Stevia has exhibited antioxidant, antihyperglycemic, antihypertensive and anti-inflammatory properties in several in vivo and in vitro models. The objective of this study was to investigate the ability of an aqueous extract of stevia (AES) to prevent experimental cirrhosis in rats and to explore its mechanism of action.
Materials and methodsLiver cirrhosis was induced by administering carbon tetrachloride (CCl4) (400mg/kg by i.p. injection 3 times a week for 12 weeks); AES was administered (100mg/kg by gavage daily) during the CCl4 treatment. Fibrosis was evaluated with histological, biochemical and molecular approaches, and liver damage was assessed with standardized procedures. The profibrotic pathways were analyzed by western blotting, qRT-PCR and immunohistochemistry.
Results and conclusionsChronic CCl4 administration increased nuclear factor kappa B (NF-κB) and proinflammatory cytokine production as well as oxidative parameters such as lipid peroxidation and 4-hydroxynonenal levels, whereas GSH and nuclear factor-E2-related factor 2 (Nrf2) levels were decreased. CCl4 induced profibrogenic mediator expression, hepatic stellate cell (HSC) activation and, consequently, extracellular matrix production. AES exhibited antioxidant, anti-inflammatory and antifibrotic properties, probably because of its capacity to induce Nrf2 expression, reduce NF-κB expression and block several profibrogenic signaling pathways, subsequently inhibiting HSC activation and preventing fibrosis induced by chronic CCl4 administration.
The past 30 years have seen major progress in the knowledge and management of liver disease, yet tens of millions of people worldwide still suffer from chronic liver conditions. The incidence and prevalence of cirrhosis is key to understanding the burden of liver disease [1]. Unfortunately, an effective pharmacological treatment is not yet available. Notably, chronic intoxication of rats with carbon tetrachloride (CCl4) reproduces many of the histological, biochemical and molecular features of human cirrhosis [2].
Stevia rebaudiana is a small perennial shrub that belongs to the Asteraceae family. It is native to the Amambay region in the northeast region of Paraguay [3] and was used by the Guarani Indians for many years [4]. In 1899, S. rebaudiana Bertoni was botanically characterized by Moisés Santiago Bertoni [3]. The leaves of stevia have traditionally been used dry [5] as a natural sweetener for hundreds of years [6] in tea and medicines or to chew [5]. The principal global producer of stevia is China; Chinese exports accounts for 80% of total production, but the biggest market for stevia is in Japan and Korea [7]. The leaves of stevia are considered the main useful parts of the plant because of their bioactive compounds [8]. The beneficial properties of stevia have important implications for future research; for example, stevioside, one of the main components of stevia, counteracts free radicals and thus might help to stabilize foods and beverages, and the addition of this compound improves the quality of beverages and other mixtures by delaying the degradation of vitamin C [9]. Because stevia exhibits important antioxidant and anti-inflammatory properties, it has been suggested that this plant may be an effective antifibrotic and anticirrhotic remedy [10,11]. Moreover, stevioside has important therapeutic benefits that make stevia a natural option for treating metabolic syndrome [12]. Stevia and stevioside are used for the treatment of many diseases, such as diabetes mellitus, obesity, and hypertension, and for the prevention of caries [13]. In addition, stevia possesses antitumor [13], antiviral [14], antimicrobial [15], antihypertensive [16], and hypoglycemic effects [14]. The protective effects of stevia products have different mechanisms of action [10,11,17]. The antioxidant and anti-inflammatory effects, two of the main characteristics of stevia, suggest that this herbal medicine is a prominent candidate to be tested in various liver disorders. Indeed, we have previously reported that stevia prevents acute and chronic CCl4-induced hepatic toxicity via upregulation of nuclear factor-E2-related factor 2 (Nrf2) and thus counteracts oxidative stress, necrosis and cholestasis by modulating proinflammatory cytokines inhibiting the nuclear factor kappa B (NF-κB) pathway [10]. The objective of this work, therefore, was to investigate the capacity of an aqueous extract of stevia (AES) to prevent CCl4-induced cirrhosis in rats and to investigate the associated mechanisms of action.
2Materials and methods2.1MaterialsS. rebaudiana Bertoni variety Morita II was commercially procured as Mayan Sweet Stevia® (Yucatan, Mexico). This product has a certification from the U.S. Department of Agriculture. Other chemicals were purchased from Sigma–Aldrich (St. Louis, MO), unless otherwise specified.
2.2Infusion preparation of steviaStevia rebaudiana Bertoni variety Morita II leaves were ground in a grinding mill, and the resulting dried stevia leaf powder was passed through a 1mm mesh sieve. After that, 500mg of this powder was dispersed in 10ml of water and incubated at 90°C for 5min to obtain the AES, as previously reported [18]. Daily, 1ml of AES containing 50mg of stevia leaf powder was orally administered to the rats by gavage.
2.3Study designMale Wistar rats (100–120g, initial weight) were randomly divided into four groups of 8 rats each. The control group received 1ml of tap water (AES vehicle) daily PO. The CCl4 group received 400mg/kg CCl4via IP injection 3 times per week, as described previously [6]. The CCl4+AES group received CCl4via the same route as the CCl4 group plus 1ml of AES PO daily. The AES-only group received 1ml of AES PO daily. The animals had free access to food (Labdiet No. 5053, Indiana, USA) and filtered water during the experiment. Body weight gain was assessed once per week. After 12 weeks of treatment, the rats were anesthetized with ketamine and xylazine, and then blood was collected by cardiac puncture and centrifuged in tubes at 3000rpm (12,000×g). The liver was rapidly removed from each rat, weighed, and stored at −75°C. All animals were treated according to Mexican official regulation NOM-062-ZOO-1999 and the technical specifications for the production, care, and handling of laboratory animals, and the protocols were in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 2011). The approval number provided by the Cinvestav Ethics Committee is 0207-16.
2.4Biochemical analysesPlasma was obtained to analyze the activity of the alanine aminotransferase (ALT), alkaline phosphatase (AP) and gamma-glutamyl transpeptidase (γ-GTP) enzymes and the concentration of bilirubin; liver sections were utilized to determine the glycogen, glutathione (GSH), and collagen concentrations and the degree of lipid peroxidation (LPO), as previously described [19].
2.5HistologyLiver sections were prepared for immunohistochemistry, hematoxylin and eosin (H&E) staining, or Masson's trichrome staining using previously reported methods [10,19].
2.6Immunohistochemistry assaysImmunohistochemical (IHC) staining was performed using a previously reported immunoperoxidase protocol [10].
2.7mRNA expression and western blot (WB) analysesTissue RNA and proteins were extracted and assayed as previously described [10,20]. The reference mRNA sequence was that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Rn01775763_g1), and the target gene was glutathione peroxidase (GPx) (Rn00577994_g1). The comparative Ct method (ΔΔCt method) was used to determine the expression of GPx [7]. Western blot analysis was performed with the appropriate primary and secondary antibodies. The antibodies utilized in the present study were against 4-HNE (Abcam, Cambridge, UK, AB46545); Col-1α (Sigma–Aldrich, Missouri, USA, C-2456); CTGF (Santa Cruz Biotechnology, CA, USA, SC-365970); IL-10 (Invitrogen, CA, USA, ARC 9102); IL-1β (Abcam, Cambridge, UK, AB18329); IL-6 (Invitrogen, CA, USA, ARC0962); MMP13 (Merck-Millipore, MA, USA, MAB13426); Nrf2 (Abcam, Cambridge, UK, AB31163); PDGF (Abcam, Cambridge, UK, AB16829); p65 (Merck-Millipore, MA, USA, MAB3026); Smad7 (Abcam, Cambridge, UK, AB90086); TGF-β1 (Merck-Millipore®, MA, USA, MAB1032); TNF-α (Ebioscience, CA, USA, BMS175); α-SMA (Sigma–Aldrich, Missouri, USA, A-5691); β-actin (Ambion, MA, USA, AM4302).
2.8ZymographyThe proteolytic activity of metalloproteinase (MMP)-2 was evaluated using gelatin-substrate gels, as previously described [19].
2.9Statistical analysesAll graphical data are presented as the means±standard errors (SE). Comparisons among multiple groups were performed using GraphPad Prism® 7.0 software (CA, USA). The results from multiple groups were analyzed using one-way ANOVA followed by Tukey's test. P≤0.05 was considered statistically significant.
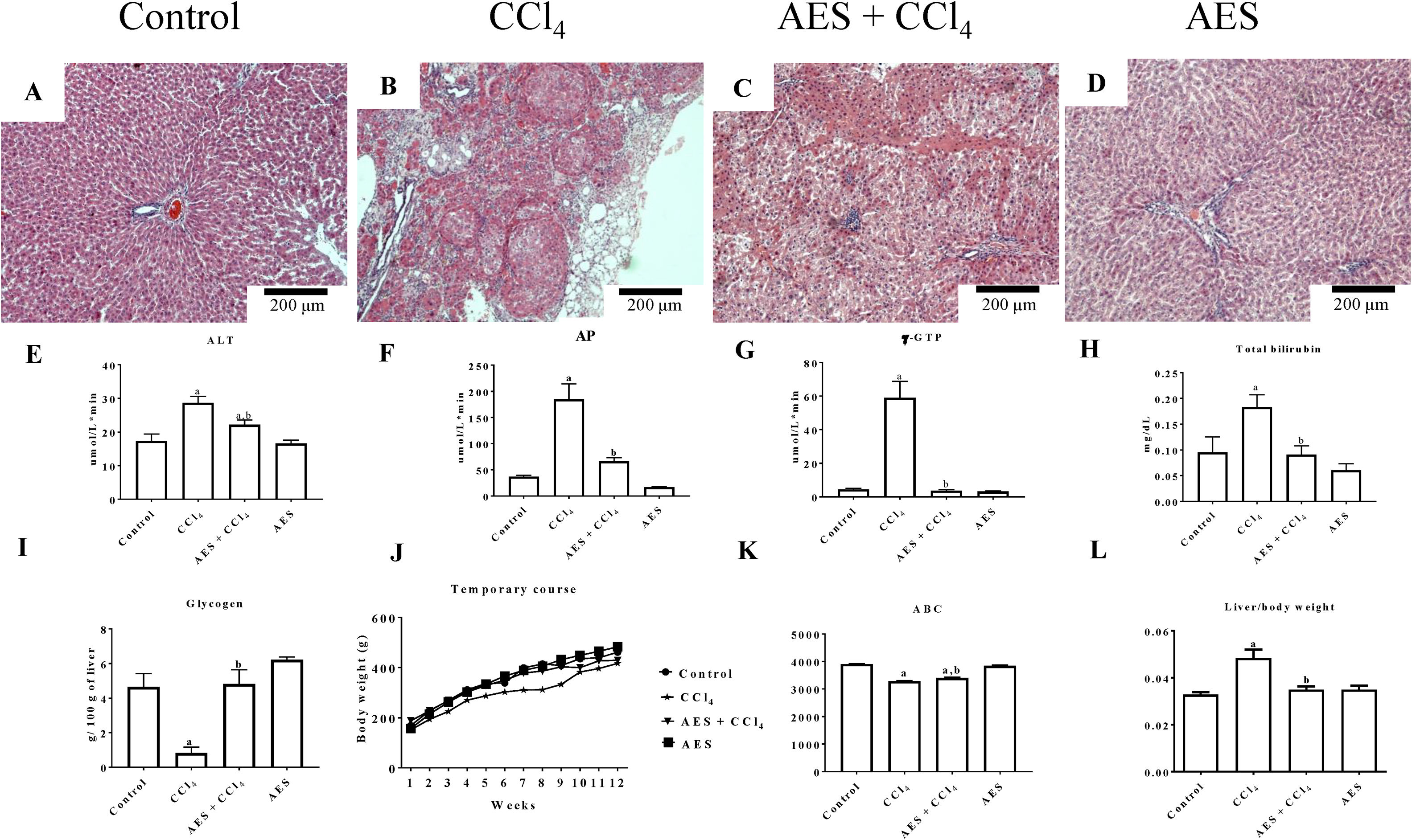
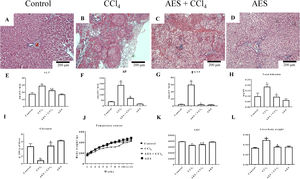
3Results3.1AES preserves liver parenchyma and markers of liver function in CCl4-treated ratsThe chronic CCl4 treatment produced steatosis, hyperchromatic nuclear hepatocytes, disruptions in the liver parenchyma and atypical and pleomorphic nuclei (Fig. 1B), whereas the control group showed no alterations (Fig. 1A). AES prevented CCl4-induced alterations in the hepatic parenchyma (Fig. 1C). Stevia tea administration to control rats did not affect liver histology (Fig. 1D). Fig. 1E–H shows that the serum enzymatic activity of ALT, AP and γ-GTP and the total bilirubin concentration increased with chronic CCl4 treatment and that stevia tea significantly prevented this effect. Hepatic glycogen, a good indicator of the liver capacity to store energy, was almost eliminated by CCl4 treatment, but AES effectively preserved the levels of this molecule (Fig. 1I). Fig. 1J exhibits a time course of the body weight gain of the treated rats over 12 weeks. Rats treated with CCl4 gained less weight than control rats, and AES treatment resulted in an intermediate amount of weight gain (Fig. 1K). The liver/body weight ratio was calculated (Fig. 1L), and it was found that chronic intoxication with CCl4 increased this ratio, while AES cotreatment completely prevented this effect. AES treatment did not affect the markers of liver damage studied in the control rats.
Effect of aqueous extract of stevia (AES) on liver histology and general markers of liver damage in cirrhotic rats. Hematoxylin and eosin staining in the livers of control (A), CCl4-treated (B), AES+CCl4-treated (C), and AES-treated (D) rats. Serum alanine aminotransferase (ALT) (E), alkaline phosphatase (AP) (F) and gamma-glutamyl transpeptidase (γ-GTP) (G) activity and the total bilirubin concentration (I) were determined. The time course of body weight gain during the experiment (J), area under the curve (AUC) (K) and body/liver weight ratios (L) are shown. Each bar represents the mean value of experiments performed in duplicate±SE (n=8). (a) P<0.05 compared with the control group; (b) P<0.05 compared with the CCl4 group.
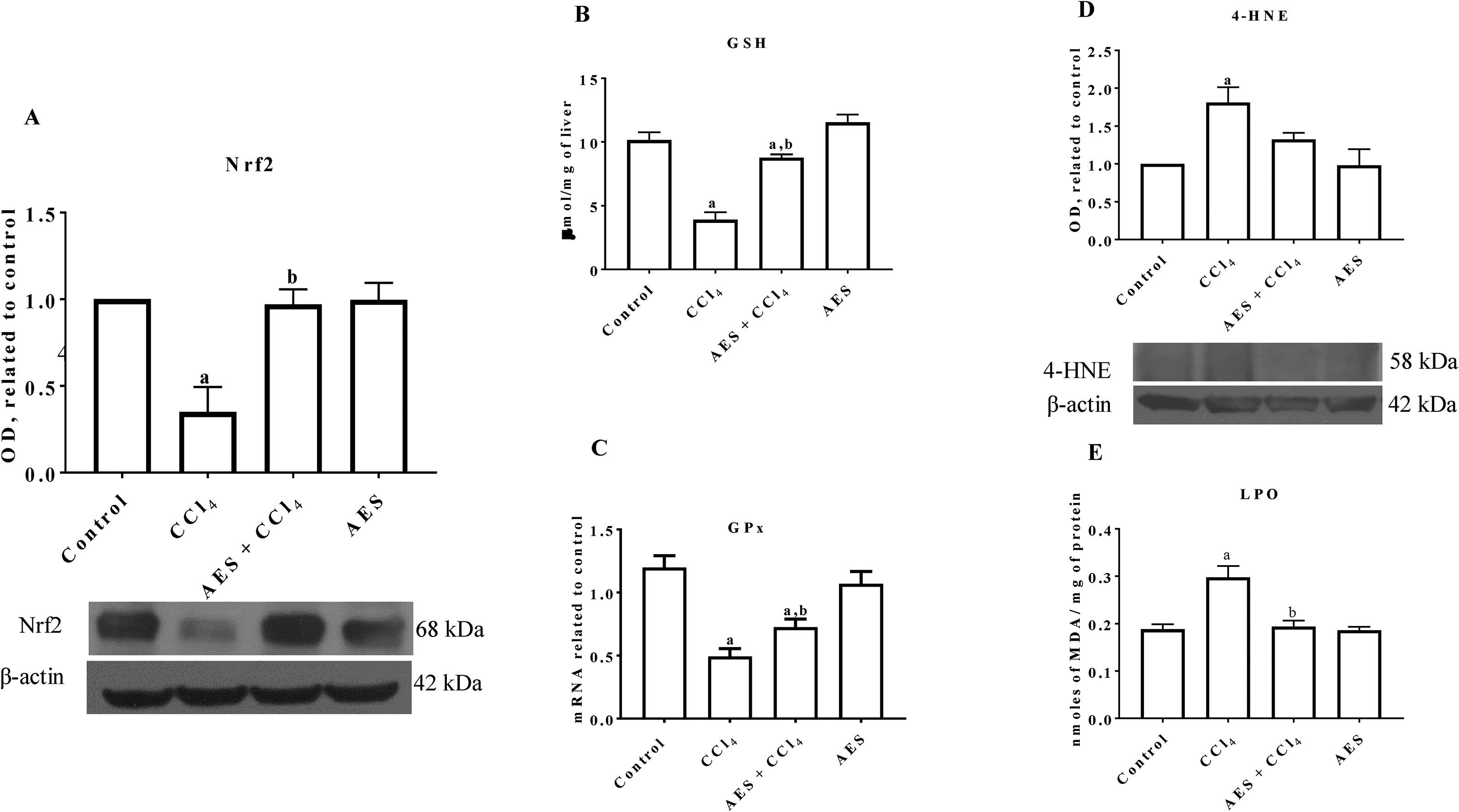
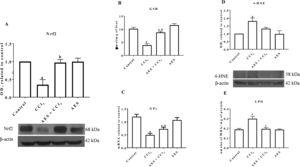
Nrf2 is the main transcription factor regulating the expression of antioxidant enzymes and factors that counteract oxidative stress in cells [21]. As shown in Fig. 2A, Nrf2 protein levels were significantly reduced in the livers of CCl4-treated rats, and AES completely prevented this reduction. As shown in Fig. 2B, CCl4 partially decreased the level of GSH, but stevia tea administration significantly prevented this effect. Chronic CCl4 treatment significantly reduced GPx mRNA levels, and AES significantly prevented this effect (Fig. 2C). 4-Hydroxynonenal (4-HNE) is a reactive lipid species [22] that is also a reliable indicator of oxidative stress damage to tissues [23]. Livers from rats with experimental cirrhosis showed increased levels of 4-HNE, and AES partially but significantly attenuated the increase in levels of this oxidative stress marker (Fig. 2D). Furthermore, CCl4 treatment increased the degree of lipid peroxidation, and AES treatment completely prevented this elevation (Fig. 2E). Stevia tea treatment had no effect on the markers of oxidative stress in the control rats.
Aqueous extract of stevia (AES) decreased Nrf2 expression and oxidative stress markers in experimental cirrhosis. Western blotting of nuclear factor-E2-related factor 2 (Nrf2) (A) and 4-hydroxynonenal (4-HNE) (D) was performed for livers derived from control, CCl4-treated, AES+CCl4-treated, and AES-treated rats (n=3). β-Actin was used as a control. The values are presented as fold increases in OD values normalized to the values of the control group (control=1). The glutathione (GSH) content (B), GPx mRNA expression (C) and degree of lipid peroxidation (LPO) (E) were determined in livers (n=8). Each bar represents the mean value±SE (n=8). (a) P<0.05 compared with the control group; (b) P<0.05 compared with the CCl4 group.
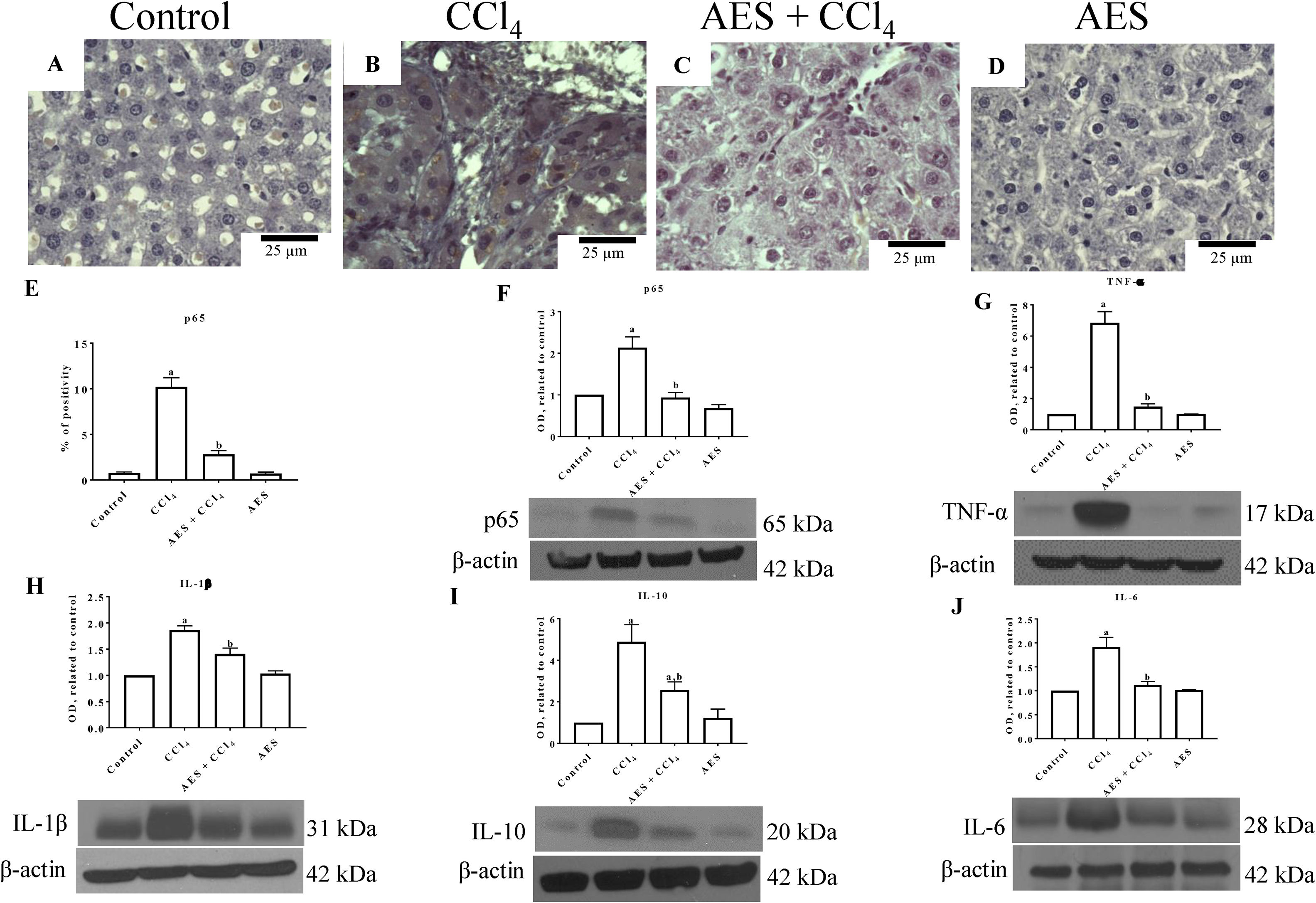
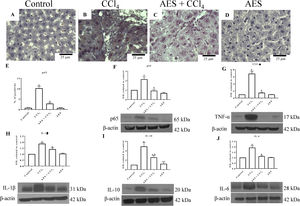
NF-κB (p65) is a key regulator of genes involved in inflammation; thus, this nuclear transcription factor is an interesting target for manipulating liver injury [24]. Liver sections from control rats exhibited low binding of the p65-specific antigen (Fig. 3A). In contrast, p65 expression was significantly increased in the CCl4 group (Fig. 3B) compared to the control group (Fig. 3A). Interestingly, liver samples from rats treated with both CCl4- and AES showed a significant prevention of the CCl4-induced upregulation of p65 (Fig. 3C, E). Consistent with the immunohistochemical findings, the western blots for p65 (Fig. 3F) revealed that CCl4 treatment significantly increased the levels of this protein and that AES completely prevented this effect. The levels of the tumor necrosis factor-alpha (TNF-α) (Fig. 3G), interleukin (IL)-1β (IL-1β) (Fig. 3H), IL-10 (Fig. 3I) and IL-6 (Fig. 3J) proteins were elevated in CCl4-cirrhotic rats, and this change was significantly attenuated by stevia tea administration. AES treatment in control rats had no effect on the levels of p65 or the cytokines regulated by this transcription factor.
The expression of NF-κB (p65) and proinflammatory cytokines was attenuated by aqueous extract of stevia (AES) in CCl4-induced cirrhosis. Representative images of p65 immunohistochemistry in liver slices from control (A), CCl4-treated (B), AES+CCl4-treated (C), and AES-treated (D) rats. Scale bar=25μm. Percentage of positivity for p65 obtained from immunohistochemistry slices (n=3) (E). The levels of the p65 (F), TNF-α (G), interleukin (IL)-1β (H), IL-10 (I), and Il-6 (J) proteins in samples of liver tissues were examined by western blot analysis (n=3). β-Actin was used as a control. The values are presented as fold increases in the OD values normalized to the values of the control group (control=1). Each bar represents the mean value±SE. (a) P<0.05 compared with the control group; (b) P<0.05 compared with the CCl4 group.
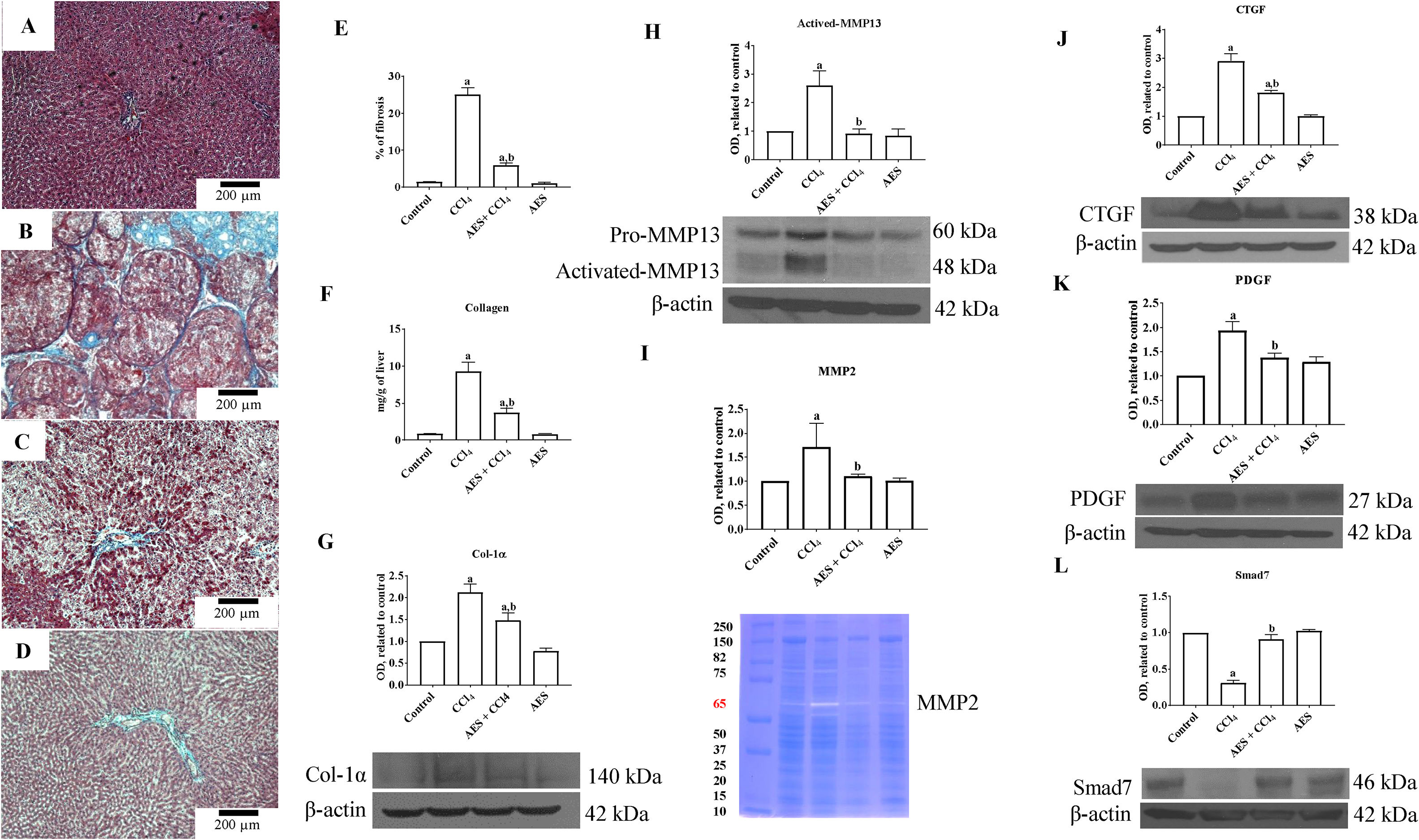
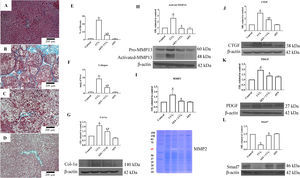
A representative liver section from a CCl4-treated rat showing substantial amounts of collagen around fibrotic nodules is shown in Fig. 4B. Administration of stevia tea to rats treated with CCl4 decreased the amount of collagen deposition (Fig. 4C). The percentages of positive fibrotic zones obtained from 3 liver slices are shown in Fig. 4E, and the observed changes reached statistical significance. As shown in Fig. 4F, chronic administration of CCl4 produced an 8-fold increase in the collagen content, as evaluated by the hydroxyproline method, and AES administration partially but significantly attenuated this increase. In agreement with this result, the WB of Col-1α showed increased levels of this protein in the livers of rats treated with CCl4, and AES significantly prevented this effect (Fig. 4G). MMPs play an important role in extracellular matrix (ECM) deposition and degradation [25]. Panels H and I in Fig. 4 show the MMP13 protein levels and the MMP2 enzyme activity, as assessed by WB analysis and zymography, respectively. In both cases, MMPs were increased by CCl4 treatment, and AES significantly blocked this effect. The cytokines connective tissue growth factor (CTGF) and platelet derived growth factor (PDGF) are well recognized for their potent profibrogenic and pro-proliferative effects, respectively [26]. The levels of the CTGF and PDGF proteins were assessed by WB analysis and are depicted in Fig. 4J and K, respectively; treatment with CCl4 increased the levels of these profibrogenic and pro-proliferative factors, but the AES cotreatment significantly prevented these effects. Smad7 inhibits the transforming growth factor-beta (TGF-β) 1 pathway by promoting TβRI degradation [27]. Notably, chronic CCl4 intoxication reduced the levels of this inhibitory protein, and stevia tea treatment of CCl4-cirrhotic rats completely preserved the normal values (Fig. 4L). AES treatment had no effect on the collagen content or the levels of these profibrogenic and proliferative factors in control rats.
Aqueous extract of stevia (AES) prevents fibrosis and profibrogenic mediators in CCl4-treated rats. Effect of AES on Masson's trichrome staining in the livers of control (A), CCl4-treated (B), AES+CCl4-treated (C), and AES-treated (D) rats. Scale bar=200μm. The percentage of fibrotic areas in the histological sections is shown (E) (n=3). The collagen levels were measured as the liver hydroxyproline content (F) (n=8). The levels of the collagen 1 alpha (Col-1α) (G), precursor and active metalloproteinase (MMP13) (H), connective tissue growth factor (CTGF) (J), platelet derived growth factor (PDGF) (K) and Smad7 (L) proteins in samples of liver tissue were determined by western blot analysis. β-Actin was used as a control. The activity of MMP2 (I) was determined by zymography (n=3). The values are presented as fold increases in OD values normalized to the values of the control group (control=1). Each bar represents the mean value±SE. (a) P<0.05 compared with the control group; (b) P<0.05 compared with the CCl4 group.
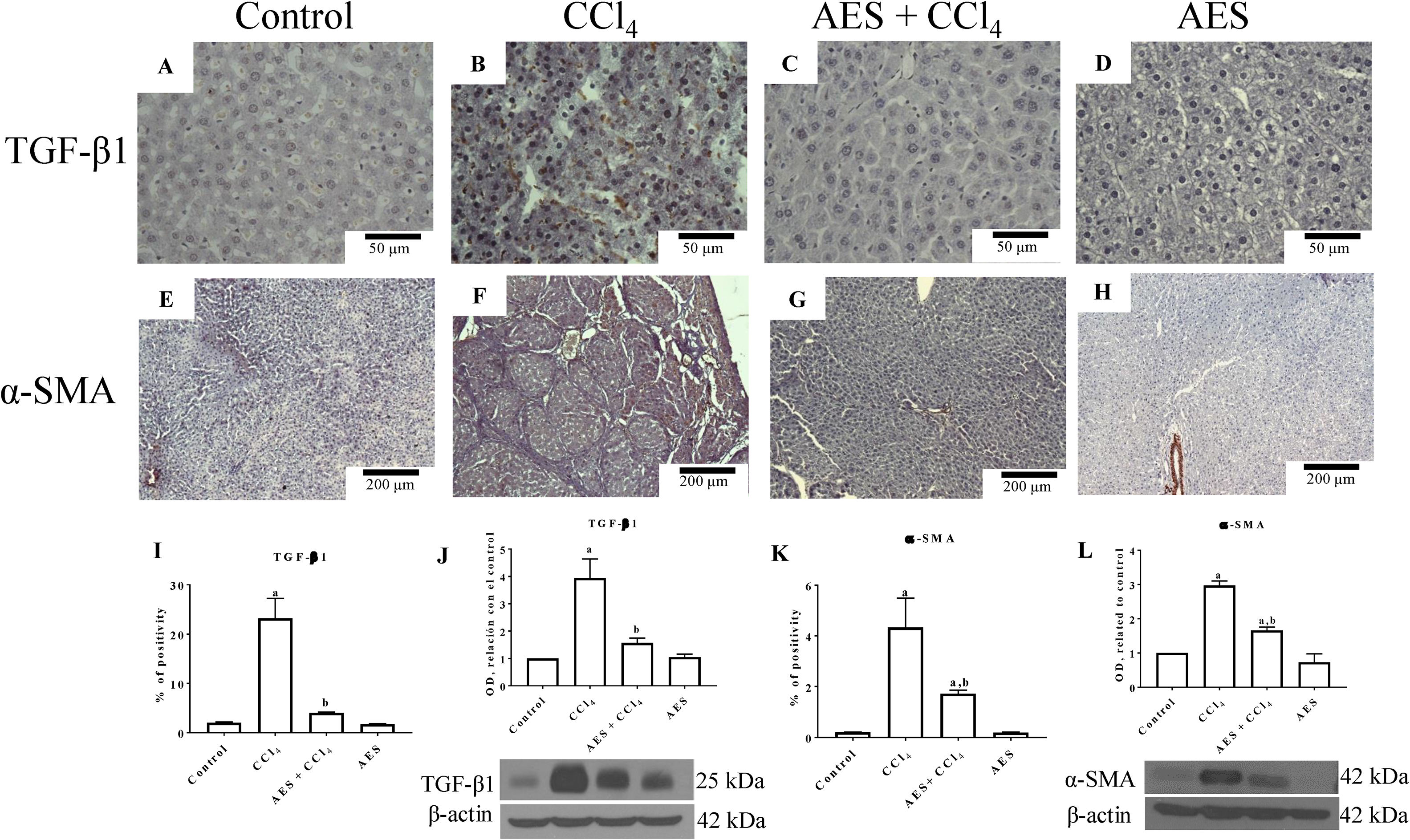
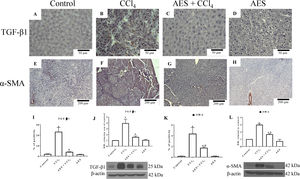
Fig. 5A depicts the IHC staining results for TGF-β1. Low positivity for TGF-β1 was observed in the control group (Fig. 5A). In contrast, TGF-β1 expression was significantly increased in the livers derived from CCl4-treated rats (Fig. 5B) compared to the livers of the control rats. Treatment with AES prevented the CCl4-induced upregulation of TGF-β1 (Fig. 5C). The percentage of positive zones obtained from 3 liver slices is shown in Fig. 5I, and the observed changes reached statistical significance. This result was confirmed by a WB analysis of TGF-β1 (Fig. 5J); rats treated with CCl4 exhibited increased levels of this cytokine, and stevia tea significantly prevented this change. IHC staining for alpha-smooth muscle actin (α-SMA), a specific indicator of activated HSCs, was performed on liver slices. Fig. 5E shows the IHC staining results for α-SMA. There was a faint signal for activated HSCs in the control group. Notably, higher α-SMA expression was observed in the CCl4 group (Fig. 5F) than in the control group. It is worth noting that treatment with stevia tea prevented the transdifferentiation of HSCs induced by chronic CCl4 intoxication (Fig. 5G). The percentage of positive zones obtained from 3 liver slices is shown in Fig. 5K, and the observed changes reached statistical significance. The WB for α-SMA (Fig. 5L) shows a similar result to that of the IHC analysis, showing that CCl4 significantly increased the levels of this indicator of HSC activation and that AES significantly prevented this increase. AES treatment did not affect TGF-β or α-SMA levels in control rats.
AES prevents HSC activation and preserves normal TGF-β1 in rats with CCl4-induced cirrhosis. Representative images of transforming growth factor-beta (TGF-β) and of alpha-smooth muscle actin (α-SMA) immunohistochemistry in control (A and E), CCl4-treated (B and F), AES+CCl4-treated (C and G), and AES-treated (D and H) rats are shown. The positive area of TGF-β positivity and the percentage of α-SMA-positive cells in slices are shown in histograms I and K, respectively (n=3). The levels of the TGF-β1 (J) and α-SMA (L) proteins in samples of liver tissue were determined by western blot analysis (n=3). β-Actin was used as a control. The values are presented as fold increases in OD values normalized to the values of the control group (control=1). Each bar represents the mean value±SE. (a) P<0.05 compared with the control group; (b) P<0.05 compared with the CCl4 group.
Previously, we reported that the administration of stevia leaves prevented acute and chronic liver damage induced by CCl4 administration in rats by improving the endogenous antioxidant system through the preservation of Nrf2 levels and by exerting anti-inflammatory activity through the downregulation of NF-κB and the proinflammatory cascade [10]. In this study, we utilized an aqueous extract of the leaves of the stevia plant and found that, in addition to exhibiting antioxidant and anti-inflammatory activity, AES also effectively prevented experimental fibrosis, probably because of its capacity to downregulate several profibrogenic signaling pathways (TGF-β, CTGF, PDGF) and to preserve the antifibrotic factor Smad7, subsequently preventing HSC activation and leading to an important reduction in ECM deposition. These results suggest that AES has the potential to improve human liver health, as stevia leaves are frequently consumed as a popular tea beverage and the CCl4 model of cirrhosis shares many similarities with human liver disease [2].
The aqueous extract of S. rebaudiana leaves possesses antihyperglycemic, antihypertensive, antioxidant, antitumor, antidiarrheal, diuretic, gastro- and renoprotective and immunomodulatory properties [3–17]. In addition, aqueous infusions of stevia leaves have been increasingly consumed in the food and cosmetic industry because of their high levels of antioxidants [18]. Accordingly, the objective of this study was to determine the efficacy of AES to prevent experimental liver cirrhosis induced by chronic CCl4 administration in rats, a model that shares several similarities with human cirrhosis [2]. We are the first to show that AES significantly prevents CCl4-induced liver fibrosis.
4.1Stevia tea prevents hepatic damage by preserving liver redoxReactive oxygen species (ROS) and electrophiles cause cellular damage and play an important role in the development of several chronic liver diseases, such as nonalcoholic steatohepatitis, hepatocellular carcinoma, viral infections by hepatitis C virus and hepatitis B virus, acetaminophen intoxication, fibrosis, and cirrhosis. Moreover, augmented production of ROS and electrophiles is able to induce a series of antioxidant genes through the activation of the Nrf2 pathway [21]. In the present study, it was found that livers derived from cirrhotic rats exhibited increased levels of lipophilic (LPO and 4-HNE) and hydrophilic (GSH) markers of oxidative stress and that concomitant treatment with AES significantly prevented these alterations. Antioxidants present in stevia tea may be, in part, responsible for the observed antioxidant effect; however, we also explored the possibility that AES may protect against free radical attack by modulating the Nrf2 system. Importantly, we found that stevia tea effectively preserved Nrf2 despite chronic intoxication with CCl4; moreover, because AES possesses low concentrations of strong antioxidants, the most likely mechanism underlying the antioxidant protective effect is the induction of Nrf2 expression [10]. In agreement with our observations, Wang et al. [28] performed in vitro studies that suggested that rebaudioside A, an important compound present in the stevia plant, enhances the antioxidative defense system by upregulating the Nrf2 signaling pathway, thus playing a protective role against CCl4-induced oxidative injury in HepG2 cells. Interestingly, ROS may induce HSC activation by paracrine activation signals, leading to the overproduction of ECM proteins [19–21]. Therefore, it seems likely that the antioxidant effects of AES may contribute to preventing the transdifferentiation of HSCs, subsequently attenuating fibrosis.
4.2AES prevents CCl4-induced experimental liver injury by downregulating NF-κBChronic liver injury of virtually any etiology triggers inflammatory and wound healing responses that eventually promote the development of hepatic fibrosis. Notably, there is a link between NF-κB, a master regulator of inflammation, and the development of hepatocellular carcinoma and liver fibrosis [29]. Interestingly, it has been reported that stevia suppresses the proinflammatory signaling pathway response by inhibiting NF-κB [30]. Here, we found that NF-κB expression was increased several-fold in cirrhotic livers and that AES effectively and completely prevented this effect, thus reducing the levels of the most proinflammatory cytokines. Furthermore, there is some evidence indicating that NF-κB directly represses Nrf2 signaling [31], which may be conducive to the elevated survival of activated HSCs because NF-κB exhibits proapoptotic properties [32] and subsequently exacerbates ECM deposition. Therefore, it seems reasonable to postulate that AES treatment may partially and indirectly protect the liver from inflammation and fibrosis induced by CCl4 by normalizing the cell redox state.
4.3AES prevents experimental liver fibrosis through a multitarget mechanismThere is strong evidence indicating that the activation of HSCs into fibrogenic myofibroblast-like cells triggers fibrogenesis in both experimental models and human hepatic fibrosis [33]. Therefore, it seems likely that the AES-mediated prevention of HSC transdifferentiation may constitute one of the most important antifibrotic mechanisms of this tea. Importantly, AES downregulated TGF-β1, which leads to HSC activation and collagen deposition through the canonical Smad pathway [34]. Moreover, it is worth noting that Smad7, which blocks TGF-β1 signaling through various mechanisms [35], was upregulated by AES treatment. On the other hand, MMP2 promotes the dissociation of IL-1β and TNF-α from the ECM, leading to the activation of NF-κB-regulated proinflammatory cytokine production. Moreover, MMP13 proteolytic activity is primarily responsible for the cleavage of CTGF, a potent profibrogenic factor, from the ECM reservoir [36]. Additionally, CTGF regulates several cellular responses, including the proliferation, migration, adhesion, and survival of HSCs [26]. Notably, stevia tea downregulated all these mediators of fibrogenesis and blocked HSC activation, providing important mechanisms by which AES exerts antifibrotic effects.
Herbal medicines have gained popularity as potential therapeutic agents for the prevention and treatment of diverse liver illnesses due to their high efficacy and few side effects. An advantage of herbal medicines in the treatment of liver diseases is their multilevel and multitarget effects; these medicines act on several molecular pathways related to the control of intracellular redox balance and inflammatory and profibrotic pathways. In this regard, silymarin [37], theanine [38], caffeine [39], curcumin [40], quercetin [19] and naringenin [41] seem to be as effective as AES in the treatment of experimental liver injury. However, it is necessary to perform clinical trials before these natural compounds can be used for the treatment of liver diseases in patients.
5ConclusionIt seems reasonable to conclude, based on the present results, that AES prevented experimental cirrhosis induced by chronic CCl4 administration in rats through antioxidative (preserving Nrf2 levels), anti-inflammatory (downregulating the NF-κB-induced inflammatory cascade), and various antifibrotic mechanisms, including the inhibition of HSC activation. Consequently, the consumption of stevia tea may be an economical strategy for the management of chronic liver disease in human patients. However, stevia should be further investigated as a novel therapy for chronic liver damage before it is recommended for human treatment.AbbreviationsAES aqueous extract of stevia carbon tetrachloride nuclear factor kappa B nuclear factor-E2-related factor 2 hepatic stellate cell extracellular matrix alanine aminotransferase alkaline phosphatase gamma-glutamyl transpeptidase glutathione lipid peroxidation hematoxylin and eosin immunohistochemical glutathione peroxidase glyceraldehyde-3-phosphate dehydrogenase metalloproteinase reactive oxygen species standard error 4-hydroxynonenal tumor necrosis factor alpha interleukin connective tissue growth factor platelet-derived growth factor transforming growth factor-beta alpha-smooth muscle actin
This work was partially supported by Conacyt grant 253037. Erika Ramos-Tovar received fellowship No. 380833 from Conacyt.
Conflict of interestThe authors have no competing interests to declare.
The authors thank Laura Dayana Buendia-Montaño, Karla M. Gil-Becerril, Rafael Leyva, Benjamín E. Chavez, and Ricardo Gaxiola for providing excellent technical assistance. The authors also acknowledge the Animal Lab Facility at UPEAL-Cinvestav and Dr. Jorge Fernández-Hernández.