The causal agents of botulism are neurotoxins produced by the Gram-positive anaerobic bacterium Clostridium botulinum. Botulinum toxin (BTX) blocks the release of acetylcholine from the presynaptic terminal of the neuromuscular junction.1 Because of its mechanism of action, BTX began to be used to treat muscle overactivity.
Since Scott described the use of intramuscular BTX type A (BTX-A) in the treatment of strabismus, BTX has been increasingly used in the treatment of various diseases, principally dermatological, ophthalmic, neurological and urological diseases.1,2
As the use of BTX increased, cases of resistance to the toxin, characterised by the absence of any beneficial effect after drug administration, began to be described.3 Antibodies (Abs) against BTX seem to be responsible for most of the cases of resistance.3 The two major commercial preparations of BTX-A are BOTOX® (Allergan, Inc., Irvine, CA, USA) and Dysport® (Ipsen Limited, Slough, UK). Both preparations have been associated with the formation of neutralising Abs.4
Antibodies against BTX can be detected by functional and laboratory tests, as well as by experimental tests involving mice.3 However, the sensitivity and specificity of these tests, as well as the clinical relevance of the results, have not been defined. We report two cases of BTX-A treatment failure secondary to the formation of neutralising Abs in two young patients, recruited from the Outpatient Clinic of the Physiatry Department. Laboratory tests were performed by the Division of Clinical Immunology and Allergy, University of São Paulo, Brazil. The Project was approved by the Institutional Review Board of our Hospital (CAPPesq) and both patients signed the free and informed consent form. We collected patients’ clinical history, a functional test (frontalis test) and a serological one (Western blot assay) to confirm the diagnosis of BTX resistance due to neutralising Abs. The clinical cases and their evaluations are presented in detail.
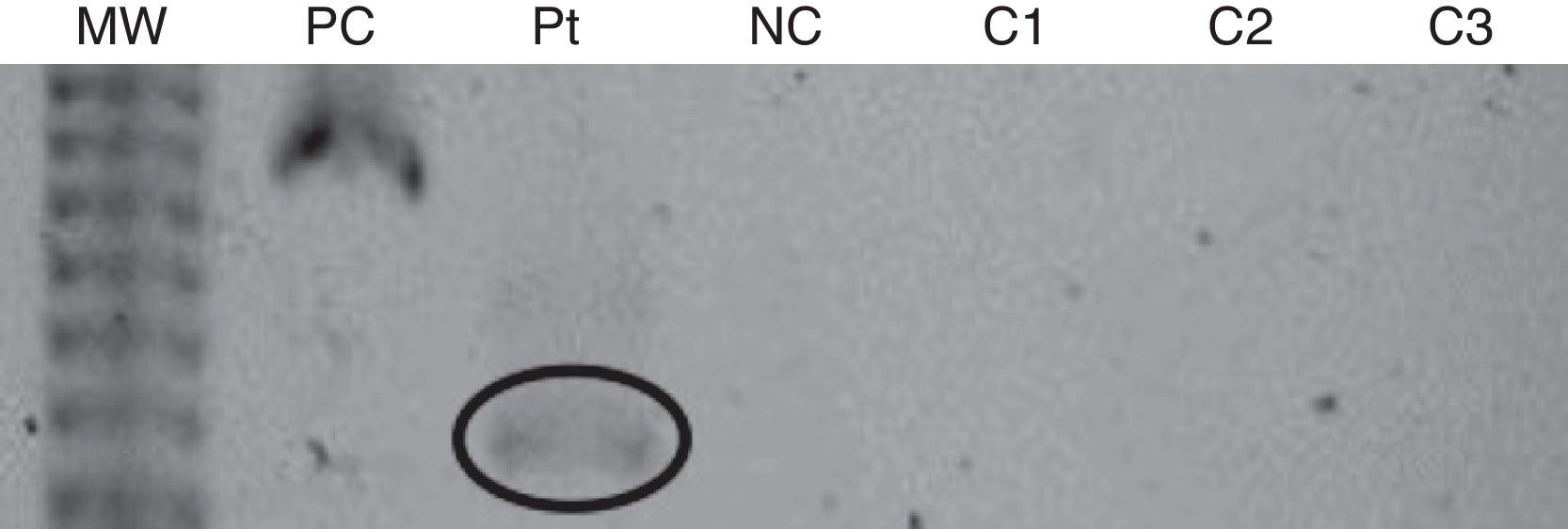
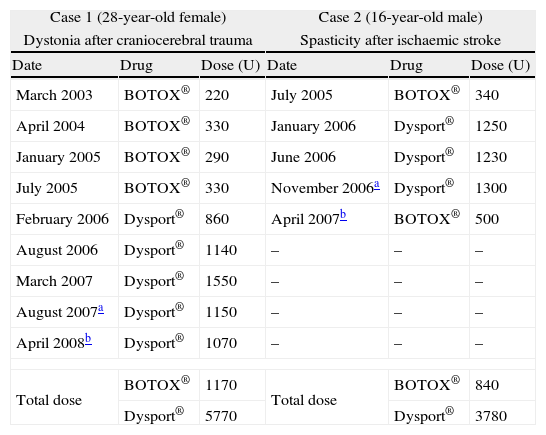
The first patient was a 28-year-old female who had been using BTX-A since 1996. In 1991, she was involved in a car accident and had craniocerebral trauma, which culminated with right-sided hemiparesis and dystonia. Despite receiving physical therapy, the right-arm dystonia remained. In 1996, BOTOX® treatment was started, with injections given once every six months. In 2001, the BOTOX® was replaced by Dysport® (Table 1). In January 2008, we observed resistance to BTX-A, as evidenced by the lack of clinical response. The frontalis test, which was performed as previously described,3 confirmed the resistance to BTX. Then, a Western blot assay revealed the presence of anti-BTX IgG Abs (Fig. 1).
Summary of the two cases of resistance to botulinum toxin A secondary to the formation of neutralising antibodies.
| Case 1 (28-year-old female) | Case 2 (16-year-old male) | ||||
| Dystonia after craniocerebral trauma | Spasticity after ischaemic stroke | ||||
| Date | Drug | Dose (U) | Date | Drug | Dose (U) |
| March 2003 | BOTOX® | 220 | July 2005 | BOTOX® | 340 |
| April 2004 | BOTOX® | 330 | January 2006 | Dysport® | 1250 |
| January 2005 | BOTOX® | 290 | June 2006 | Dysport® | 1230 |
| July 2005 | BOTOX® | 330 | November 2006a | Dysport® | 1300 |
| February 2006 | Dysport® | 860 | April 2007b | BOTOX® | 500 |
| August 2006 | Dysport® | 1140 | – | – | – |
| March 2007 | Dysport® | 1550 | – | – | – |
| August 2007a | Dysport® | 1150 | – | – | – |
| April 2008b | Dysport® | 1070 | – | – | – |
| Total dose | BOTOX® | 1170 | Total dose | BOTOX® | 840 |
| Dysport® | 5770 | Dysport® | 3780 | ||
Western Blot assay from the first patient. MW: molecular weight; PC: positive control (botulinum toxin and antibody anti-toxin); Pt: patient reported; NC: negative control (botulinum toxin alone); C1: control 1 (normal individual without previous contact with the toxin); C2: control 2 (responsive patient with previous contact with the toxin); C3: control 3 (responsive patient with previous contact with the toxin).
The second patient was a 16-year-old male, who received BTX-A injections from 2005 to 2007. In 2004, he presented with ischaemic stroke and subsequently developed left spastic hemiparesis. No illnesses that could be associated with stroke in a young patient were identified. From the outset, he received multidisciplinary physical therapy and acetylsalicylic acid. In 2005, treatment was started with injections of BOTOX® and Dysport® (Table 1). In November 2006, the clinical response was minimal. In April 2007, there was no improvement after the administration of the toxin. The frontalis test and the Western blot assay confirmed the resistance to BTX-A due to IgG Abs.
In the Western blot assays, three controls were used: two patients treated with botulinum toxin, with no evidence of resistance, and a normal individual without previous contact with the toxin. Serological tests from controls were all negative.
The clinical use of BTX has played an extremely important role in the treatment of various pathological and cosmetic conditions that had once been difficult to address. However, because it is a protein conjugate of high molecular weight, BTX can stimulate the immune response and lead to the production of specific Abs, which can neutralise the therapeutic effects of BTX.1
Various tests are available to detect anti-BTX Abs, including functional tests, serological tests and experimental neutralisation assays in laboratory animals.3 There are three major laboratory animal tests that can be used to detect these Abs: the mouse lethality assay, mouse protection assay and mouse phrenic nerve-hemidiaphragm preparation, considered to be the gold standard.4 However, these tests are expensive and difficult to perform in clinical practice.
The functional tests most commonly used to confirm clinical resistance to BTX-A are: the frontalis test, extensor digitorum brevis test, sternocleidomastoid test and sudomotor test.3
In order to confirm the association between resistance to BTX and the presence of Abs, any serological tests can be used. However, none of them are commercially available. They are only used in research and their accuracy has not been determined yet. We used Western blot assay because it is a laboratory test with which we have more extensive experience. The patients described probably developed therapy failure due to neutralising Abs, since the controls had negative tests. In other words, the Western blot tests performed were not false positives.
The great difficulty in diagnosing resistance to BTX lies not only in the limited availability of in vivo, in vitro and experimental tests, but also in establishing a clinical correlation. While in vivo functional tests do not confirm the presence of Abs, serological tests do identify Abs, but they do not distinguish between neutralising and non-neutralising Abs. Since BTX is not formulated as a pure protein, the production of Abs against proteins conjugated with the toxin, such as haemagglutinins, can occur, and non-neutralising Abs can be formed.3,5 In addition, all serological tests can yield false-negative results. Therefore, such tests are useful only when the results are correlated with a clinical profile of resistance and with the results of an in vivo functional test.
Most studies investigating resistance to BTX have shown that the production of blocking Abs is the main cause of such resistance; in many patients, however, it is not possible to document Ab formation, possibly because the serological test systems are ineffective.4,6
In an attempt to find possible risk factors for the development of Abs, researchers evaluated 503 patients experiencing secondary therapy failure to BTX-A.4 There was no significant difference between BOTOX® and Dysport® with regard to the presence of anti-BTX Abs. In addition, of the 503 patients evaluated, 224 (44.5%) presented neutralising Abs, suggesting that resistance is associated with other factors. Therefore, no recommendations can currently be made as to which commercial preparation should be used for primary prevention.
Other possible causes for the loss of effectiveness of the medication include insufficient dose, inappropriate site of administration, inappropriate drug storage and down regulation of BTX receptors.1
Various studies have shown that the prevalence of anti-BTX Abs in patients receiving BTX-A due to cervical dystonia and torticollis ranges from 2.5 to 15.3%.6 Nevertheless, as previously mentioned, the presence of Abs does not necessarily indicate that they are blocking Abs. It has recently been shown that over 40% of the patients with dystonia who respond well to BTX-A have detectable Abs.7
Table 2 shows the principal risk factors for the development of neutralising Abs.1,4–6 These factors seem to explain why resistance due to Abs is rarely seen in cases in which BTX is recommended for the treatment of dermatological or ophthalmic conditions.4 For such conditions, the doses of BTX are considerably lower than those administered in patients with dystonia or spasticity.4
Risk factors for the development of anti-botulinum toxin neutralising antibodies.
| High doses of botulinum toxin |
| Short interval between injections (<3 months) |
| Booster or “touch-up” injections (<6 weeks between injections) |
| Total cumulative dose of botulinum toxina |
| Young age |
aMore than 40 months of treatment; >6000U for Dysport®.
In the cases reported here, the high dose, the total cumulative dose and the fact that they were young might have triggered the development of neutralising Abs. They were identified within a group of hundreds of patients in various age brackets. Although patients of any age can synthesise these antibodies,4,8 we evaluated patients of all ages receiving botulinum toxin for spasticity, according to the same protocol, and we noticed that only two young patients were producers of neutralising antibodies.
Therefore, clinicians should administer the lowest possible dose, at intervals greater than three months, avoiding “booster” injections.
After treatment failure secondary to Ab formation has occurred, there are few available strategies to reverse the profile. Replacing BTX-A with BTX-B, BTX-C or BTX-F has been described.1 However, this strategy has little impact, because the other types of BTX are not readily available, their long-term effectiveness has yet to be established and, principally, because cross-reactions occur.1 In patients who previously received BTX-A injections, BTX-B, which is strongly correlated with Ab formation, also induces resistance.5
An increase in the dose administered has been able to restore the effectiveness of the toxin in patients with resistance due to the presence of Abs.9 However, this is a very expensive treatment, and the long-term safety of high doses has yet to be determined. Other alternatives have been described and include the use of immunosuppressants, plasmapheresis and intravenous human immunoglobulin.10 These treatments have also yet to be evaluated in-depth in terms of their cost, effectiveness and, principally, safety in such patients.
The formation of neutralising Abs is a rare occurrence, but it constitutes a major complicating factor, principally for the treatment of patients with dystonia or spasticity. Further studies are required in order to determine whether age is a risk factor for the development of blocking Abs and how to treat patients who develop resistance to BTX. Currently, treatment with lower doses and longer intervals seems to be the only truly effective prophylactic measure.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.