Annexin A1 (ANXA1) is an important anti-inflammatory mediator that may play a significant role in bronchial asthma. MiR-196a2 can target ANXA1 and therefore may play a role in the pathogenesis of asthma.
Aim of studyThis is the first study which aimed to evaluate the expression of miR-196a2 in the serum of asthmatic children and correlate its expression with ANXA1 serum level and asthma severity.
Subjects and methodsThe study included 100 asthma patients who were subdivided into three groups (mild, moderate and severe) and 50 healthy control subjects. Assessment of miR-196a2 expression and ANXA1 serum level were done using quantitative reverse transcriptase PCR (RT qPCR) and Elisa techniques, respectively.
ResultsCompared to the control group, asthmatic children showed an increased ANXA1 serum level and decreased expression of miR-196a2 (p=0.001). However, ANXA1 serum level was lower and miR-196a2 expression was higher in severe asthmatic patients compared to moderate asthmatic ones (p=0.01, 0.03). Pearson's correlation coefficient revealed no significant correlations between ANXA1 serum level and miR-196a2 expression in the patient group (p=0.9).
ConclusionsAltered miR-196a2 expression and serum ANXA1 concentration may play a role in the pathogenesis of asthma. In addition, ANXA1 and miR-196a2 may represent potential diagnostic biomarkers for asthma and future targets for therapy.
Asthma is a chronic inflammatory respiratory disease. It is characterized by airway obstruction due to hyper-responsiveness of smooth muscles associated with inflammatory reactions.1 It is considered as one of the leading chronic diseases in children and its management during exacerbation represents an economic health problem, especially in developing countries such as Egypt.1,2 It remains challenging to generate a risk assessment model and determine proper treatment for asthmatic children. Therefore, several biomarkers have been developed to understand the pathogenesis of the disease for better management.
MicroRNAs (miRNAs) are small (∼22-nucleotide) non-protein-coding RNAs that pair with 3′-untranslated regions of messenger RNAs (mRNAs) to regulate their expression.3 There is accumulating evidence recently which supports the vital role of miRNAs in the development and progression of various human diseases.4,5 Moreover, emerging evidence also indicates the role of miRNAs in allergic airway diseases by modulating the expression of various inflammatory genes.2,4
MiR-196a2 can target annexin A1 (ANXA1), also known as lipocortin 1 and calpactin II.5 ANXA1 is an important endogenous anti-inflammatory mediator that may play a significant role in bronchial asthma through regulating the expression and function of different inflammatory enzymes such as phospholipase A2, cyclooxygenase 2 (COX2), and inducible nitric oxide synthase (iNOS). Therefore, through regulating annexin A1, miR-196a2 may exert a prominent effect in asthmatic children.6 However, prior studies of miR-196a2 in asthma have only focused on the polymorphisms in the miR-196a2 gene in asthmatic patients.2,4 The current study is the first study that aimed to study the expression of miR-196a2 in the serum of asthmatic children and correlate its expression with ANXA1 serum level and asthma severity.
Subjects and methodsSubjectsThis case control pilot study included 100 asthmatic children chosen randomly, albeit respecting the study's inclusion and exclusion criteria from Abu-El Reish Hospital, Kasr-El Aini Faculty of Medicine, Cairo University. Asthma was diagnosed in accordance with the Global Initiative for Asthma (GINA) guidelines.7 The severity of asthma was assessed based on the spirometric staging according to GINA guidelines7 and accordingly, patients were subdivided into mild (16 patients), moderate (50 patients), and severe (34 patients) groups.
Patients having any of the following criteria were excluded: a diagnosis of chronic obstructive pulmonary disease or any current respiratory disorder other than asthma, receiving antihistamine, systemic, or topical corticosteroids in the month prior to the study, having associated heart, renal, or liver diseases, diabetes mellitus, or any other systemic autoimmune, malignant, or metabolic disease.
The study also included 50 age-and sex-matched children with no previous or family history of respiratory, allergic, or other systemic autoimmune or endocrinal diseases.
Informed consent was obtained from the parents of all included subjects. The study was performed in accordance with the 1983 Helsinki Declaration.
Methods- -
All participants were subjected to full clinical examination and routine laboratory investigations.
- -
Serum ANXA1 level was measured using an ELISA kit supplied by Novus Biologicals, USA; catalog number: NBP2-60538.8
- -
Expression of miR-196a2 was assessed using reverse transcriptase quantitative PCR (qRT-PCR) technique. RNA was isolated using RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions for miRNA purification. Then the extracted miRNA was reverse transcribed using a miScript reverse transcription (RT) kit (Qiagen, Hilden, Germany) and the cDNA specimens were stored at −80 C. We performed qRT-PCR analysis using miScript SYBR green PCR kit (Qiagen, Hilden, Germany) for the detection of miR-196a2 (Assay ID MS00008960). Quantitative PCR was run on Applied technologies, Stratagene Mx3000P, according to the manufacturer's recommendations. Melting curves were used to evaluate the reliability of PCR results. The resulted miRNA data were calculated in relation to the endogenous control RNU6B primer. Relative quantity (RQ) of miR-196a2 was calculated by the formula: (RQ=2−ΔΔCt)9
Analysis of data was performed using SPSS version 17 software for Windows. Qualitative data were presented as frequencies, while quantitative data were presented as mean and standard deviation (SD). Non-parametric distributed data including miR-196a2 expression and serum ANXA1 were presented as median and interquartile range (IQR). One-way ANOVA analysis was used for multiple group comparisons. Pearson's correlation coefficient was used for correlating miR-196a2 expression and serum ANXA1 with other quantitative parameters. Regression analysis was performed to confirm the association of significant miR-196a2 expression and serum ANXA1 with bronchial asthma after the adjustment of associated covariates. Receiver operating characteristic (ROC) analysis was used to assess the biomarker potential for asthma in children. A p-value<0.05 was considered statistically significant.
Insilco analysis- -
The network of gene–gene interaction for ANXA1 was utilized through String online server (http://string-db.org/).
- -
The network of miRNA interaction for ANXA1 was utilized through miRTargetLink Human (https://ccb-web.cs.uni-saarland.de/mirtargetlink/index.php)
- -
Genomic data for miR-196a2 were retrieved from GeneCard Human Gene Database (https://www.genecards.org) and mirbase database (http://www.mirbase.org).
- -
The predicted miRNA target genes were analyzed for gene ontology (GO) terms and Kyoto encyclopedia of genes and genomes (KEGG) enrichment pathway analysis using miRPathDB v1.1 (https://mpd.bioinf.uni-sb.de/overview.html).
- -
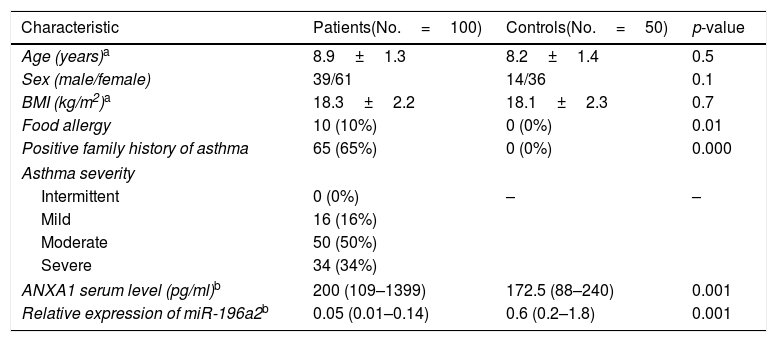
The demographic, clinical, and laboratory characteristics of the subjects included in this study are shown in Table 1. There were no statistical differences between the studied groups regarding age, sex, or body mass index (BMI) (p>0.05).
Table 1.Characteristics of the studied groups.
Characteristic Patients(No.=100) Controls(No.=50) p-value Age (years)a 8.9±1.3 8.2±1.4 0.5 Sex (male/female) 39/61 14/36 0.1 BMI (kg/m2)a 18.3±2.2 18.1±2.3 0.7 Food allergy 10 (10%) 0 (0%) 0.01 Positive family history of asthma 65 (65%) 0 (0%) 0.000 Asthma severity Intermittent 0 (0%) – – Mild 16 (16%) Moderate 50 (50%) Severe 34 (34%) ANXA1 serum level (pg/ml)b 200 (109–1399) 172.5 (88–240) 0.001 Relative expression of miR-196a2b 0.05 (0.01–0.14) 0.6 (0.2–1.8) 0.001 BMI=Body mass index, ANXA1=Annexin A1.
- -
Serum ANXA1 and miR-196A2 levels in the control group ranged from 85 to 270 (pg/ml) and from 0.1 to 2.8, respectively. Compared to the control group, asthmatic children showed increased serum AnXA1 level and decreased miR-196a2 expression (Table 1). The level of miR-196a2 expression was four-fold higher in healthy subjects compared to asthmatic children.
- -
Linear regression analysis confirmed the association of serum ANXA1 level and relative expression of miR-196a2 with asthma after the adjustment of age, sex, and BMI (p=0.01 and 0.0001, respectively).
- -
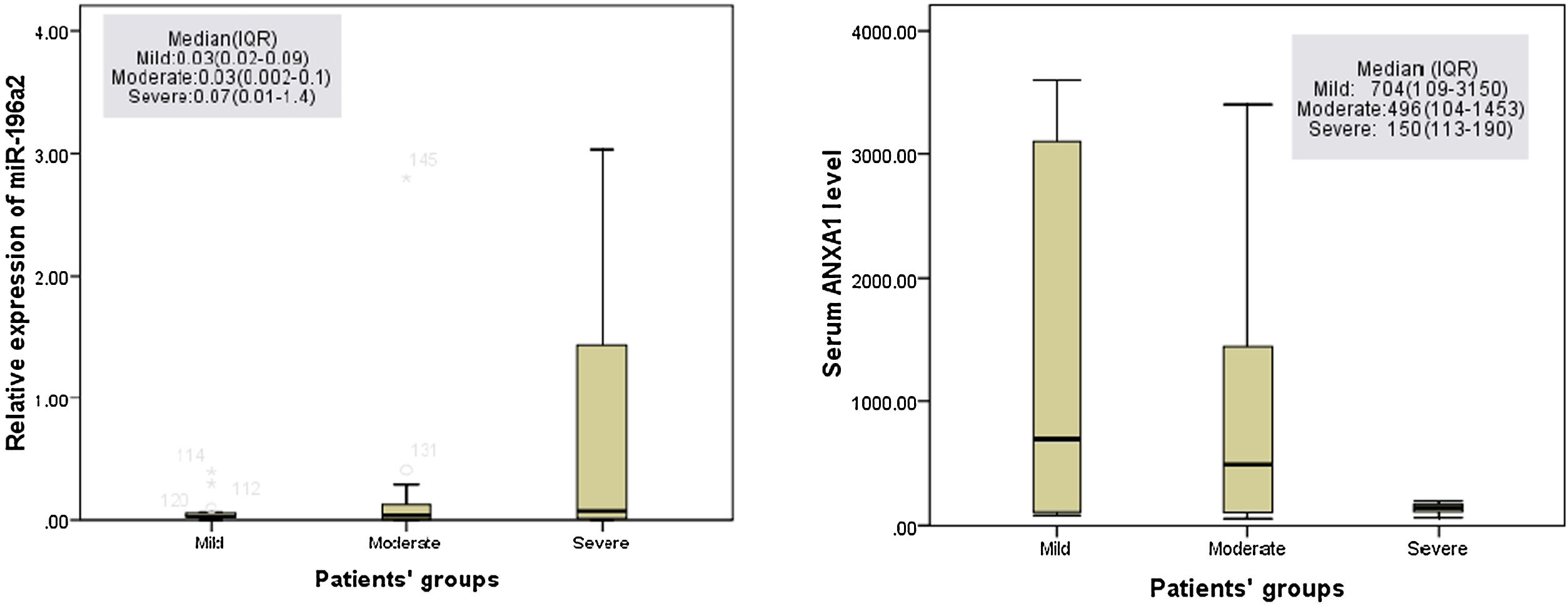
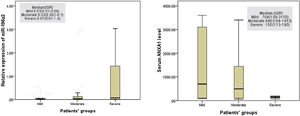
Regarding the patient group, no significant difference was detected in serum ANXA1 level between mild and moderate (p=0.3) or severe asthmatic patients (p=0.1). However, serum ANXA1 level was lower in severe asthmatic patients than that in moderate asthmatic ones (p=0.01) (Fig. 1).
Figure 1.Annexin A1 (ANXA1) protein level in the serum of asthmatic patients’ groups showed that no significant difference was detected in serum ANXA1 level between mild asthmatic patients and moderate (p=0.3) or severe asthmatic patients (p=0.11), while ANXA1 serum level was lower in severe asthmatic patients compared to moderate asthmatic patients (p=0.01). On the other hand, the expression of miR-196a2 showed only a statistical difference between moderate and severe asthma patients (p=0.03). No statistical difference was detected between mild asthmatic patients and moderate (p=0.9) or severe asthmatic patients (p=0.1). Data was shown as median and IQR (interquartile range).
- -
The only statistical difference reported in the expression of miR-196a2 was between moderate and severe asthmatic patients (p=0.03). Severe asthmatic patients showed seven-fold higher miR-196a2 expression than moderate asthmatic patients. However, no difference in the gene expression was detected between mild, and moderate (p=0.9) or severe asthmatic patients (p=0.1) (Fig. 1).
- -
Regarding the patient group, using Pearson's correlation coefficient revealed no significant correlations between ANXA1 serum level and miR-196a2 expression (p=0.9).
- -
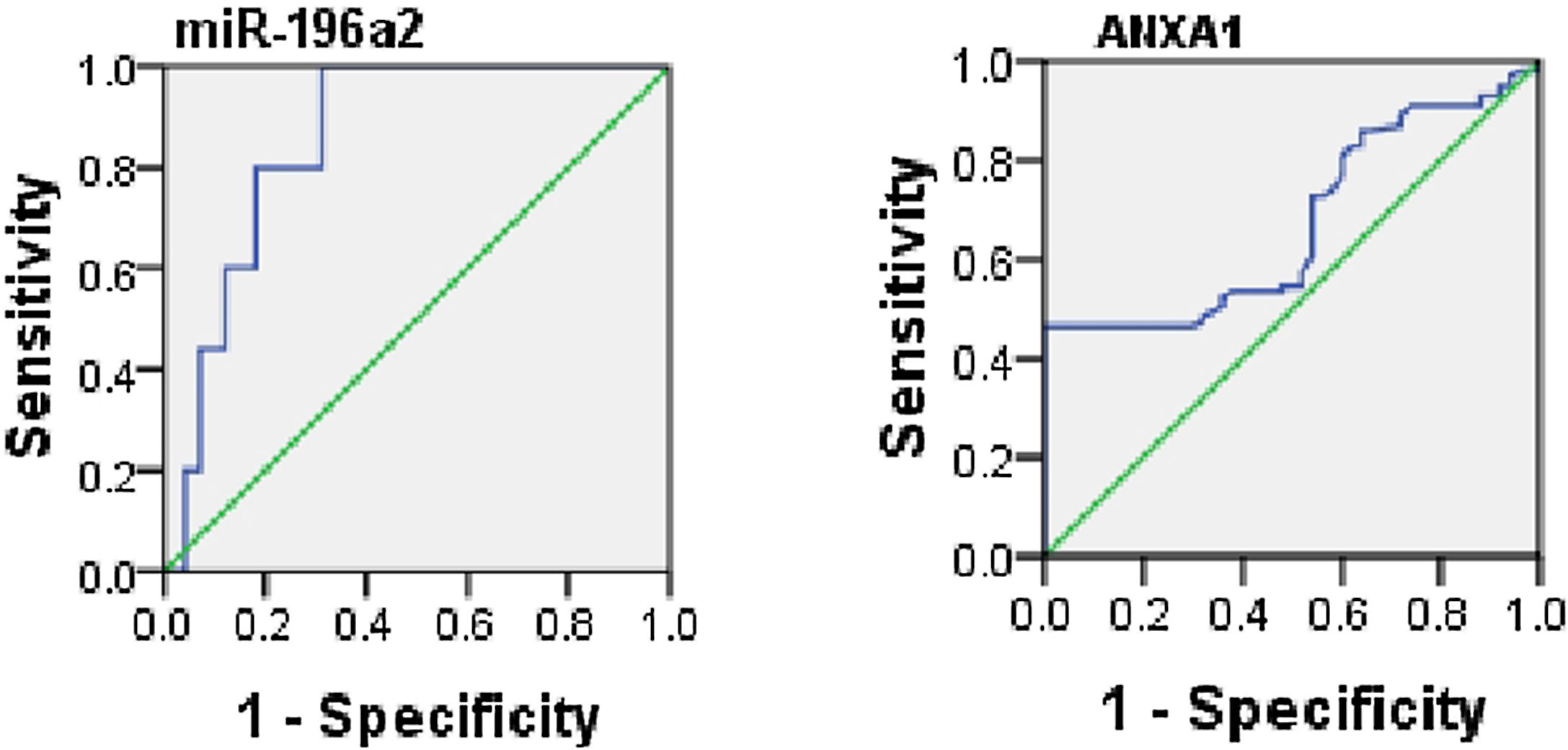
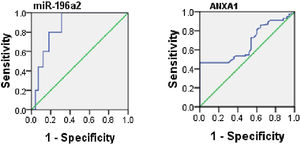
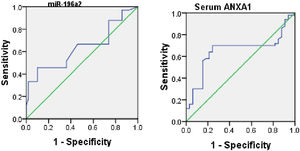
ROC analysis was performed to evaluate the usefulness of serum ANXA1 and miR-196a2 expression as potential blood-based biomarkers for asthma and it yielded AUC of 0.66 for serum ANXA1 (95% CI, 0.6–0.77; p=0.001) and 0.8 for miR-196a2 expression (95% CI, 0.7–0.9; p=0.001) (Fig. 2).
- -
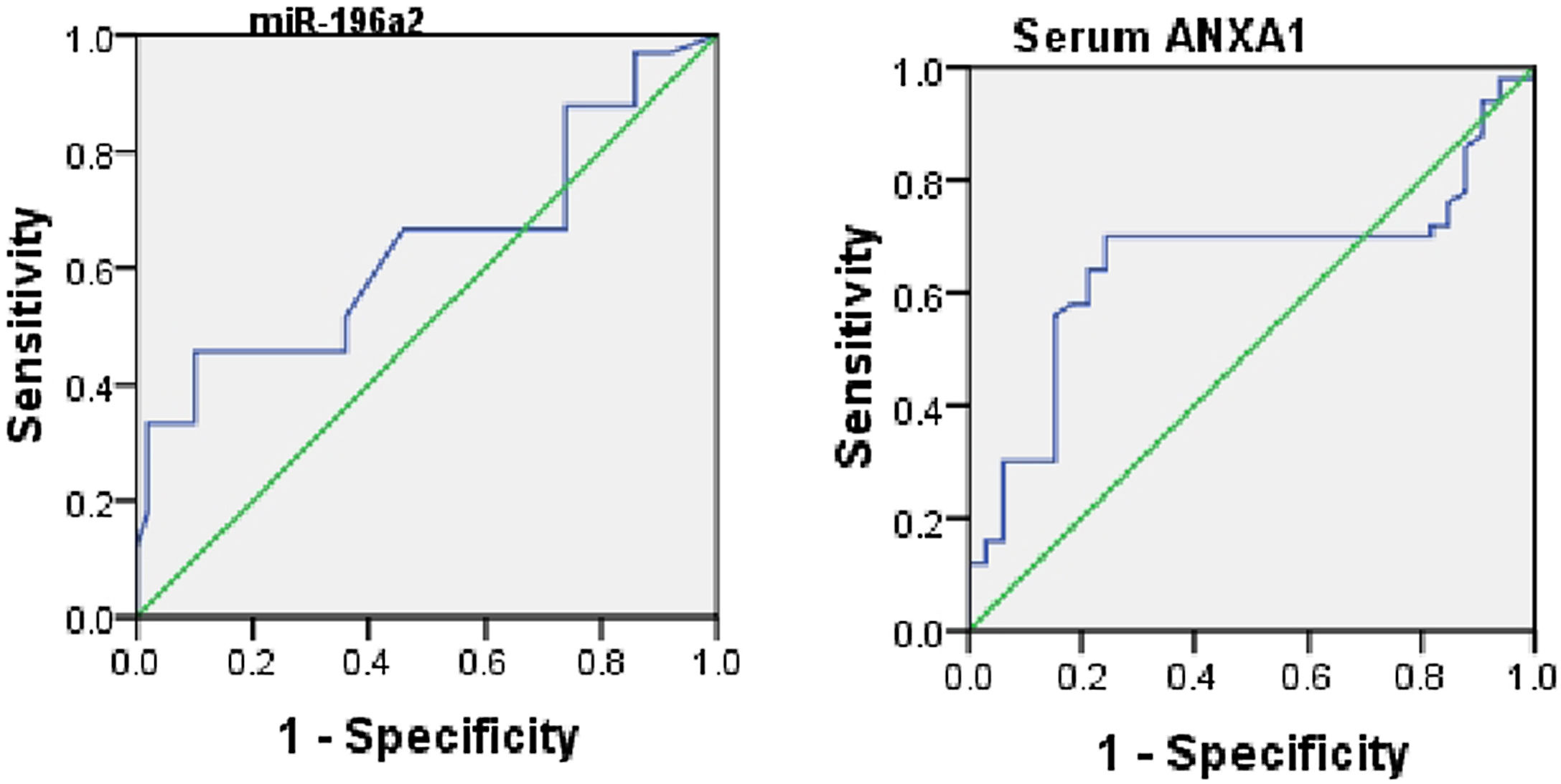
When assessing the ability of ANXA1 and miR-196a2 in differentiating between moderate and severe asthmatic patients, an ROC analysis was performed, which yielded AUCs of 0.6 for ANXA1 (95% CI, 0.53–0.77; p=0.01) and 0.65 for miR-196a2 (95% CI, 0.58–0.76; p=0.03) (Fig. 3).
- -
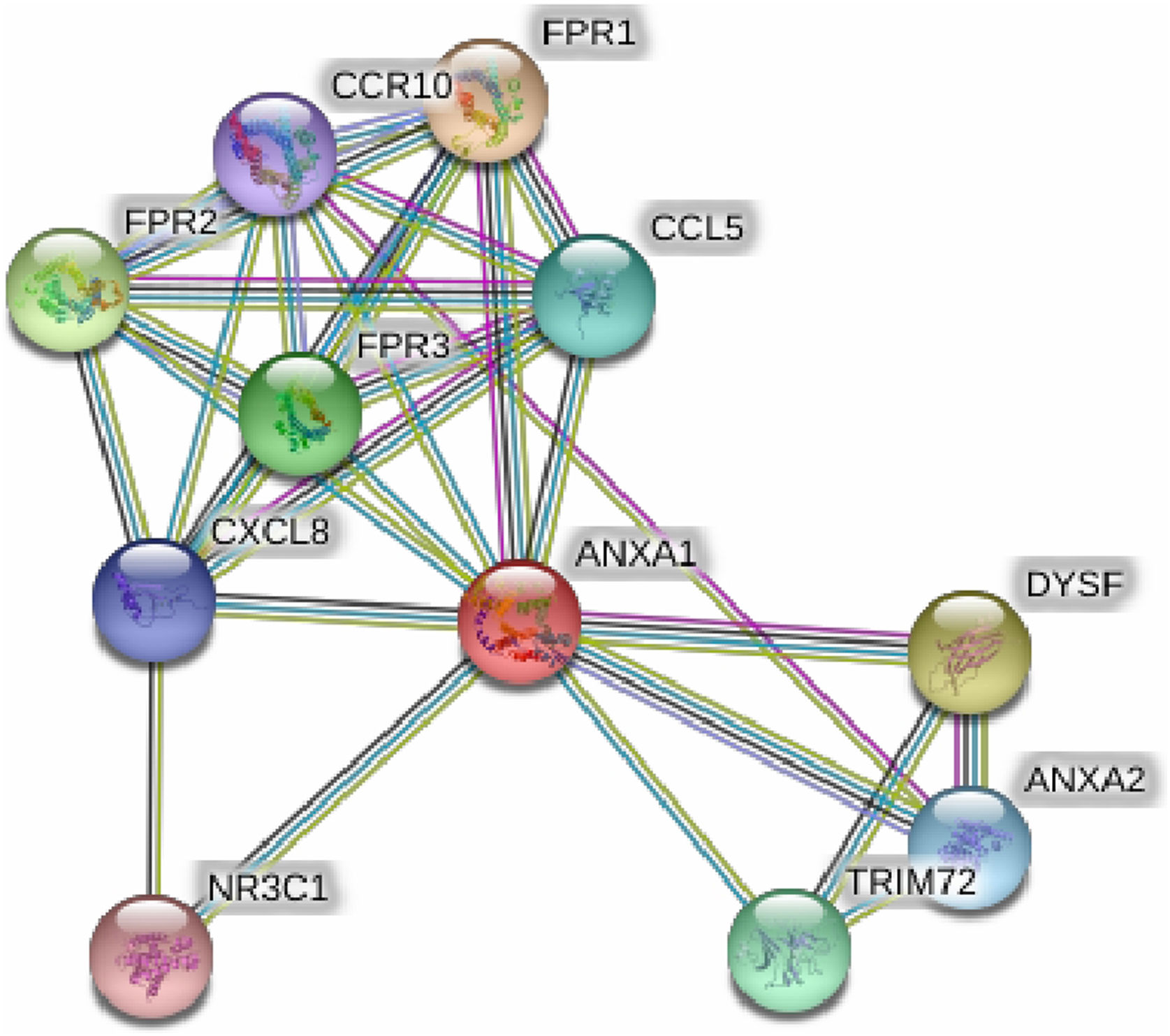
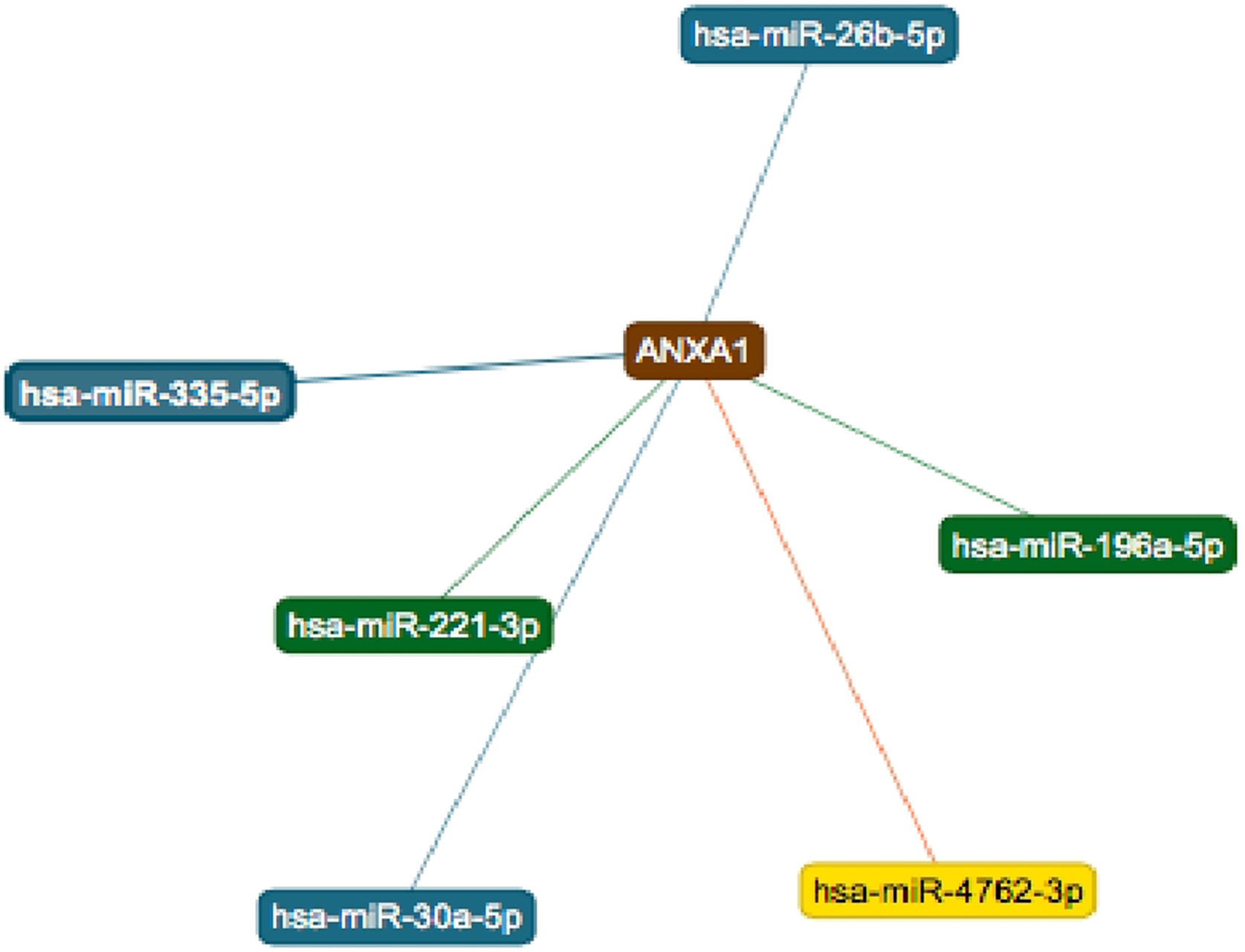
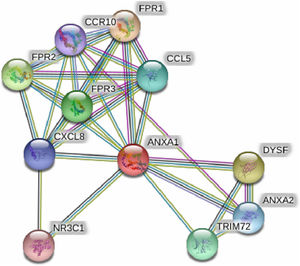
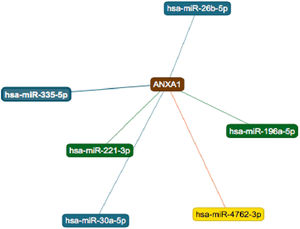
String online server indicated that ANXA1 gene interacts with numerous genes, playing a role in the inflammatory reaction in bronchial asthma. The network of at least 10 gene–gene interactions has been illustrated in Fig. 4. Moreover, miRTargetLink found other microRNAs that target ANXA1 in addition to miR-196a2 which showed strong support interaction (Fig. 5).
Figure 4.Human Annexin A1 interactions network with other genes that play a significant role in bronchial asthma obtained from String server. At least 10 genes have been indicated to correlate with Annexin A1 gene and play a significant role in bronchial asthma. These genes included: FPR1; fMet-Leu-Phe receptor, FPR2; N-formyl peptide receptor 2, FPR3; N-formyl peptide receptor 3, DYSF; Dysferlin, TRIM72; Tripartite motif-containing protein 72; CCL5; C-C motif chemokine 5, ANXA2; Annexin A2, CXCL8; Interleukin-8; CCR10; C–C chemokine receptor type 10, NR3C1; Glucocorticoid receptor an Isoform Alpha-D3.
Figure 5.Six microRNAs that target ANXA1. The two strong interactions, including its interaction with miR-196, appear in green. The three interactions with weak support appear in blue and the one predicted interaction appear in yellow. Source: (https://ccb-web.cs.uni-saarland.de/mirtargetlink/index.php).
Asthma is the most common chronic illness in children; this has a major impact on the lifestyle. Despite the advances in asthma research, the factors contributing to the pathogenesis and exacerbation of this disease remain unclear, especially the epigenetic factors.
Previous publications have shown that miR-196a2 could target many genes enriched in cell cycle regulation and apoptosis and it has been reported to be deregulated in asthma and various cancer types.10,11 MiR-196a2 has recently gained a lot of attention in the research field of asthma. It can contribute in the disease pathogenesis by targeting multiple molecular and signaling pathways involved in the inflammatory and immunological biological processes which play a significant role in bronchial asthma. However, based on the previously published data, miR-196a2 has only been studied in asthmatic children on DNA level and these studies have revealed the association of miR-196a2 polymorphism with susceptibility to asthma.2,4 The present study is the first one to report decreased miR-196a2 expression in the serum of asthmatic patients compared to healthy subjects. However, moderate asthmatic patients showed higher miR-196a2 expression compared to severe asthmatic ones. One of the miR-196a2-targeted genes is ANXA1 which is a glucocorticoid induced 37-kDa protein with a wide range of physiological and pathological functions.12 It stimulates cell motility and cancer cell invasion via interaction with specific receptors,5 so it is deregulated in different types of human cancer.13 ANXA1 overexpression has been reported in pancreatic and hepatocellular carcinoma,14 while its down-regulation has been reported in breast and prostate cancer, suggesting the complexity of its function and regulatory mechanism.15–17 Furthermore, ANXA1 has also anti-inflammatory and immunosuppressive actions, and it is abundantly released in respiratory secretions.18,19 In this study, serum levels of ANXA1 were increased in asthmatic patients compared to healthy controls, which is in agreement with previous studies.5 This increase could be explained by the compensatory anti-inflammatory effects of ANXA1 in asthma. However, ANXA1 levels were also higher in bronchoalveolar lavage fluids in smokers20 and patients with cystic fibrosis.21 Eke et al. demonstrated a decreased ANXA1 level in a sample taken from wheezy children mentioning that this decreased level predisposes to a wheezy chest.8 However, increased level of ANXA1 may be associated with defective function of ANXA1 as it is reported that the inflammatory conditions of lung are associated with the release of defective ANXA1 with a molecular weight of 33 kDA rather than 37kDA ANXA1.22 Interestingly, we also reported a lower serum ANXA1 level in severe asthmatic patients compared to moderate asthmatic patients. This finding indicates that the circulating ANXA1 may be decreased due to ANXA1 increase in the target inflammatory site and this reduction may predispose to increased asthma degree due to the associated decrease in ANXA1 anti-inflammatory effect. The absence of significant differences in miR-196a2 expression and ANXA1 level between mild asthmatic patients and other patients could be attributed to two reasons: the first is the small sample size of mild asthmatic patients due to the nature of sample type (random sample), and the second is the high frequency of moderate and severe asthma between admitted patients due to a combination of poor access to health care and increased exposure to environmental factors which predisposes to asthma, such as passive smoking and perennial allergen exposure.23 An inverse correlation has been reported previously between miR-196a2 expression and ANXA1 expression and subsequently its protein level.10,24 Although we observed an inverse correlation between miR-196a2 expression and serum ANXA1 level, Pearson's correlation coefficient and regression analysis failed to confirm this relationship. Different mechanisms could be responsible for the regulation of ANXA1 expression other than miR-196a2 mediated repression and subsequently, these mechanisms may also affect its serum protein level. These mechanisms include: genomic deletions or coding mutations of the ANXA1 gene, promoter hypermethylation, defect in post-translational modifications of the protein by proteolysis or phosphorylation, and other miRNAs or gene-gene interactions as shown in Figs. 4 and 5.13 Thus, the qualitative and quantitative changes of ANXA1 may vary between tissues according to the nature of disease. Álvarez-Teijeiro also mentioned that miR-196 overexpression and ANXA1 under-expression offer a pathogenic mechanism in cancer. However, this inverse relationship is specific but not unique because both miR-196a2 and ANXA1 target other genes, including transcription factors that could modify their promoters to affect the transcriptional activity. Another reason is that the opposing qualitative effects are dependent on the cancer type studied.13
Conclusions and recommendationsOur findings showed that altered miR-196a2 expression and serum ANXA1 concentration may play a role in the pathogenesis of asthma. The expression of miR-196a2 and serum ANXA1 concentration may represent potential diagnostic biomarkers for asthma and future targets for therapy. However, prospective studies with a larger sample size are recommended to investigate the potential mechanism of miR-196a2 in asthma.
Ethical approvalThe research proposal was revised and approved by the ethical committee of the National Research Centre (NRC), Cairo, Egypt and in accordance with the 1975 Helsinki Declaration, as revised in 1983.
Conflict of interestAll authors declared no conflict of interest.
Informed consentInformed consent was obtained from all individual participants included in the study.