 or 6 and in 56 (29.6%; p < 0.001) of the 189 with score ≤ 4. Patients with cirrhosis had a significantly higher grading than those without cirrhosis (median = 8, IQR 6-11 vs. Median = 6, IQR = 4-8, respectively, p < 0.001). A multivariate logistic regression analysis showed that age, sex and anti-HBc positivity were independent predictors of histological cirrhosis.
Conclusion. Our data support the idea that in patients with chronic hepatitis C the presence in serum of anti-HBc is associated with histological cirrhosis and is therefore a marker of clinical value.
The hepatitis C virus (HCV) infects over 170 million people worldwide and is a leading cause of cirrhosis and hepatocellular carcinoma (HCC).1 After the acute infection about 60-80% of patients develop chronic hepatitis.2 Roughly one quarter of these patients will develop liver cirrhosis within 20-30 years.3 The rate of progression of chronic hepatitis C (CHC) is, however, highly variable among patients and over time: some individuals show a benign clinical course for decades and others rapidly progress to end-stage liver disease.3 Several factors have been linked to the severity of CHC, including virus-related factors (genotype, viral load, duration of infection), co-morbidities (human immunodeficiency virus coinfection, hepatitis B virus coinfection, insulin-resistance, high body mass index, immunosuppression), and iron overload.4–8 Hostrelated factors, such as age at the onset of infection and gender, and lifestyle factors (alcohol intake) have also been implicated in the severity of CHC.5 It has been demonstrated that eradication of infection with antiviral therapy is associated with a reduced progression to cirrhosis and even with its regression.9,10
The development of sensitive assays to detect small amounts of HBV DNA has favored the identification of occult hepatitis B infection, which is characterized by undetectable HBsAg in serum and HBV DNA detectable in the liver tissue.11 In patients with chronic hepatitis C, occult HBV infection was found to be associated with more severe liver damage,12,13 a higher risk of developing HCC14–19 and HBV reactivation in immunosuppressed patients,20,21 such as onco-hematological patients receiving chemotherapy.22 This virological condition has been more frequently found in patients with serological markers of previous HBV infection (anti-HBc, anti-HBs) than in those without.23 However, it is still debated whether the presence of anti-HBc in patients with HCV-related hepatitis is linked to a greater risk of severe hepatitis and HCC.24
ObjectivesThe aim of our study was to evaluate whether the presence of anti-HBc antibodies and, therefore, previous contact with the hepatitis B virus, was associated with cirrhosis in a group of patients with biopsy-proven HCV-related chronic hepatitis.
Material and MethodsPatients#We reviewed the charts of all patients with chronic hepatitis C admitted for a liver biopsy to the Department of Mental Health and Public Medicine, Section of Infectious Diseases (Second University of Naples), and to the Department of Public Medicine and Social Security-Section of Infectious Diseases (University of Naples “Federico II”, Italy) from 01.01.2000 to 30.06.2012. These two centers have cooperated in several clinical investigations using the same clinical approach and the same laboratory methods.25
Inclusion criteria:
- •
Positivity for anti-HCV.
- •
HCV RNA in serum and the availability of liver biopsy data.
Exclusion criteria:
- •
HBsAg and/or anti-HIV positivity.
- •
Incomplete liver panel (including test for the anti-HBc antibody).
- •
Hepatocellular carcinoma, ascites, liver transplantation, insufficient liver tissue for staging.
Two hundred and twenty-two consecutive Italian patients with anti-HCV/HCV RNA-positive chronic hepatitis for 18-36 months were included in the study. Patients underwent complete physical examination, full liver function tests, blood cell counts, viral marker tests (HBV, HCV, hepatitis delta virus-HDV, human immunodeficiency virus-HIV) and liver ultrasound scan.
The laboratory tests were performed within 1 month of the date of the liver biopsy. Alcohol consumption was defined as drinking more than 30 g per day of alcohol for at least 6 months. This was corroborated by the patient’s family.
Liver biopsy was performed for all patients with an 18-gauge needle under ultrasound guidance. Liver specimens were fixed in formalin, embedded in paraffin and stained with hematoxylin-eosin and Masson’s trichrome stain. In each case, the liver specimens were more than 2 cm in length and had more than 11 portal tracts. Liver biopsies were examined by a pathologist (G.P.) who, unaware of the clinical and laboratory data, used the Ishak scoring system to grade necroinflammation and fibrosis.26 Patients with fibrosis score 5 or 6 were considered as having histological cirrhosis.
All procedures used were in accordance with the international guidelines and with the Helsinki Declaration of 1975 and revised in 1983.
Routine analysisHBV and HDV serum markers were sought using commercial immunoenzymatic assays (Abbott Laboratories, North Chicago, IL, USA, for HBsAg, anti-HBs and anti-HBc, and DiaSorin, Saluggia, VC, Italy, for anti-HDV). The anti-HCV antibody was sought using a 3rd generation commercial immunoenzymatic assay (Ortho Diagnostic Systems, Neckargemund, Germany). Antibodies to HIV 1 and 2 were sought using a commercial ELISA (Abbott Lab., North Chicago, Ill, USA). Liver function tests were performed by the routine methods.
HCV genotype and viral loadThe HCV genotype was determined using the HCV genotype assay Lipa (Bayer, France), following the manufacturer’s instructions. Viral RNA was extracted from 140-µL samples of plasma using a microspin column (QIAamp RNA viral kit, Qiagen GmbH, Hilden, Germany). HCV RNA was quantified by performing a real-time polymerase chain reaction (PCR) in a Light cycler 1.5 (Roche Diagnostics, Branchburg, NJ, USA), as previously described;27 by this method, the detection limit in plasma samples is estimated at around 40 IU/mL.
Statistical analysisThe Kolmogorov-Smirnov test was used to assess the distribution of the quantitative variables. These variables are reported as mean ± standard deviation (SD), while in the case of a non-Gaussian distribution they are reported as median and interquartile range (IQR). For comparisons, for the Gaussian distribution, the Student t-test for unpaired variables was applied, while the Mann-Whitney U test was used for a non-Gaussian distribution or for quantitative ordinal variables. The χ2 test with the Yates correction (or Fisher’s exact test where appropriate) was used for qualitative variables. The correlation between quantitative ordinal variables was assessed with the Spearman ñ test. P < 0.05 in a two-sided test was considered statistically significant. Any independent variable (or with P ≤ 0.2 at univariate analysis) with a biological plausibility to the aim of the study was included in the binary logistic regression analysis using the forward conditional stepwise method. The cut-off values used for the stepwise method were: P = 0.05 for entry to the model, and P = 0.10 for removal. All statistical analyses were carried out using the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc. Chicago, 1ll).
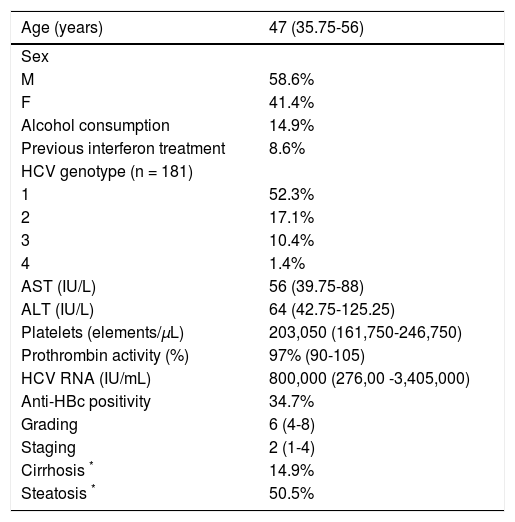
ResultsThe demographic, biochemical, serological, virological and histological data obtained at the time of the diagnostic liver biopsy are shown in table 1. The patients were relatively young (median and range: 47 years, 35.75-56) and prevalently (58.6%) males. Thirty-three (14.9%) admitted alcohol consumption and 19 (8.6%) had been previously treated with interferon (Table 1). HCV genotype 1 prevailed (52.3% of cases), the median HCV RNA in serum was 800,000 IU/mL (range 276,000-3,405,000) and 77 patients (34.7%) showed anti-HBc in serum (Table 1). The median grading of necroinflammation was 6 (IQR 4-8) and fibrosis staging 2 (IQR 1-4) according to the Ishak scoring system; 33 patients had fibrosis score 5 or 6, and therefore histological cirrhosis (Table 1).
Demographic and clinical characteristics of the patients (n = 222).
| Age (years) | 47 (35.75-56) |
|---|---|
| Sex | |
| M | 58.6% |
| F | 41.4% |
| Alcohol consumption | 14.9% |
| Previous interferon treatment | 8.6% |
| HCV genotype (n = 181) | |
| 1 | 52.3% |
| 2 | 17.1% |
| 3 | 10.4% |
| 4 | 1.4% |
| AST (IU/L) | 56 (39.75-88) |
| ALT (IU/L) | 64 (42.75-125.25) |
| Platelets (elements/µL) | 203,050 (161,750-246,750) |
| Prothrombin activity (%) | 97% (90-105) |
| HCV RNA (IU/mL) | 800,000 (276,00 -3,405,000) |
| Anti-HBc positivity | 34.7% |
| Grading | 6 (4-8) |
| Staging | 2 (1-4) |
| Cirrhosis * | 14.9% |
| Steatosis * | 50.5% |
The data are given as median (interquartile range) for quantitative variables and as a percentage for qualitative variables.
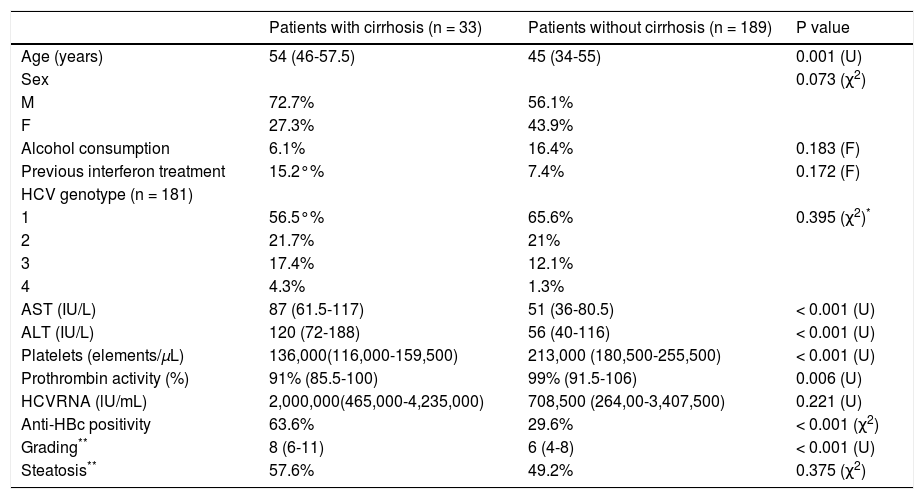
Table 2 shows the demographic, biochemical, virological and histological characteristics associated with histological cirrhosis. Compared to the patients without histological cirrhosis, those with fibrosis score 5 or 6 were more frequently older (median age [IQR]: 54 [46-57.5] vs. 45 [34-55] years, p < 0.001), had a higher mean serum value of AST (87 [61.5-117] vs. 51 [36-80.5] IU/mL, p < 0.001), ALT (120 [72-188] vs. 56 [40-116] IU/mL, p < 0.001), a lower platelet count (136,000 [116,000-159,600] vs. 213,000 [180,500-255,500] cells/mL, p < 0.001) and prothrombin activity (91% [85.5-100] vs. 99 [91.5-106], p < 0.001) (Table 2). The presence of anti-HBc was associated with histological cirrhosis, as it was identified in 21 (63.6%) of the 33 patients with a fibrosis score 5 or 6 and in 56 (29.6%; p < 0.001) of the 189 with a score ≤ 4 (Table 2). A significant correlation was found between the grading and staging scores (ρ = 0.498; p < 0.001). In addition, patients with cirrhosis had a significantly higher grading than those without cirrhosis (median = 8, IQR 6-11 in patients with cirrhosis vs. median = 6, IQR = 4-8 in patients without cirrhosis, p < 0.001). However, the patients with anti-HBc antibodies had a similar grading to those without (median = 4, IQR 4-8 vs. median = 7, IQR = 4-8, respectively, p < 0.138).
Demographic and clinical characteristics of the patients with and without cirrhosis (n = 222).
| Patients with cirrhosis (n = 33) | Patients without cirrhosis (n = 189) | P value | |
|---|---|---|---|
| Age (years) | 54 (46-57.5) | 45 (34-55) | 0.001 (U) |
| Sex | 0.073 (χ2) | ||
| M | 72.7% | 56.1% | |
| F | 27.3% | 43.9% | |
| Alcohol consumption | 6.1% | 16.4% | 0.183 (F) |
| Previous interferon treatment | 15.2°% | 7.4% | 0.172 (F) |
| HCV genotype (n = 181) | |||
| 1 | 56.5°% | 65.6% | 0.395 (χ2)* |
| 2 | 21.7% | 21% | |
| 3 | 17.4% | 12.1% | |
| 4 | 4.3% | 1.3% | |
| AST (IU/L) | 87 (61.5-117) | 51 (36-80.5) | < 0.001 (U) |
| ALT (IU/L) | 120 (72-188) | 56 (40-116) | < 0.001 (U) |
| Platelets (elements/µL) | 136,000(116,000-159,500) | 213,000 (180,500-255,500) | < 0.001 (U) |
| Prothrombin activity (%) | 91% (85.5-100) | 99% (91.5-106) | 0.006 (U) |
| HCVRNA (lU/mL) | 2,000,000(465,000-4,235,000) | 708,500 (264,00-3,407,500) | 0.221 (U) |
| Anti-HBc positivity | 63.6% | 29.6% | < 0.001 (χ2) |
| Grading** | 8 (6-11) | 6 (4-8) | < 0.001 (U) |
| Steatosis** | 57.6% | 49.2% | 0.375 (χ2) |
The data are given as median (interquartile range) for quantitative variables and as a percentage for qualitative variables. U: Mann-Whitney U test. χ2: chisquared test. F: Fisher’s exact test.
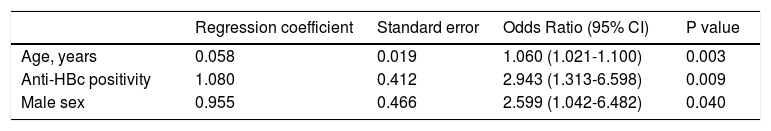
To confirm the association between positivity for anti-HBc and histological cirrhosis, a multivariate logistic regression analysis was done. The analysis included the presence of anti-HBc and other potential confounding factors (age and sex). After all the other factors were taken into account, anti-HBc positivity, age and sex were independent predictors of histological cirrhosis (Table 3).
Factors independently associated with cirrhosis at logistic regression analysis (n = 222).
| Regression coefficient | Standard error | Odds Ratio (95% CI) | P value | |
|---|---|---|---|---|
| Age, years | 0.058 | 0.019 | 1.060 (1.021-1.100) | 0.003 |
| Anti-HBc positivity | 1.080 | 0.412 | 2.943 (1.313-6.598) | 0.009 |
| Male sex | 0.955 | 0.466 | 2.599 (1.042-6.482) | 0.040 |
CI: confidence interval.
Analyzing only the 77 patients who were anti-HBc positive, cirrhosis was detected in 21 (27.3%) cases. Among these 77 subjects, patients with cirrhosis had significantly higher levels of AST (79 IU/L, IQR: 62-117 vs. 48 IU/L, IQR: 36-80, P = 0.009), ALT (120 IU/L, IQR: 62-188 vs. 52 IU/L, IQR: 44-104.5, P = 0.003) and grading (9, IQR: 6-11 vs. 6, IQR: 4-8, P = 0.0031), and a lower platelet count (140,000/µL, IQR: 116,500-167,500 vs. 226,000/µL, IQR: 190,500-256,750, P ≤ 0.001) compared to those without cirrhosis. All other variables in this subgroup of patients did not differ significantly in presence or absence of cirrhosis. Particularly age was non-significantly higher in patients with cirrhosis than in those without cirrhosis (52 years, IQR:46-58.5 vs. 50.5 years, IQR 42.25-56, p = 0.227).
DiscussionThe data from the present study indicated that in the patients with chronic hepatitis C, positivity for anti-HBc was independently associated with severe fibrosis. In fact, of the 33 patients with histological cirrhosis, 63.6% were anti-HBc-positive, a higher percentage than that (29.6%) observed in the 189 patients without cirrhosis. Other factors associated with severe fibrosis were an older age and male sex, as already reported in other studies.5,25,28 Moreover, a significant correlation was found between the grading and staging scores, even though the grading was similar in the patients with anti-HBc antibodies and in those without. Instead, the steatosis score was not associated with histological cirrhosis.
The presence of anti-HBc in the serum without serological HBsAg is indicative of a resolved HBV infection. However, it may be considered a surrogate marker of occult HBV infection, as suggested by several authors.6,12,13,30 In a previous study on 89 HBsAgnegative/anti-HCV-positive patients, the occult HBV infection was detected by a highly sensitive PCR in plasma or peripheral blood mononuclear cells or liver tissue in about 60% and 80% of the anti-HBs-positive/anti-HBc-positive and anti-HBs-negative/anti-HBc-positive patients, respectively, and in only about 10% of those who were negative for both anti-HBs and anti-HBc.31 However, the absence of demonstration of HBV in liver tissue in this study was a limitation. An indirect demonstration of the role of anti-HBc as a surrogate marker of occult HBV infection was that the reactivation of HBV infection in HBsAg-negative patients with immunosuppression was observed exclusively in the anti-HBc-positive.21,22,32
Our data comply with the data of some previous studies12,13,33–35 in which occult HBV infection was described as associated with the more severe stages of the liver disease. For example, in a previous study of ours, the 88 anti-HBc-positive patients with HCV-related chronic infection showed a higher percentage of severe liver disease than the 97 anti-HBc-negative patients (72.7 vs. 46.4%, respectively, P < 0.0005).13 The mechanism leading to more severe fibrosis in anti-HBc-positive patients with CHC has not been elucidated completely. However, the data in the literature allow us to hypothesize that the hepatocytes with a low-level occult HBV replication may be a suitable target for cytotoxic T cells previously sensitized to viral antigens.36 As regards the entity of the immune reaction, it should be remembered that in chronic hepatitis B the cytotoxic T cell-mediated attack is frequently strong in cases with a low to moderate exposure of the viral antigens on the membrane of the hepatocytes and weak in cases with a high expression. The persistence of low-level HBV replication in the hepatocytes and the consequent immunoreactions may bring about increased severity of the liver disease and more marked fibrosis. However, it is not possible to rule out that the anti-HCV-positive/HBsAg-negative, but anti-HBc-positive patients may be at the last step in a hypothetical sequence of events leading to a progressive suppression of HBV replication during HBV-HCV chronic coinfection, as suggested by Tanaka, et al.37 In fact, HCV or HBV chronic carriers who became super-infected with HBV or HCV, respectively, experienced a strong inhibition of the pre-existing viral genome, but a severe liver disease.38–42 Thus, an accumulative effect of HBV and HCV infection was hypothesized for these patients. This hypothesis seems to be confirmed by the data from this study showing a similar grading in anti-HBc-positive and -negative patients; thus, we might hypothesize that histological cirrhosis was more frequent in our anti-HBc-positive patients because of an accumulative effect of HBV and HCV infection, as in chronic dual coinfection.6,34,43
ConclusionOur data indicating that the anti-HBc-positive patients had a high prevalence of severe liver disease further support the idea that anti-HBc positivity is a marker of clinical value in patients with chronic hepatitis C.
Abbreviations- •
CHC: chronic hepatitis C.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HDV: hepatitis D virus.
- •
HIV: human immunodeficiency virus.