Thyroid and parathyroid surgery (TPTS) is associated with risk of injury to the recurrent laryngeal nerve, superior laryngeal nerve and voice changes. Intraoperative neuromonitoring (IONM), intermittent or continuous, evaluates the functional state of the laryngeal nerves and is being increasingly used. This means that points of consensus on the most controversial aspects are necessary.
ObjectiveTo develop a support document for guidance on the use of IONM in TPTS.
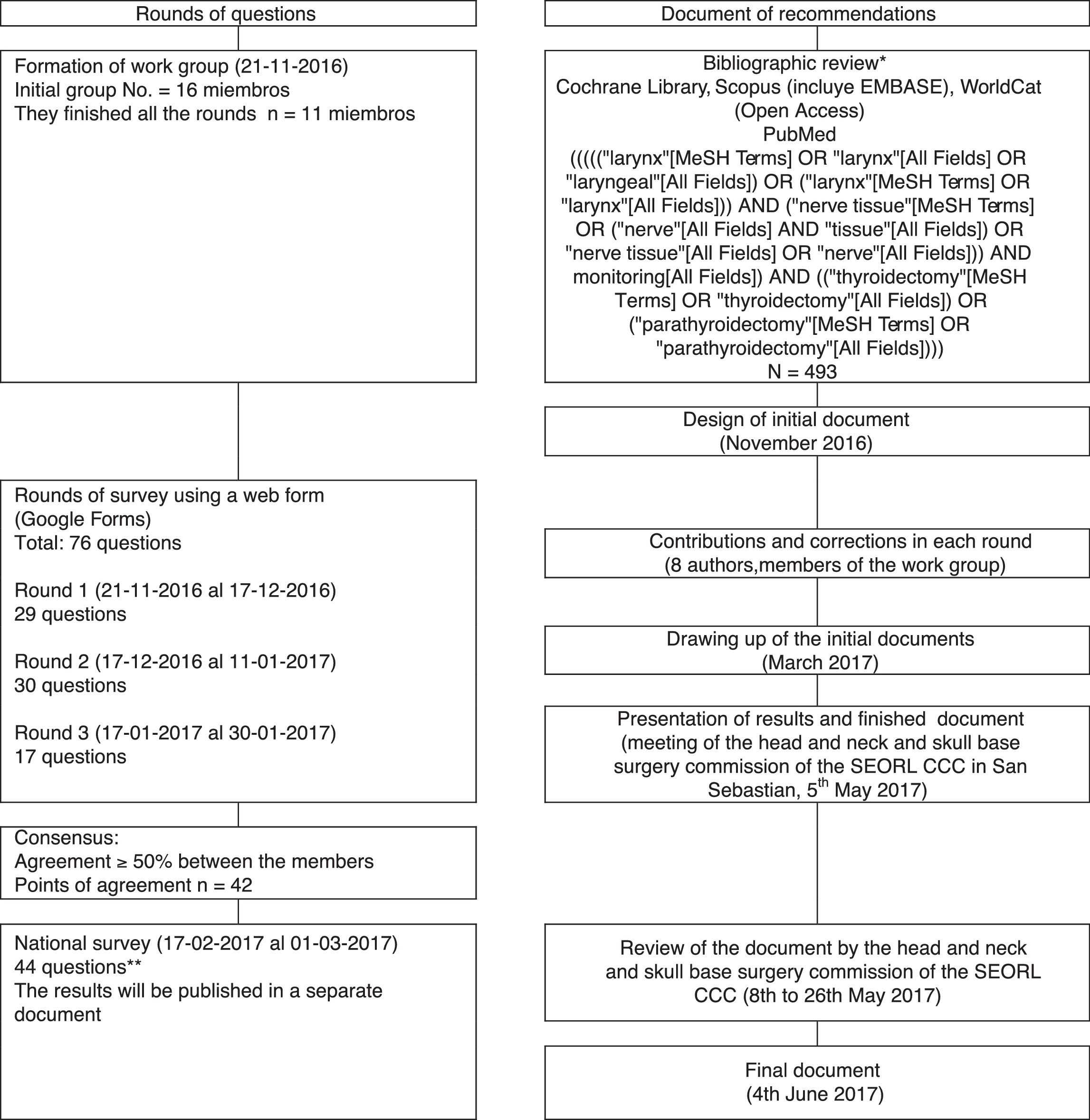
MethodWork group consensus through systematic review and the Delphi method.
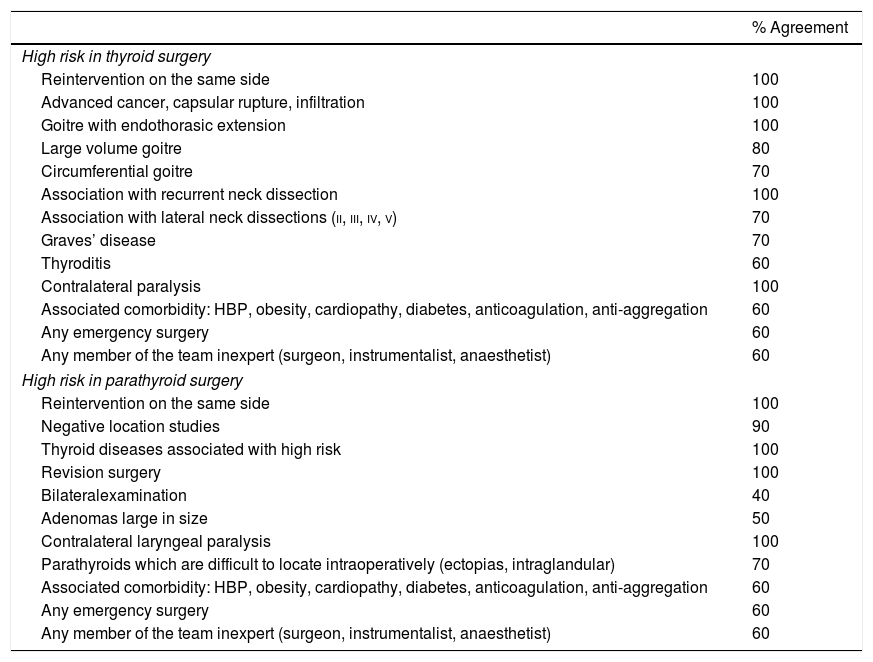
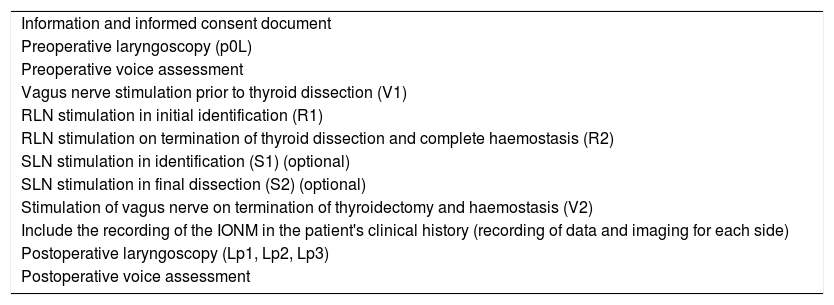
ResultsSeven sections were identified on which points of consensus were identified: indications, equipment, technique (programming and registration parameters), behaviour on loss of signal, laryngoscopy, voice and legal implications.
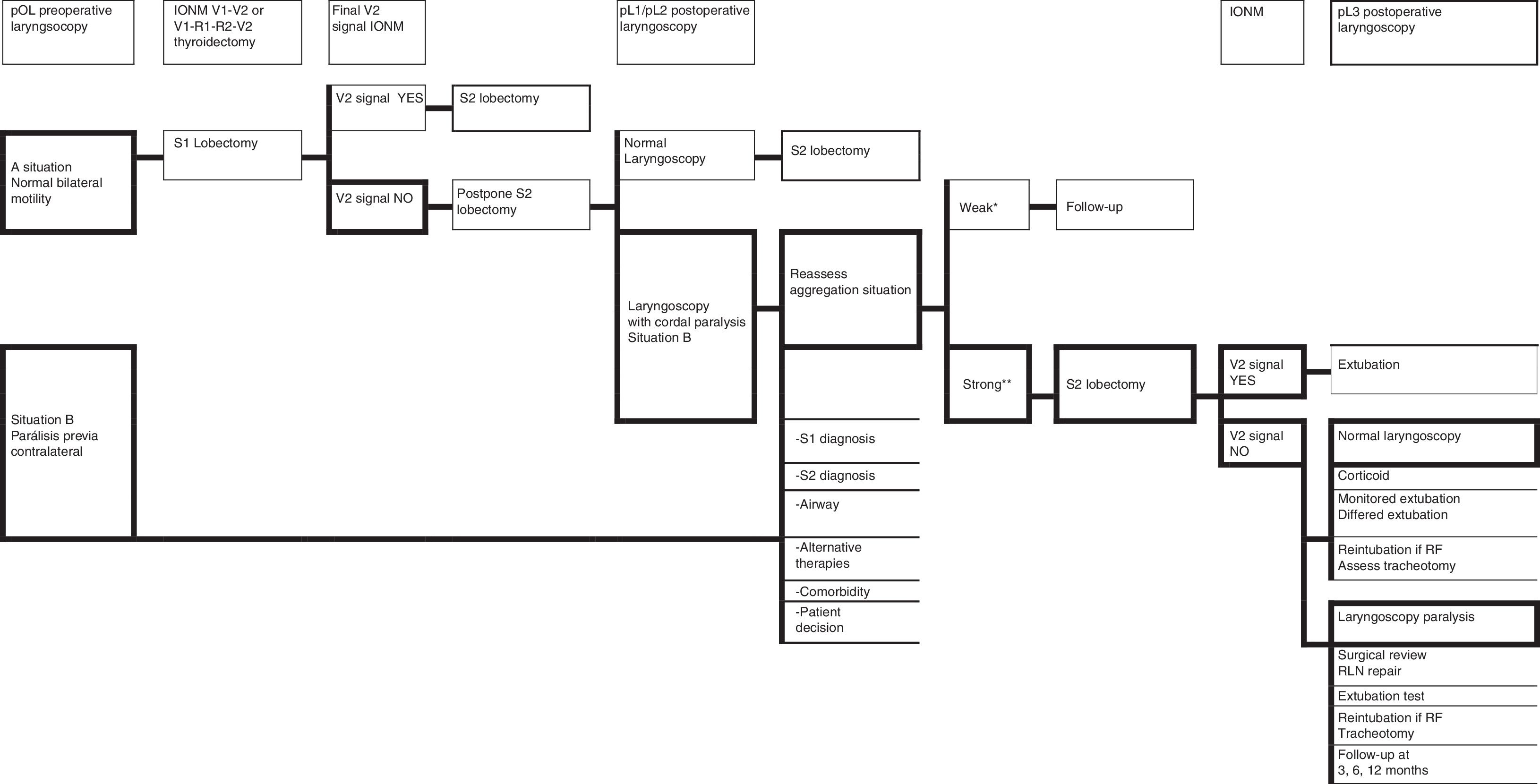
ConclusionsIONM helps in the location and identification of the recurrent laryngeal nerve, helps during its dissection, reports on its functional status at the end of surgery and enables decision-making in the event of loss of signal in the first operated side in a scheduled bilateral thyroidectomy or previous contralateral paralysis. The accuracy of IONM depends on variables such as accomplished technique, technology and training in the correct execution of the technique and interpretation of the signal. This document is a starting point for future agreements on TPTS in each of the sections of consensus.
La cirugía de tiroides y paratiroides (CTPT) se asocia a riesgo de lesión del nervio laríngeo recurrente, nervio laríngeo superior y cambios en la voz. La neuromonitorización intraoperatoria (NMIO), intermitente o continua, en CTPT evalúa el estado funcional de los nervios laríngeos y se utiliza progresivamente con más frecuencia. Esto obliga a adoptar puntos de acuerdo en los aspectos más controvertidos.
ObjetivoElaborar un documento de ayuda para orientar en la utilización de la NMIO en CTPT.
MétodoConsenso en grupo de trabajo mediante revisión sistemática y método Delphi.
ResultadosSe identificaron 7 secciones sobre las que se establecieron puntos de acuerdo: indicaciones, equipo, técnica (parámetros de programación y registro), conducta en pérdida de señal, laringoscopia, voz e implicaciones legales.
ConclusionesLa NMIO ayuda en la localización e identificación del nervio laríngeo recurrente, ayuda durante su disección, informa sobre su estado funcional al finalizar la cirugía y permite tomar decisiones en caso de pérdida de señal en el primer lado operado en una tiroidectomía bilateral programada o si había parálisis contralateral previa. La precisión de la NMIO depende de variables como la técnica realizada, la tecnología utilizada y la formación para la correcta ejecución de la técnica e interpretación de la señal. El documento presentado es un punto de inicio para futuros acuerdos en CTPT en cada una de las secciones de consenso.