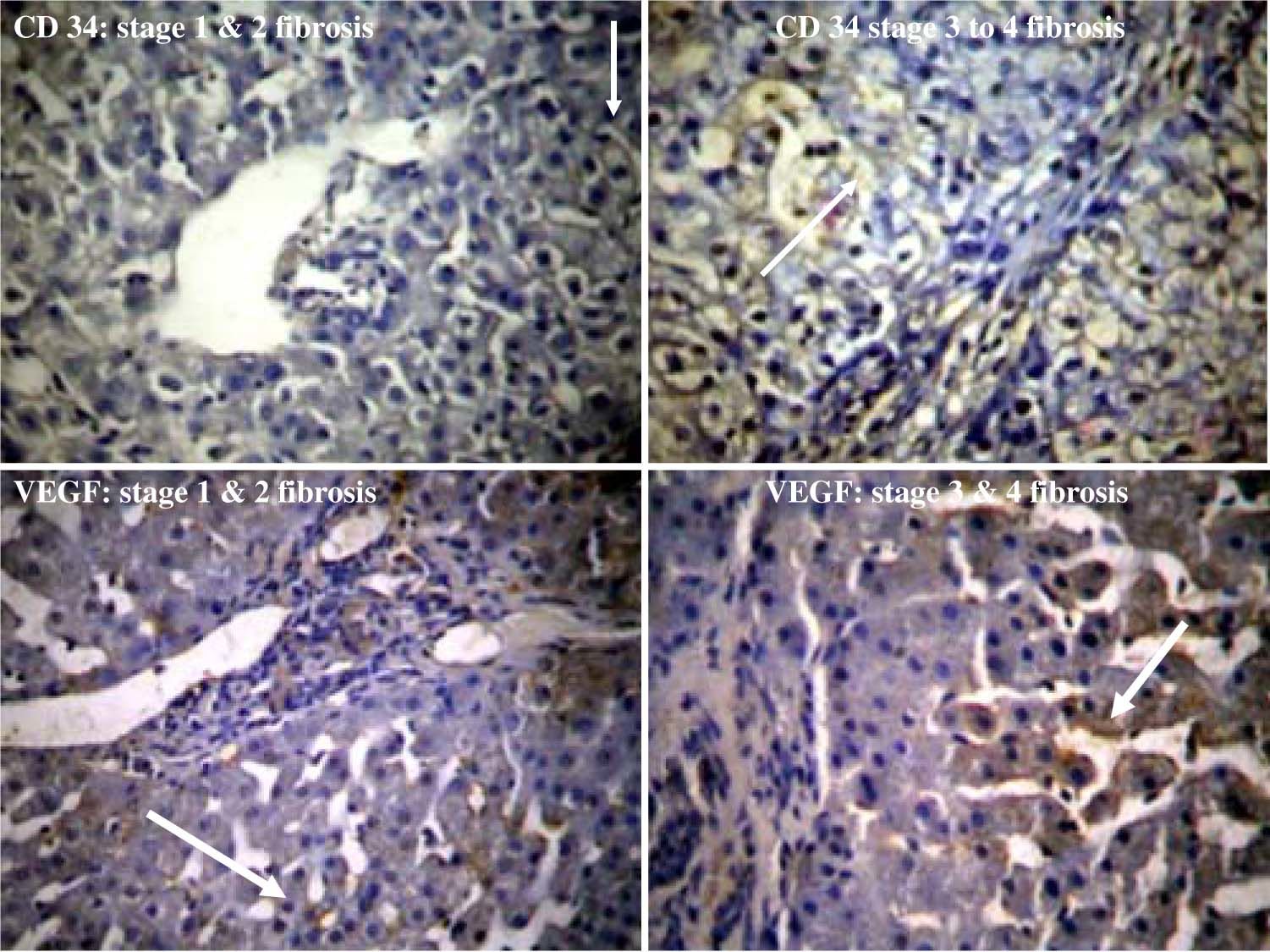
Background: Chronic liver disease is characterized by inflammation and fibrosis. As a consequence angiogenesis leading to new vasculature may have prognostic value in disease progression. Interfering with angiogenesis may be a potential target to avoid progression of liver disease. Hence we planned to evaluate the CD34 and vascular endothelial growth factor (VEGF), the markers for angiogenesis in chronic liver disease.Method: Liver biopsies from 79 patients of chronic liver disease and 21 cases of HCC (M: F = 4:1, age range 22 to 80) were stained for routine HE, CD 34 and VEGF immunostaining (Dako Corp & Santa Cruz respectively). Etiologies of chronic liver disease were alcoholic liver disease, HBV, HCV infection, NAFLD, autoimmune liver disease, and cryptogenic liver disease. Thirty biopsies from normal liver obtained at autopsy were taken as controls. Expressions of CD 34 and VEGF were compared with the stage of fibrosis. Results: Out of 79 patients, angiogenesis was seen in 45.5% cases of chronic liver disease. None of the case with normal liver histology was CD 34 or VEGF positive. No significant correlation of angiogenesis was found between any etiologies of chronic liver disease. CD 34 was positive in 18/21 (85.7%) cases of hepatocellular carcinoma. CD 34 and VEGF positivity was 20.9% and 46.5% in stage 1 and 2 fibrosis while it was 75% and 80% in stage 3 and 4 fibrosis respectively. VEGF appeared more common as compared to CD 34 in early fibrosis. Conclusion: Angiogenesis was present in 45.5% cases of chronic liver disease. It was proportional to the increase in stage of fibrosis. Expression of VEGF was commonly found in early stages of fibrosis. Hence, therapeutic strategies of inhibiting VEGF expression may be of importance in preventing the progression of chronic liver disease in its early stage.
Angiogenesis is an integral part of the tumor progression. It also plays a major role in chronic inflammation. Accumulation of inflammatory infiltrate and development of fibrosis increases resistance of the tissue to blood flow and delivery of oxygen, resulting into hypoxia. Under these circumstances, angiogenesis switch occurs leading to up regulation of proangiogenic factors which are responsible for vascular remodelling and neovessel formation.1-5 VEGF is an important proangiogenic factor. It acts as a link between angiogenesis, immune system and tissue remodelling. Expression of VEGF is enhanced in angiogenesis.6 CD 34 which is a marker for endothelial cells is not expressed by normal endothelial cells however when endothelial cells alter their phenotype, they are able to express CD 34.7
Angiogenesis in liver is characterized by capillarization of the sinusoids. The process of angiogenesis has been well documented in hepatocellular carcinoma (HCC). Liver disease such as chronic hepatitis B (HBV) or C infection (HCV), non alcoholic steatohepatitis (NASH) or autoimmune hepatitis are known to progress to cirrhosis. Later they may develop hepatocellular carcinoma. These chronic liver diseases respond poorly to conventional immunosuppressive or anti inflammatory therapy. Understanding the process of angiogenesis might suggest an effective therapeutic target to reverse the inflammation and prevent progress of chronic liver disease.8 Hence the aim of this study was to evaluate expression of CD 34 and vascular endothelial growth factor (VEGF) in chronic liver disease and compare it with the stage of fibrosis.
Material and methodsTotal 79 patients of chronic liver disease and 21 cases of HCC undergoing liver biopsy were included in this study over the period of 2 years. Thirty biopsies from normal liver obtained at autopsy with no evidence of liver disease were taken as controls. Institutional research committee approval was taken before carrying out this study. History and clinical examination findings were noted from hospital recorded. Laboratory tests such as liver function tests, HBsAg, anti HCV, autoantibodies and workup for Wilson’s disease was done for all the patients. Depending on the history and laboratory findings, patients were categorized into different etiologies for chronic liver disease. Etiology of chronic liver disease was as follows; 21 patients of alcoholic liver disease, 29 HBV infection, 03 HCV infection, 17 NAFLD, 05 autoimmune liver disease and 04 were cryptogenic liver disease. The diagnosis of HCC in 21 cases was done with the help of USG, alpha fetoprotein and confirmed on his-topathology.
Liver biopsy slides of all the patients were stained with routine HE stain and evaluated by experienced hepatic pathologist. Staging of chronic liver disease was done as per Ishak Scoring system.9 Immunohistochemistry was performed in all the cases by using standard avidine biotin peroxidase method for the expression of endothelial cell markers CD 34 and VEGF. Monoclonal antibody CD 34, clone QB End-10 (Dako Corp.) was used at 1: 50 dilution and VEGF (Santa Cruz) at 1: 80 dilution. Positive staining was taken as any cell that stained brown with a dotty, linear, semicircular or circular pattern and was clearly separate from an adjacent one. Larger vessels and vessels with thick muscular walls were not included. The cases of chronic alcoholic liver disease and HCC were stained with only CD34. Semi quantitative assessment of CD 34 and VEGF was done. Minimum 10 high power fields were search for presence of sinusoidal capillarization which was noted as present or absent and then correlated with the stage of fibrosis. Statistical analysis using Chi square test was done to evaluate the significant difference in angiogenesis between stage 1 & 2 and stage 3 & 4 fibrosis.
ResultsPatients of chronic liver disease were between 22 to 60 years (mean age 42 ± 3 SD years) and of HCC upto 80 years of age (mean 65 ± 5 SD years). The clinical presentations were jaundice in 12 (15.1%), accidentally detected HBV or HCV infection in 12 (15.1%), chronic alcoholism with or without complications of cirrhosis 21 (26.5%), and miscellaneous (malaise, loss of appetite, loss of weight, fever, abdominal pain) in 34 (43%) cases. Liver function tests showed mean serum bilirubin (total) 1.7 ± 2.9 SD, mean SGOT 63.8 ± 49.2 SD and mean SGPT 94.4 ± 40 SD. Esophageal varices were present in only 2 patients of non alcoholic chronic liver disease and 8 cases of alcoholic cirrhosis. Out of 79 patients of chronic liver disease, angiogenesis was seen in 45.5% cases. None of the case with normal liver histology was CD 34 or VEGF positive. Angiogenesis found in chronic liver disease (by VEGF / CD 34) was as follows: Angiogenesis was seen in 16/21 (76.1%) cases of alcoholic liver disease, 19/29 (65.5%) cases of HBV infection, 02/ 03 (66.6%) HCV infection, 05/ 17 (29.4%) NASH, 3/5 (60%) autoimmune hepatitis, and 4/4 (100%) cases of cryptogenic cirrhosis. However due to small sample size it was difficult to derive any specific conclusion regarding angiogenesis in different etiologies of chronic liver disease. CD 34 was positive in 18/21 (85.7%) cases of hepatocellular carcinoma. Considering the stage of disease, CD 34 positivity was seen in 9/43 (20.9%) and 27/36 (75%) cases of stage I & II and stage III and IV fibrosis respectively (Figure 1). VEGF was positive in 20/43 (46.5%) cases of stage I and II fibrosis and 12/15 (80%) in stage III and IV fibrosis (Table I). Hence we observed that angiogenesis correlated significantly with the degree of fibrosis (p value < 0.05). VEGF appeared more common as compared to CD 34 in early fibrosis.
DiscussionThe process of angiogenesis in chronic liver disease starts with the development of fibrosis, laying down of extracellular matrix leading to hypoxia which acts as a stimulus for neovascularization.10 While comparing different etiologies of chronic liver disease; we have not found any specific etiology of chronic liver disease showing significantly high positivity for CD 34 and VEGF. This is due to small sample size in each etiology of chronic liver disease.
Amongst viral hepatitis, HCV infection has been documented to show more angiogenesis and has been suggested to represent a risk factor for HCC in patients with chronic HCV infection.11 Salcedo X et al12 have studied markers for angiogenesis in chronic HCV patients and have concluded that serum VEGF, Ang - 2and Tie - 2 levels could be useful as non invasive markers of response to therapy and disease progression in hepatitis C patients. Messerini L et al13 have compared angiogenesis in chronic hepatitis B and C infection. Results of this study have shown that angiogenesis was more frequent in HCV positive patients as compared to HBV infected individuals or controls. As against this some of the studies have failed to demonstrate a significant correlation between microvascular density (MVD) and HCC associated with either HCV/ HBV infection. 14,15 Information regarding angiogenesis in other chronic liver diseases is very limited.16-19 There are certain controversial issues regarding tendency to vasopenia in autoimmune liver disease and reduced peribiliary capillary plexus in PBC, PSC and autoimmune hepatitis. Medina J et al16 has shown marked expression of VEGF in the periportal areas in cases of PBC as compared to control group. The exact molecular mechanism involved in chronic viral hepatitis and angiogenesis is not clearly identified. Local production NO in chronic hepatitis B or C patients has been shown to participate in angiogenesis response by inducing vasodilatation. Possibly activated cytotoxic T cells may up regulate expression of vascular adhesion molecules which enhances angiogenesis.
In our study none of the cases of normal livers were positive for CD 34/VEGF. This is in concordance with most of the studies in the literature suggesting that capillarization and phenotypic changes within hepatic sinusoids occur with inflammation and liver fibrosis.20 Hepatocellular hypoxia and angiogenesis progress together with fibrogenesis after liver injury. An enhanced hepatic vascular proliferation with pathological angiogenesis has been described in association with HCC in comparison with chronic hepatitis and cirrhosis.21-25 We have also found significantly high expression of CD 34 in HCC as compared to chronic liver disease (85.7% vs 45.5%). While comparing the stage of fibrosis, CD 34 as well as VEGF expression was significantly more in stage 3 and 4 as compared to stage 1 and 2 fibrosis. It was also observed that VEGF appeared more common as compared to CD34 in early fibrosis (stage 1 and 2). It is the fact that the VEGF is not only a proangiogenic agent but also acts exclusively on endothelial cells and is known as a survival factor for endothelial cells.6 Yoshiji H et al26 while studying a model of CCL 4 induced hepatic fibrosis have shown that VEGF significantly stimulate proliferation of hepatic stellate cells and sinusoidal endothelial cells which are the prerequisite for liver fibrosis development. Similar to our study, recently Yoshiji H et al27 have also shown that VEGF expression increases significantly during liver fibrogenesis and carcinogenesis. The result of this study has shown stepwise increase mRNA expression of VEGF and CD31 from adjacent non neoplastic liver to adenoma and HCC. Kitade M. et al28 have recently shown angiogenesis in development of liver fibrosis and carcinogenesis in NASH in Leptin deficient rats. Leptin mediated neovascularization was shown to be correlated with VEGF and it increased significantly with progression of liver fibrogenesis and carcinogenesis. Hence understanding the process of angiogenesis is of great help in developing new therapeutic approaches for the chronic liver disease patients.
AcknowledgementWe are very much thankful to Bombay Hospital & Medical research centre, Mumbai for the financial assistance to carry out this study.