Our study aims to assess the importance of serum eosinophil cationic protein (ECP) levels as a non-invasive marker of bronchial hyperresponsiveness (BHR) in children with asthma, and may predict objectively the asthmatic severity and sensitivities.
MethodsThis study, which was carried out on 75 asthmatic patients from a paediatric population (average age: nine years old, sex-ratio M/F: 1.64), is based on both interrogation conducted by the clinician and biological explorations, essentially serological testing of ECP and eosinophilia determination, as well as the measurement of serological IgE amounts.
ResultsThe analysis of the questionnaires and the biological results allowed us to evaluate the clinico-biological relations within this population. ECP, more than eosinophilia, proves to be a relevant marker of asthma severity (p<0.05) and sensitivities within this given population (r=0.65).
ConclusionWe were able to show that the evaluation of the serological levels of ECP seems to be a good biological marker of asthma.
It is currently well established that an inflammatory reaction is the basis of allergic diseases. The cell populations involved in this process are the lymphocytes Th2 determining the allergic reaction and the effective cells among which, after mastocytic activation, eosinophils play a predominant role within the mid-late phase of IgE-dependent allergy.1
These cells contain various proteins among which the major basic protein (MBP), located inside the crystalloid of the granules, as well as the neurotoxin (EPX-EDN), the peroxidase (EPO) and the eosinophil cationic protein (ECP), all three found in the granule matrix. ECP quantity increases in cases of asthma, rhinitis, atopic dermatitis, parasitic infections and auto-immune diseases.2 Nevertheless, blood testing and expectoration are principally suggested when it comes to the estimation of inflammatory allergy (asthma and rhinitis).3,4The aim of this work was to determine the role of the eosinophilic activity in the bronchial inflammation and allergenic sensitivity within an asthmatic paediatric population. Thus, after establishing a general description of the population under study through the questionnaire, we evaluated the eosinophilia and serological ECP values in order to correlate them with the clinical parameters and study their variations depending on the age of the patients.
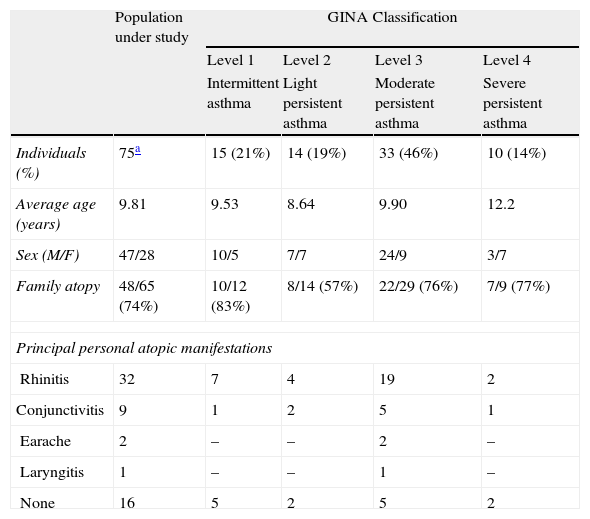
PatientsDuring their medical consultations, specialised doctors (ENT doctors, paediatricians, lung specialists) proceeded to the selection of the patients and the elaboration of the questionnaire. The study concerns 75 asthmatic people of Annaba (Algeria) between 4 and 18 years old (average of 9 years old) including 47 males and 28 females (sex-ratio of 1.64 in favour of the males). Table 1 shows the general description of the population under study.
General description of the patients.
| Population under study | GINA Classification | ||||
| Level 1 | Level 2 | Level 3 | Level 4 | ||
| Intermittent asthma | Light persistent asthma | Moderate persistent asthma | Severe persistent asthma | ||
| Individuals (%) | 75a | 15 (21%) | 14 (19%) | 33 (46%) | 10 (14%) |
| Average age (years) | 9.81 | 9.53 | 8.64 | 9.90 | 12.2 |
| Sex (M/F) | 47/28 | 10/5 | 7/7 | 24/9 | 3/7 |
| Family atopy | 48/65 (74%) | 10/12 (83%) | 8/14 (57%) | 22/29 (76%) | 7/9 (77%) |
| Principal personal atopic manifestations | |||||
| Rhinitis | 32 | 7 | 4 | 19 | 2 |
| Conjunctivitis | 9 | 1 | 2 | 5 | 1 |
| Earache | 2 | – | – | 2 | – |
| Laryngitis | 1 | – | – | 1 | – |
| None | 16 | 5 | 2 | 5 | 2 |
On the basis of the questionnaire, two different scores were obtained: A first one, named “allergological”, with values ranging from 1 to 4, takes into account all of the atopic manifestations associated with the patients’ bronchial hyperreactivity. The second score resents asthma severity and its values, ranging from 1 to 4, which correspond to the four levels described by the GINA classification.5
Determination of eosinophiliaA blood sample had to be collected on EDTA in order to determine the leukocytic formula. A smear test was then carried out and fixed with methanol for 2 to 3min before exposure to the May–Grünwald–Giemsa coloration procedure. We could then proceed to the calculation of the eosinophil percentage by counting at least 200 cells, all leukocytic cell categories included, under the microscope. Eosinophilia is expressed as an absolute value (number of elements per μl), after carrying out a globular enumeration using a coulter. We consider 500elements/μl as the threshold value.6
Measurement of the eosinophil cationic proteinA blood sample was taken and immediately centrifuged at 12,000rpm for 10min; ECP amounts could then be measured by chemiluminescence as described previously in the Immulite 2000 (DCP).7 The normal value is 20ng/ml.
Measurement of serological IgEMeasurement of total IgE (IgEt)This sequential immunoenzymatic test (‘sandwich’ type) has been described previously by Addison,8 It was carried out using the Beckman Coulter™ immunoanalysis Access system. The results are given in UI/ml (1UI/ml corresponding to 2.4ng of IgE). The values considered as normal are the following: 0.01–60UI/ml (from 1 to 5 years old); 0.01–90UI/ml (from 6 to 9 years old); 0.01–200UI/ml (from 10 to 15 years old); 0.01–100UI/ml (over 15 years old).
Measurement of specific IgE (IgEs)We carried out an automated qualitative test using the VIDAS system (Stallertest),9 allowing the detection in serum of specific IgEs from a determinate mixture of the 10 allergens most frequently involved in allergies: tree pollen (birch: T3 and olive tree: T9), grass (orchard grass: G3), herbaceous (parietary: W21 and mugwort: W6), animal hair (cat: E1 and dog: E2), an acarid (Dermatophagoïdes pteronyssinus: D1), a fungus (Alternia: M6) and an insect (Blattela germanica: I6). A ‘value from the test’ (VT) is automatically calculated by the device against a pre-memorised standard S1. The result of the test is positive when VT ≥0.70. Moreover, IgEs specific to two individualised pneumallergens (D1: D. pteronyssinus and I6: B. germanica) are detected (Immulite 2000, DPC).10 In this last case, the limit of detection is 0.35kU/L.
Ethical approvalThe study was performed in accordance with Good Clinical Practice and was approved by the local Ethics Committee. Written informed consent was obtained from each patient's parents or legal guardian.
Statistical studyPearson's correlation coefficient as well as Student's t-test results was obtained from the statistical analysis software Minitab 13. Differences between the qualitative variables were analysed using the χ2 and Fisher's exact tests through the software SAS version 8.02. A value of p<0.05 is statistically significant.
ResultsClinico-biological correlationsThe mean values of the different parameters under study are shown in Table 2. The GINA score was considered positive when over the first level and a positive allergological score was obtained when at least two allergological symptoms were observed.
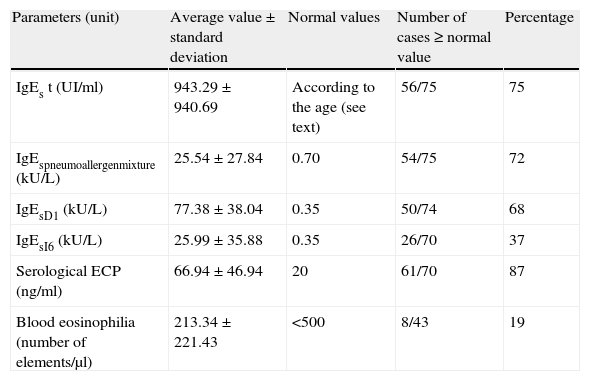
Immunobiological parameters studied: average values and number of positive cases.
| Parameters (unit) | Average value±standard deviation | Normal values | Number of cases≥normal value | Percentage |
| IgEs t (UI/ml) | 943.29±940.69 | According to the age (see text) | 56/75 | 75 |
| IgEspneumoallergenmixture (kU/L) | 25.54±27.84 | 0.70 | 54/75 | 72 |
| IgEsD1 (kU/L) | 77.38±38.04 | 0.35 | 50/74 | 68 |
| IgEsI6 (kU/L) | 25.99±35.88 | 0.35 | 26/70 | 37 |
| Serological ECP (ng/ml) | 66.94±46.94 | 20 | 61/70 | 87 |
| Blood eosinophilia (number of elements/μl) | 213.34±221.43 | <500 | 8/43 | 19 |
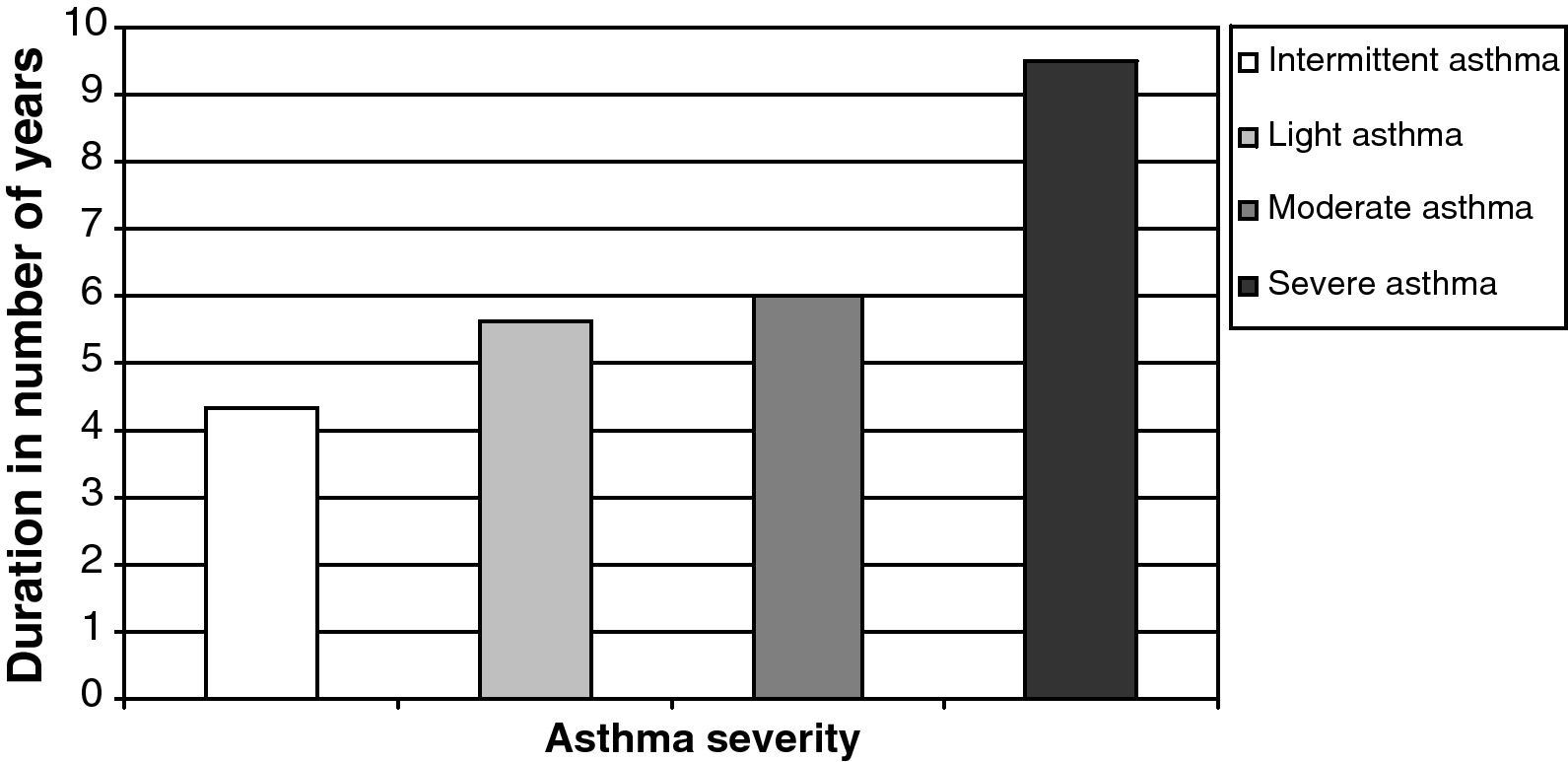
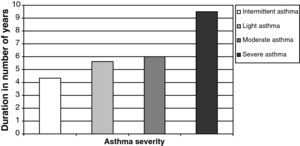
We thus noticed a relation between asthma severity, depicted by the GINA score, and the ECP (p=0.03). However, we did not notice any significant relation between asthma severity and blood eosinophilia. Eosinophilia only correlated with the length of time the patient has suffered from the disease (Pearson's correlation=0.389, p=0.013). Thereby, the duration of the disease increases along with the GINA levels from four years for the first level up to an average of nine and a half years for the last one (Fig. 1).
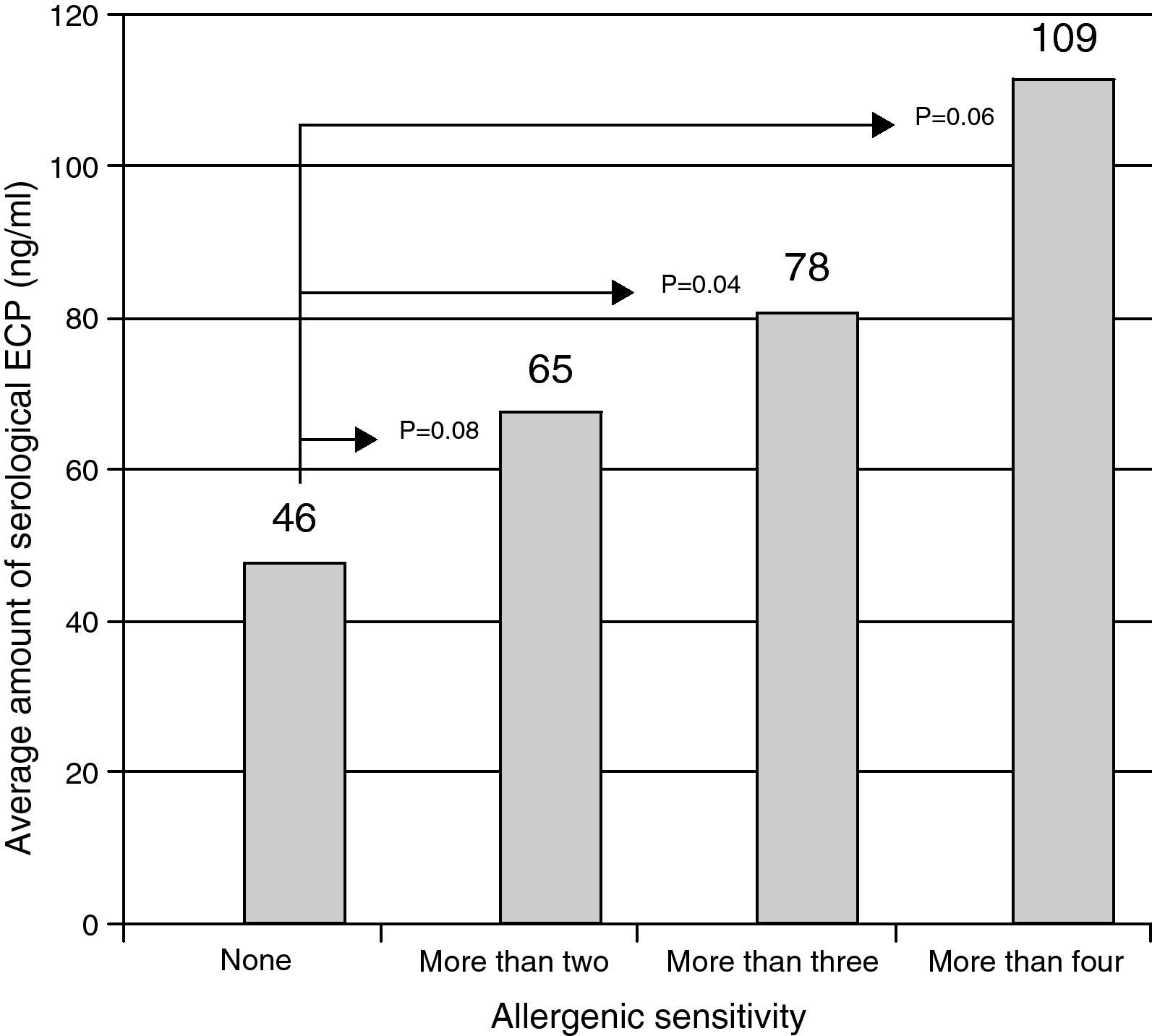
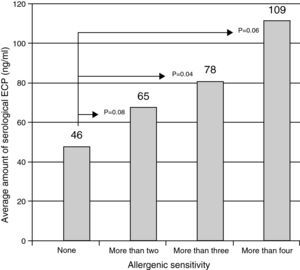
The variation of ECP levels in function of the number of known sensitivities among the children. There was a significant increase of serological ECP (ng/ml) as a function of allergen sensitisation. In fact p=0.04 for children over three sensitisations compared with children who have no awareness detected by the tests we made.
The ECP values are correlated with the total IgEs values (r=0.54) and with those of the IgEs against the pneumoallergens (r=0.65). Fig. 2 illustrates the increase in the ECP average value in function of the level of children's sensitivity.
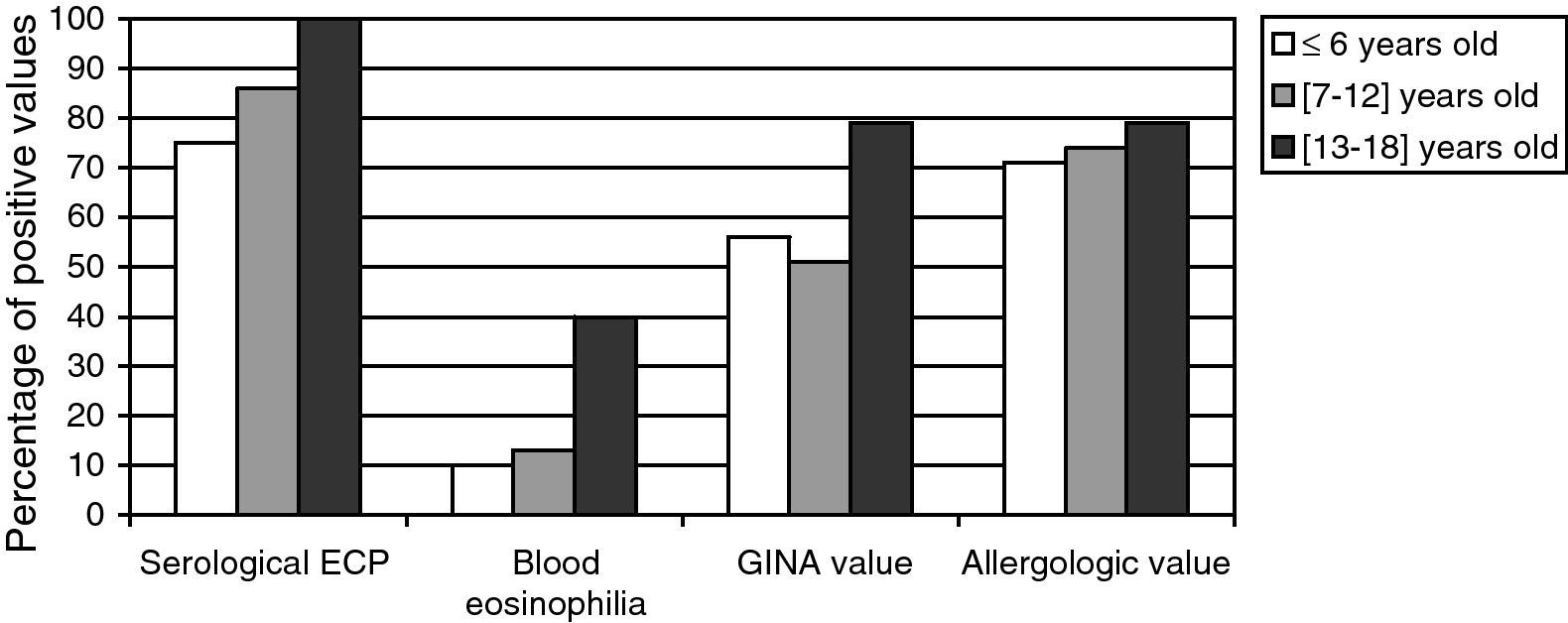
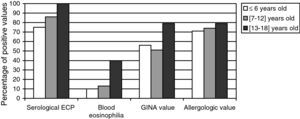
Relation between the age and both the biological and clinical parametersWe divided the whole population into three age brackets: from 4 to 6, 7 to 12, and 13 to 18 years old. The results are expressed in terms of values obtained which were higher than the normal ones, for each age bracket (Fig. 3). All the considered parameters (asthma severity and asthma-associated symptoms) increase with the age of the patients. Moreover, the eosinophilic activity also seems to increase with age.
DiscussionLooking at the general description of the population under study, we notice that most of the children suffer from a grade 3 moderate persistent asthma (46%) with an average age of nine. Very few suffer from severe asthma (14%) and their average age is higher, around 12 years old. Yet, precisely, most types of asthma in children are light or moderate during adolescence. Nevertheless, asthma can become worse for various reasons, such as: the loss of the referral doctor, the denial of the disease, smoking habits and even a bad diagnostic or a cessation of the basic medical treatment.
Associated allergic signals, such as rhinitis and conjunctivitis (being the most frequently observed, 32 and 9 times, respectively), lead to suspicion of an atopic component. Indeed, the association of rhinitis and bronchial hyperreactivity is frequent11 and the role of the inflammation of the superior airways of the respiratory system on the appearance of bronchial hyperreactivity has been widely described in literature.12,13 This can be explained by the existence of direct interactions between the inflammatory nasal suffering and the superior airways of the respiratory system, probably through the diffusion of inflammation mediators from the nasal cavity.14 Our study concerned children with high familial atopic risk, as the family atopic field seems more pronounced than in other paediatric studies, with 74% of family atopy antecedents compared to 70.5% for Moneret-Vautrin for example.15 We also observe that paternal atopy is more predominant (1.6 times) than the maternal one.
We know that neither the diagnostic nor the appreciation of the intensity of the allergic inflammation depends on the amount of inflammation acute proteins, synthesised by the liver, as cytokines Th2 do not induce this synthesis. Therefore, the biological parameters leading to the identification of the allergic inflammation are the cytokines Th2 along with the mediators of the effective cells, especially eosinophils. The screening of the latter is thus proposed for the evaluation of bronchial hyperreactivity. Just et al.16 have previously shown that among children with asthma, eosinophilic inflammation was closely related to respiratory allergy. Several other studies17,18 suggest the existence of a relation between eosinophilic inflammation (ECP) and asthma severity. In our study, eosinophilia was correlated with the duration of the disease. In fact, the persistence of asthma over a lengthy period could be associated with a restructuration of the superior respiratory airways due to the activation of eosinophils and the resulting release of deleterious proteins such as ECP, proceeding from the intracytoplasmic granules which, once liberated, cause damage to the epithelium of the airways, shedding, and an increase in bronchial hyperreactivity.19 Indeed, we notice that the ECP serological rate is related to the asthma severity as already described by several authors.20,21 However, we have not found any significant correlation between blood eosinophilia and asthma severity.
The sensitivity level and allergenic environment of the children that have been discussed in a previous study,22 will not be contemplated here. In addition, we notice that the rates of total and specific IgEs are both in accordance with the ECP amounts. We observe an increase in the average amount of serological ECP with the sensitivity level of the children. Our results seem to confirm those obtained in the study conducted by Fauquert et al., in which the authors analysed the value of a selective measurement of serological ECP and observed that its average amount was significantly higher in cases of allergy provoked by mites.23 The same observation was made in several other studies showing that polysensitive asthmatic children, especially those allergic to dust mites, pollen and domestic animals, showed high ECP levels compared to monosensitive ones.
Various studies suggested that during childhood, the appearance of allergic sensitivities and then the development of clinical signals follow a particular chronology, which depends on family inheritance as well as on exposition, more or less important or lasting, to environmental allergens.22,24 In our work, the children are divided within three age brackets which differentiate roughly the preschool, school, and adolescence phases. We find an increase in eosinophilic activity, asthma severity and associated symptoms in accordance with age. This observation suggests that, for asthmatic children, a high ECP serological value could represent a risk factor in asthma persistence and may be a potential marker of the severity of the inflammation.25
ConclusionIn conclusion, the present data show that eosinophilic activity represented by ECP (more than eosinophilia itself) seems to be a good biological marker of allergic asthma, reflecting its severity as well as the allergenic sensitivity level. In addition, we notice a statistically significant increase in accordance with age, affecting not only the amounts of IgEs against the pneumallergen mixture, acarids and cockroaches but also the global sensitivity level of the children.
Conflict of interestThe authors have no conflict of interest to declare.
We are sincerely grateful to the “Agence Universitaire de la Francophonie” for its support in the form of a grant allocation for research training as well as to the firm “Siemens” (ex-DPC), especially to Mrs S. Grillère (from Company Gen-Probe), for kindly providing us with the major part of the reagents used for this study. We thank the Doctors from Annaba who participated in the recruitment of the patients: Aidaoui, Amiri, Bechtella, Bouhadeb, Bouhouche, Boukertouta, Boumaza, Chahlef, Debez, Demmak, Derouiche, Fezzari, Guedjati, Malki, Nacer and Tarfaya. Finally, we also thank the heads of department and all the technicians from Chekat and Bensalah laboratories as well as those from the immunology laboratory of the CHU Clermont Ferrand.