Storage mites (SMs) occur in house-dust and the rate of sensitisation to them is high. We aimed to investigate if past and current living conditions are associated with the risk of SM sensitisation.
MethodsIn total, 321 patients (70% females) aged 33.6±11.9 years (range: 14–68 years) were studied at our allergy unit between September 2009 and December 2010. Patients with persistent or intermittent rhinitis and/or asthma were included in the study. Skin prick tests (SPTs) for SMs (Lepidoglyphus destructor, Tyrophagus putrescentiae, and Acarus siro) and other common aeroallergens were performed. Demographic data and characteristics of the patients’ homes were assessed via a questionnaire.
ResultsIn all, 102 (31.8%) patients were sensitised to ≥1 SM, of whom 43.1% were also sensitised to Dermatophagoides pteronyssinus. Comparison between the SPT-negative group (n=129) and the SM-positive only group (n=33) showed that having lived in a village during the first years of life was associated with SM sensitisation. Current place of residence was not significantly associated with any of the study variables.
ConclusionsLiving conditions have been changing and SM sensitisation may be associated with a history of village residence. The high rate of SM sensitisation observed in the study population might indicate the necessity of including those mite species in SPT panels, but the clinical relevance of sensitisation remains unclear. The clinical importance of SM sensitisation in urban areas should be investigated further.
Allergy to storage mites (SMs) is reported to be high in farmers and in the general population.1–3 Although SMs are generally thought to occur in occupational settings, they have also been isolated from house dust.3,4 However, the percentage of SM-positive dust samples in farm homes was reported to be considerably higher than in urban homes.5 Farm workers, grain elevator workers, and bakers are occupationally exposed to SMs and can become sensitised.6 A considerable proportion of German farmers suffering from symptoms of rhinitis and/or asthma were found to be sensitised to storage mites.7,8
Microscopic examination of house dust samples from Kütahya, Turkey showed the most prevalent SMs to be Tyrophagus putrescentiae 29%, Lepidoglyphus destructor 25%, Glycyphagus domesticus 23%, Acarus siro 17%, and unidentified 6%.9 Another study from Turkey reported SMs to be extremely rare in house dust samples in which almost three-quarters of the samples surveyed were collected from cities where apartment flats constitute the major type of building.10
SMs are most commonly found in humid and damp environments.11 In Europe, SMs were once the most common species in house dust, but they have been overtaken by Dermatophagoides pteronyssinus (DP). This shift in prevalence was explained by changes in indoor living conditions such as cleaning habits, food storage, introduction of wall-to-wall carpeting, and greater tendency to live in the cities; however, none of these variables has been compared in the same patient population.12 As such, the present study aimed to investigate if past and current living conditions are associated with the risk of SM sensitisation in Turkey.
Materials and methodsParticipantsThis cross-sectional study included 321 patients (70% females) aged 33.6±11.9 years (range: 14–68 years) that were studied at our allergy unit between September 2009 and December 2010. Consecutive patients that were diagnosed with persistent/intermittent rhinitis and/or asthma, and provided informed consent to participate were included in the study. Demographic data, the characteristics of the homes the patients lived in at least until the age of two years, and the homes they were currently living in for ≥1 years were assessed via a structured questionnaire administered by a trained physician. The questionnaire collected data on level of education, method of home heating, number of individuals living in the home, the presence of wall-to-wall carpeting, and the presence of domestic animals and livestock (cows, sheep, and chickens). Location of the home (inland or coastal) was also recorded. All participants provided written informed consent and the study protocol was approved by the local Ethics Committee.
Skin prick testsSkin prick tests (SPTs) to common aeroallergens, including DP and three SMs (A. siro, L. destructor, T. putrescentiae), pollens (Phleum pratense, Artemisia vulgaris, Parietaria officinalis, Corylus avellana, Olea europaea, and Betula verrucosa), moulds (Aspergillus fumigatus, Cladosporium herbarum, and Alternaria alternata), animal dander (dog and cat), latex, and Blatella orientalis, were performed. Standardised allergenic extracts for SMs were obtained from ALK Abello (Denmark) and the other allergenic extracts were obtained from Allergopharma (Germany). Histamine (10mg/mL) and saline were used as positive and negative controls, respectively. All the patients underwent SPTs and standard procedures were applied.13 A test was considered positive if the mean wheal diameter was ≥3mm and controls showed adequate reactions. Values calculated for each allergen were recorded on a standardised data collection form.
Statistical analysisSPSS v.15.0 was used for statistical analysis. Data are expressed as frequency for categorical variables and as mean±standard deviation for continuous variables. Patients sensitised to ≥1 SM constituted the SM-positive group; those that were sensitised only to SMs constituted the SM-positive only group; those that were sensitised only to DP constituted the DP-positive only group; and patients with negative SPTs constituted the SPT-negative group. Between-group comparisons of categorical variables were made using the chi-square test. Associations between SM-positivity only and DP-positivity only that were statistically significant based on univariate analysis were adjusted for age and gender, and then subjected to logistic regression analysis to assess the independent associations. The SPT-negative group was considered as the reference group in these comparisons, and p values were not adjusted for multiple comparisons. Age seemed to be a confounder. Older patients were more commonly living in a village. Age was grouped as <40 years and ≥40 years. Childhood home characteristics were compared with those of the current home using Cohen's kappa statistics. Kappa values >0.75, 0.40–0.75, and <0.40 were considered to have excellent, fair-to-good, and poor agreement, respectively. For all comparisons p<0.05 was considered statistically significant.
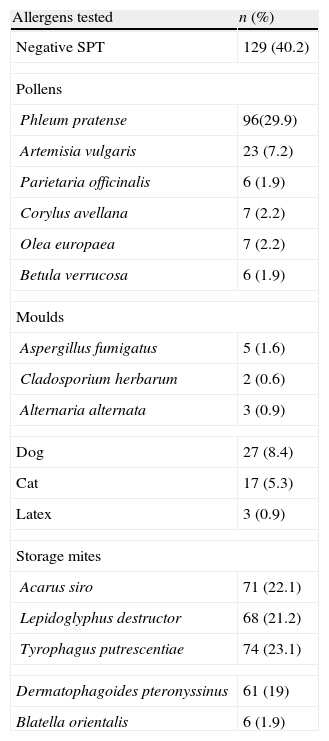
ResultsMean age of the study population was 33.62±11.9 (16–68), and 70% were women. SPT results are shown in Table 1. Mean wheal size of ≥3mm was considered positive. In total, 162 (50.5%), 25 (7.8%), and 134 (41.7%) patients were diagnosed with rhinitis, asthma, and asthma with rhinitis, respectively.
Skin prick test results.
| Allergens tested | n (%) |
| Negative SPT | 129 (40.2) |
| Pollens | |
| Phleum pratense | 96(29.9) |
| Artemisia vulgaris | 23 (7.2) |
| Parietaria officinalis | 6 (1.9) |
| Corylus avellana | 7 (2.2) |
| Olea europaea | 7 (2.2) |
| Betula verrucosa | 6 (1.9) |
| Moulds | |
| Aspergillus fumigatus | 5 (1.6) |
| Cladosporium herbarum | 2 (0.6) |
| Alternaria alternata | 3 (0.9) |
| Dog | 27 (8.4) |
| Cat | 17 (5.3) |
| Latex | 3 (0.9) |
| Storage mites | |
| Acarus siro | 71 (22.1) |
| Lepidoglyphus destructor | 68 (21.2) |
| Tyrophagus putrescentiae | 74 (23.1) |
| Dermatophagoides pteronyssinus | 61 (19) |
| Blatella orientalis | 6 (1.9) |
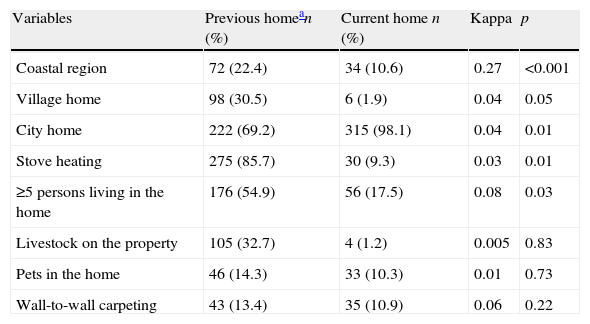
Table 2 shows the comparison of the homes the patients lived until age two years and those they lived in at the time of the study. In all, six (1.9%) patients were currently living in a village home, whereas 98 (30.5%) had done so as children. Along with this change in place of residence, the number of livestock outside their homes decreased. Additionally, the childhood homes had more individuals living in them than the current homes did. All the estimated kappa values were <0.40.
Comparison of previous and current home.
| Variables | Previous homean (%) | Current home n (%) | Kappa | p |
| Coastal region | 72 (22.4) | 34 (10.6) | 0.27 | <0.001 |
| Village home | 98 (30.5) | 6 (1.9) | 0.04 | 0.05 |
| City home | 222 (69.2) | 315 (98.1) | 0.04 | 0.01 |
| Stove heating | 275 (85.7) | 30 (9.3) | 0.03 | 0.01 |
| ≥5 persons living in the home | 176 (54.9) | 56 (17.5) | 0.08 | 0.03 |
| Livestock on the property | 105 (32.7) | 4 (1.2) | 0.005 | 0.83 |
| Pets in the home | 46 (14.3) | 33 (10.3) | 0.01 | 0.73 |
| Wall-to-wall carpeting | 43 (13.4) | 35 (10.9) | 0.06 | 0.22 |
The rate of any SM sensitisation was 31.8% (n=102) and SM sensitisation only rate was 10.3% (n=33). Comparison of the variables between SM-positive group and the remaining patients (n=219) showed that living in a coastal region during the first two years of life was associated with SM sensitisation (p=0.017); however, the DP (n=44, 43.1%) sensitisation rate was significantly high in the SM-positive group (p<0.001). The association of coastal residence with significant SM sensitisation was due to high rate of DP sensitisation in SM-positive group. When the SM-positive only group was compared with the remaining patients the association of coastal residence with SM sensitisation disappeared. There were no significant differences in the characteristics of the current homes between the patient groups.
In all, 17 (5.3%) patients were sensitised only to DP. Comparison of those 17 patients with the SPT-negative group showed that living in a coastal region and the presence of wall-to-wall carpeting in the home that was lived in until two years of age were associated with DP sensitisation (p=0.024, p=0.048). Paradoxically, stove heating was associated with a lower rate of DP sensitisation (p=0.018). When current living conditions were compared between DP-positive only and SPT-negative group, more of the DP-positive only patients lived in a coastal region (23.5% versus 8.5%) and had wall-to-wall carpeting (23.5% versus 10.9%), but the differences were not significant (p= 0.136, p=0.270, respectively).
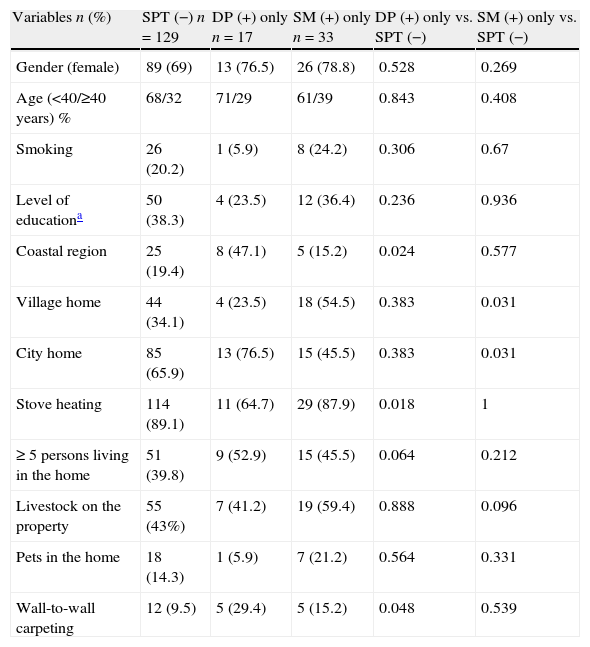
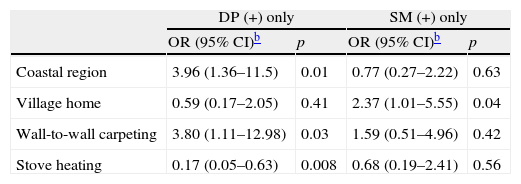
In total, 30.5% of the patients’ childhood homes were village homes, whereas 1.9% of the patients’ current homes were village homes. Comparison of childhood home characteristics in the SPT-negative, DP-positive only, and SM-positive only groups is shown in Table 3. Comparison of the SPT-negative group and SM-positive only group showed that living in a village home until two years of age was associated with SM sensitisation (p=0.031). None of the patients that were sensitised to any SM were currently living in a village home. Significant variables in univariate analysis associated with DP sensitisation only and SM sensitisation only were adjusted for age and gender in the logistic regression model, which showed that living in coastal region and having wall-to-wall carpeting were associated with DP sensitisation (p=0.01 and 0.03, respectively). Living in a country house during the first years of life was associated with SM sensitisation (p=0.04) (data shown in Table 4).
Comparison of past living conditions between the SPT-negative, DP-positive only, and SM-positive only groups.
| Variables n (%) | SPT (−) n=129 | DP (+) only n=17 | SM (+) only n=33 | DP (+) only vs. SPT (−) | SM (+) only vs. SPT (−) |
| Gender (female) | 89 (69) | 13 (76.5) | 26 (78.8) | 0.528 | 0.269 |
| Age (<40/≥40 years) % | 68/32 | 71/29 | 61/39 | 0.843 | 0.408 |
| Smoking | 26 (20.2) | 1 (5.9) | 8 (24.2) | 0.306 | 0.67 |
| Level of educationa | 50 (38.3) | 4 (23.5) | 12 (36.4) | 0.236 | 0.936 |
| Coastal region | 25 (19.4) | 8 (47.1) | 5 (15.2) | 0.024 | 0.577 |
| Village home | 44 (34.1) | 4 (23.5) | 18 (54.5) | 0.383 | 0.031 |
| City home | 85 (65.9) | 13 (76.5) | 15 (45.5) | 0.383 | 0.031 |
| Stove heating | 114 (89.1) | 11 (64.7) | 29 (87.9) | 0.018 | 1 |
| ≥ 5 persons living in the home | 51 (39.8) | 9 (52.9) | 15 (45.5) | 0.064 | 0.212 |
| Livestock on the property | 55 (43%) | 7 (41.2) | 19 (59.4) | 0.888 | 0.096 |
| Pets in the home | 18 (14.3) | 1 (5.9) | 7 (21.2) | 0.564 | 0.331 |
| Wall-to-wall carpeting | 12 (9.5) | 5 (29.4) | 5 (15.2) | 0.048 | 0.539 |
Analysis of the variables strongly associated with DP or SM sensitisation, based on a logistic regression model adjusted for age and gender.a
In the present study, the SM sensitisation rate was 31.8% in a population of patients diagnosed with asthma, rhinitis, or both, and 43.1% of the patients sensitised to a SM were also sensitised to DP. Vidal et al. reported that in a general adult population the SM sensitisation rate was 24.4% and that 90% of the participants that were sensitised to DP were also sensitised to SMs, which is the highest rate ever reported. Interestingly, their entire study population was of rural and village origin. They concluded that SM sensitisation may be of clinical importance, but probably not as important as of DP sensitisation, and that the high rate of sensitisation to both DP and SMs may have been due to cross reactions.14 Heide et al. reported that extracts of DP inhibited IgE binding to all SMs (A. siro, L. destructor, and T. putrescentiae) up to 60%, whereas IgE binding to DP was only minimally inhibited by extracts of SMs, which was largely due to cross reactions between different mite species.15
It was reported that SMs were present in dust samples taken from the homes of 55 asthmatic children, but that the number of DP observed in the samples was greater. Additionally, the mite sensitisation rate was 38% and 50% of house-dust mite-sensitised patients were also sensitised to a SM and only one patient was sensitised to SMs only.3 The researchers reported that there was an association between the presence of SMs in homes and sensitisation to them; however, almost all the patients in their study that were sensitised to SMs were also sensitised to house-dust mites. In the present study 10.3% of patients were sensitised to SMs only, and when compared to the SPT-negative patients, living in a village home during the first years of life was associated with this sensitisation. Village homes in Turkey are generally damp, overcrowded, and not centrally heated, and there are livestock on the property; when those variables were individually analysed, no association between any of them and SM sensitisation was noted.
It was reported that in a region of high humidity in Spain inclusion of glycyphagid mite extracts might be necessary for proper diagnosis and treatment of patients exposed to both DP and SMs.16
The Turkish Ministry of Agriculture and Rural Affairs reported that 74.9% of the Turkish population lived in rural areas in 1950, whereas in 2009 it was 24.4%.17 In their report rural areas included small towns with a population <20,000. In all, 1.9% of the patients in the present study were currently living in a village home, versus 30.5% during childhood. This finding is indicative of the tendency for Turks to move from villages to cities. Although SMs are isolated from urban dwellings, the patients may have been sensitised to them in the past. The low number of SMs in house-dust may not cause symptoms; on the other hand cross-reactions with DP may have resulted in the observed positive SPT results.
Comparison of the SPT-negative patients and DP-positive only patients showed living in a coastal region and the presence of wall-to-wall carpeting in the childhood home were associated with DP sensitisation. Homes in coastal regions of Turkey are generally humid, which may account for the high rate of DP sensitisation observed in the present study. The most prevalent mite species isolated from house-dust samples in Turkey was reported to be DP (94.95%), SMs were extremely rare in dust samples, and mites were more frequently isolated in samples from humid coastal regions.10 All these data indicate that the conditions necessary for SM and DP sensitisation may differ, and that some cases of SM sensitisation may be due to cross reactions with DP allergens as well.
None of the patients in the present study that were sensitised to any SM were currently living in a village home. Thus, comparison of data for current living conditions was not possible, which is a limitation of the present study. Additionally, we did not perform mite counts from village and urban homes for comparison. In the patients that were sensitised to SMs we had expected SM specific provocation test results to be positive; however, at the time the study was conducted those patients were living in cities and were probably not exposed to SMs in their homes. Based on a previous study from Turkey, we know that SMs are extremely rare in homes in cities.10
In conclusion, in Turkey the general trend is relocation from villages to cities and as such SM sensitisation may due to a history of village residence. The high rate of SM sensitisation observed in the present study suggests that those mite species should be included in SPT panels, but the relevance of sensitisation with symptoms remains unclear. A history of village residence appeared to be associated with SM sensitisation; additional research is necessary to more clearly understand the clinical significance of SM sensitisation in urban regions.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interests to declare.
AcknowledgementWe thank Associate Professor Ahmet Ugur Demir (Hacettepe University, School of Medicine, Department of Chest Diseases) for data analysis.