This case report discusses a case of a patient who experienced acute autonomic dysfunction during the parainfectious phase of COVID-19, attributed to Guillain-Barre Syndrome (GBS). This is the first well-documented case of such an association.
Case presentationA 64-year-old woman, previously infected with COVID-19, was admitted to the emergency department due to altered mental status. Brain computed tomography (CT) revealed bilateral occipital diffuse hypodensity. Throughout her hospitalization, she exhibited elevated blood pressure necessitating intravenous treatment.
The initial brain MRI revealed T2-weighted image hyperintensity at the parietal and occipital levels, indicative of vasogenic edema. These neuroimaging findings were suggestive of posterior reversible encephalopathy syndrome (PRES). Euvolemic hyponatremia with concurrent low serum osmolality and high urine osmolality and sodium was observed, indicating a syndrome of inappropriate antidiuretic hormone (SIADH). Throughout the patient's stay, her level of consciousness exhibited significant improvement. However, she developed ascending symmetrical limb weakness and progressive loss of reflexes, along with a severe motor deficit and gait disturbance, accompanied by arterial blood pressure fluctuations and other signs of autonomic dysfunction.
Clinical manifestations, neurophysiological findings, and laboratory results were indicative of Guillain-Barre Syndrome (GBS), leading to a conclusive diagnosis of GBS with dysautonomia triggered by a COVID-19 infection.
ConclusionThis case reveals the relevance of diagnosing autonomic dysfunction (including PRES) as the initial manifestation of GBS linked to COVID-19 infection and the importance of early diagnosis to prevent potential complications.
Este caso clínico describe un paciente que presenta disfunción autonómica aguda durante la fase parainfecciosa de COVID-19, secundaria al Síndrome de Guillain-Barré (SGB). Este es el primer caso bien documentado de tal asociación.
Presentación del casoUna mujer de 64 años que previamente presentó infección por COVID-19, fue admitida en urgencias debido a un bajo nivel de conciencia. La tomografía computarizada (TC) cerebral reveló hipodensidad difusa bilateral en las regiones occipitales. Durante su hospitalización, presentó hipertensión arterial que requirió tratamiento intravenoso.
La resonancia magnética cerebral inicial mostró hiperintensidad en secuencias T2 a nivel parietal y occipital, indicativa de edema vasogénico. Estos hallazgos en la neuroimagen sugieren un síndrome de encefalopatía reversible posterior (PRES, por sus siglas en inglés). Se observó hiponatremia euvolémica con baja osmolalidad sérica concurrente y alta osmolaridad y sodio en la orina, lo que indicaba un síndrome de secreción inapropiada de hormona antidiurética (SIADH, por sus siglas en inglés). Durante la estancia de la paciente, su nivel de conciencia mostró una mejora significativa. Sin embargo, desarrolló debilidad simétrica ascendente en las extremidades y una pérdida progresiva de reflejos, junto con un déficit motor severo y alteraciones en la marcha, acompañadas de fluctuaciones en la presión arterial y otros signos de disfunción autonómica.
Las manifestaciones clínicas, los hallazgos neurofisiológicos y los resultados de laboratorio fueron indicativos del Síndrome de Guillain-Barré (SGB), lo que llevó a un diagnóstico concluyente de SGB tras infección por COVID-19 con disautonomía como síntoma inicial.
ConclusiónEste muestra la importancia de diagnosticar la disfunción autonómica (incluyendo PRES) como manifestación inicial del SGB vinculado a la infección por COVID-19 y la importancia de un diagnóstico temprano para prevenir posibles complicaciones relacionadas con la neuropatía autonómica.
The COVID-19 infection has the potential to trigger autoimmune disorders such as Guillain-Barré syndrome (GBS). GBS is known for its association with autonomic dysfunction and may present as the initial manifestation, correlating with increased mortality and unfavorable functional outcomes. Despite the necessity for regular screening of autonomic nervous system neuropathy, it is typically overlooked. This report is the first case of PRES and SIADH in a patient with COVID-19 infection-related GBS.
Case reportA 64-year-old Caucasian woman with a history of high blood pressure, no other medical conditions, and no prior medical treatment, presented to the hospital with altered consciousness. She had a recent COVID-19 infection with mild respiratory symptoms (cough, fever, and headache) ten days before admission.
Upon initial examination, conducted within an hour of symptom onset, her blood pressure was 172/92mmHg, and oxygen saturation was 98% on ambient air. Neurological assessment revealed altered mental status with confusion and mutism. Motor examination indicated normal tone and force, with sensory examination showing normal deep tendon reflexes. The patient exhibited motor agitation with disorientation within the subsequent 24h.
Laboratory results showed a sodium level of 131mEq/L (normal range: 136–145). Cerebrospinal fluid (CSF) analysis revealed normal glucose and cell count, but elevated protein (70.2mg/dL, normal range: 15–45). CSF viral serology, gram stain, and culture were negative. The oropharyngeal SARS-CoV-2 PCR test remained positive.
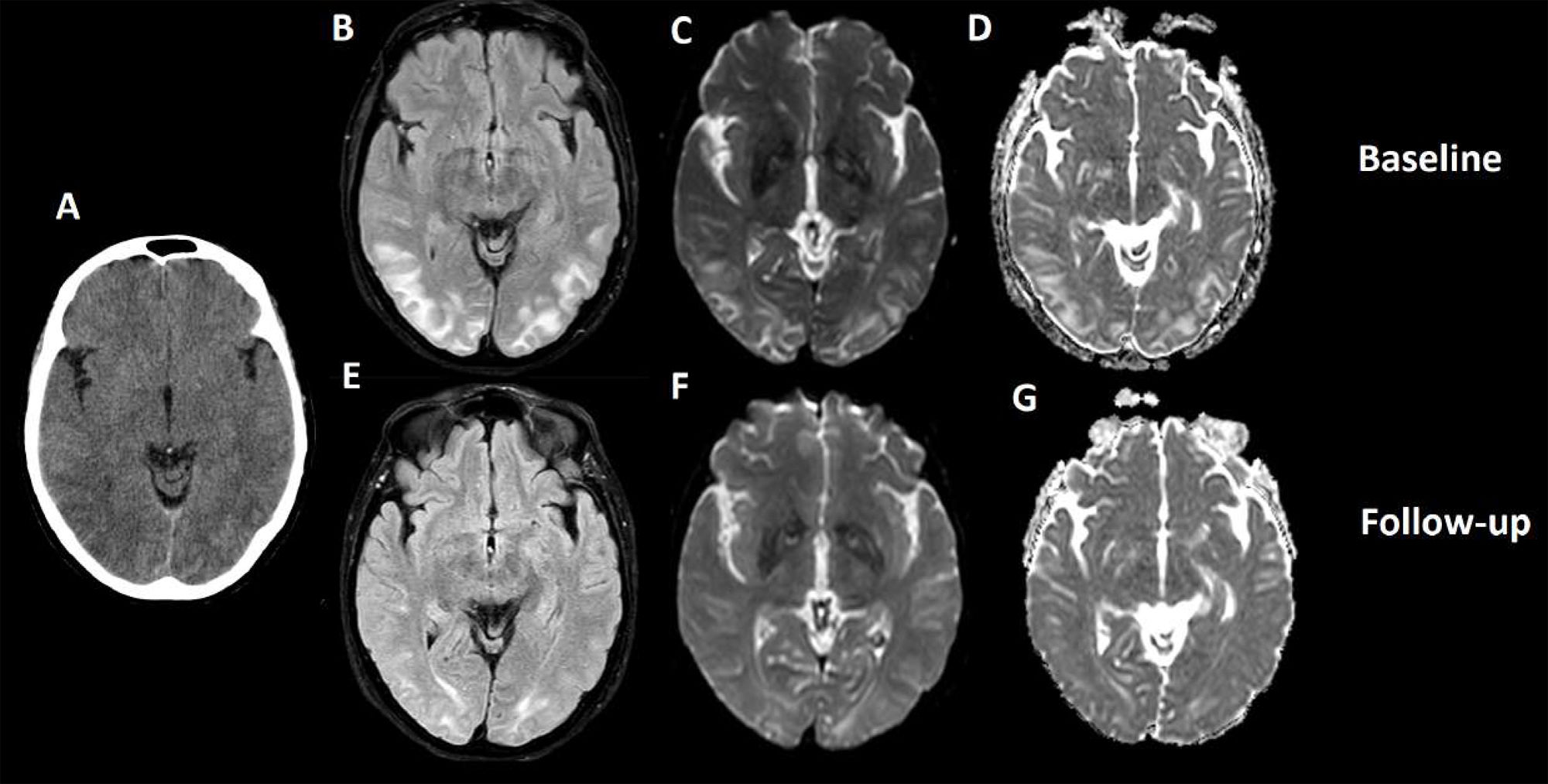
Brain computed tomography (CT) displayed bilateral occipital diffuse hypodensity (Fig. 1A). Initial brain MRI exhibited T2-weighted image hyperintensity at the parietal and occipital levels, seen in FLAIR, DWI, and ADC (Fig. 1B–D), suggesting vasogenic edema. These neuroimaging findings indicated posterior reversible encephalopathy syndrome (PRES) with compatible neurological symptoms.
(A) Cranial CT scan: diffuse and symmetrical hypodensity in bilateral occipital regions. (B) T2/FLAIR hyperintensity in bilateral parietal and occipital regions. (C and D) Hyperintensity in T2 sequences in DWI and ADC respectively, with symmetrical distribution in bilateral parietal and occipital regions, a similar distribution of the hyperintensity observed in FLAIR sequences. (E and F) Control cranial MRI study, 7 days after the previous study. Again, hyperintensity is observed in T2 sequences (FLAIR and DWI respectively), predominantly in the occipital regions, with a marked decrease in the extension of the lesion observed in T2 lines. Conclusion: hyperintensity in T2 sequences, observed in DWI, ADC, and FLAIR, suggestive of vasogenic edema in these locations, with significant improvement in the extension in the control study 7 days later, associating significant clinical improvement. The described radiological characteristics, distribution, and evolution were compatible with PRES.
Throughout the hospital stay, the patient had elevated blood pressure (systolic > 200mmHg), necessitating intravenous treatment (sodium nitroprusside, urapidil, and labetalol). Euvolemic hyponatremia (122mEq/L) with low serum osmolality and high urine osmolality and sodium (>40mmol/L) was observed, suggesting the syndrome of inappropriate antidiuretic hormone (SIADH). Serum cortisol, renal function, and thyroid function tests were normal. SIADH was managed with hypertonic saline infusion due to altered mental status followed by fluid restriction. No underlying medication or medical conditions commonly associated with SIADH were identified.
A follow-up brain MRI 7 days after the onset of neurological deficit showed a significant decrease in vasogenic edema (Fig. 1E and F).
Furthermore, during the patient's stay, mutism and consciousness showed marked improvement. However, she developed ascending symmetrical limb weakness and progressive reflex loss, along with severe motor deficit and gait disturbance, accompanied by arterial blood pressure fluctuations and signs of autonomic dysfunction.
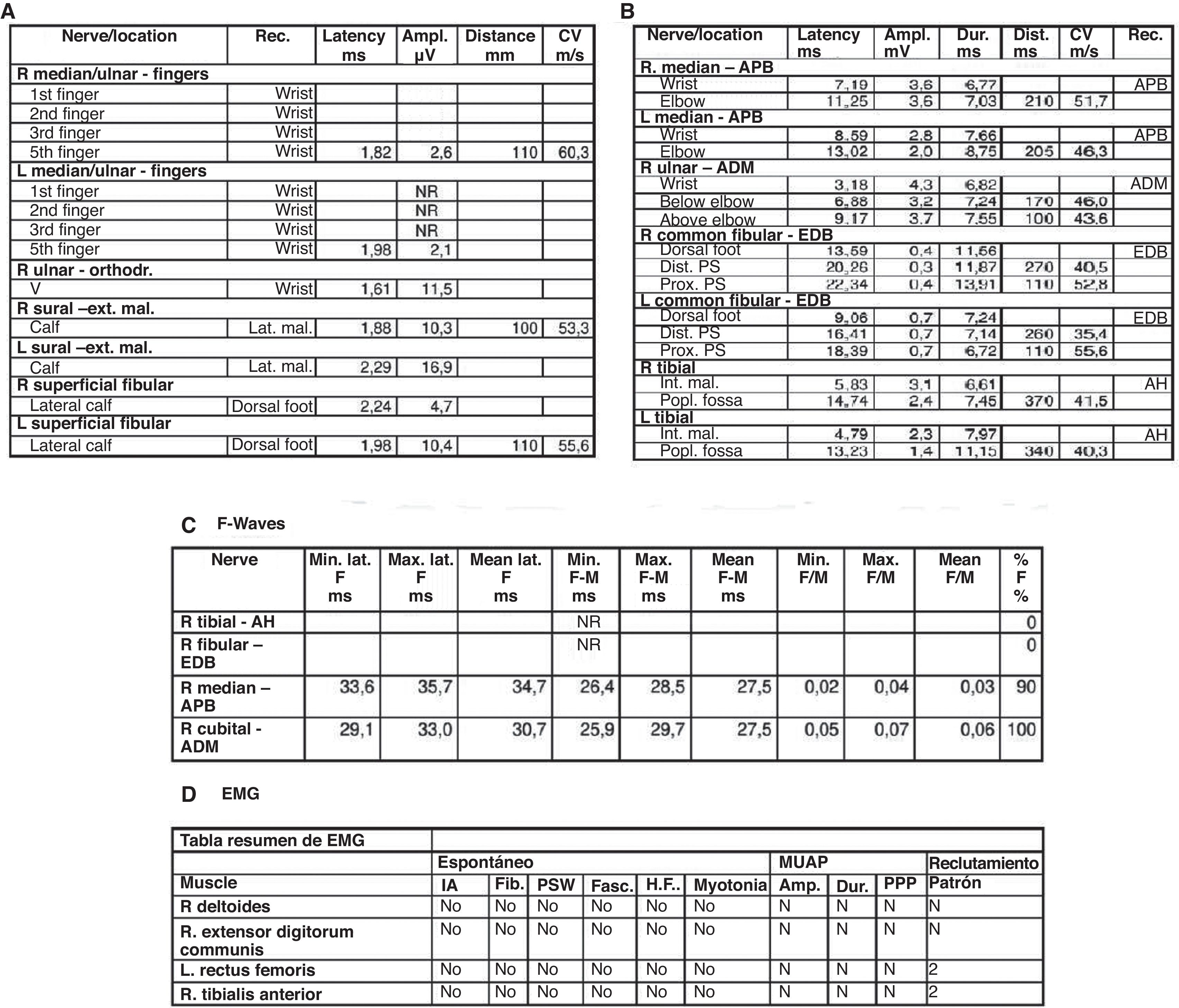
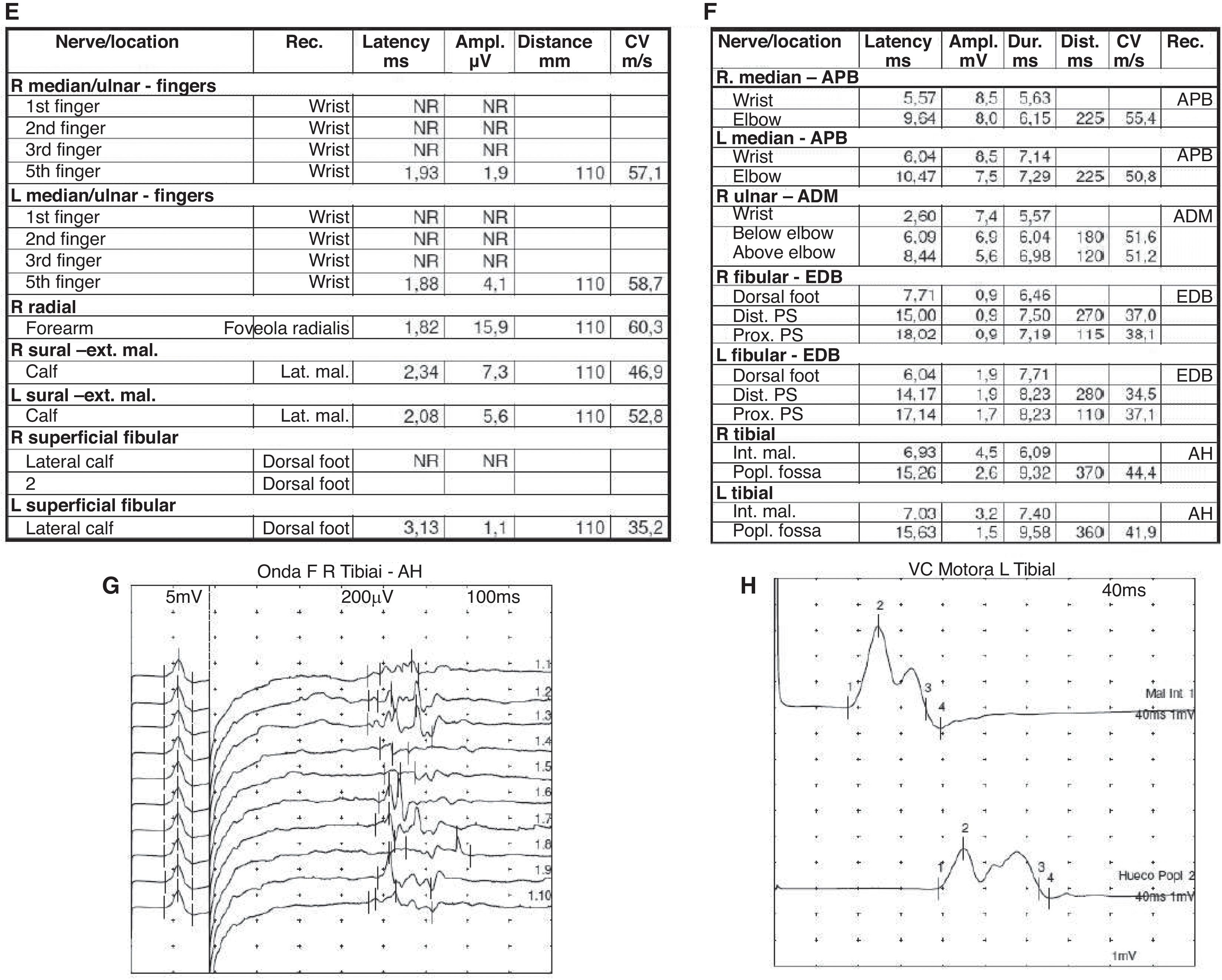
Electromyography (EMG) performed 15 days after symptom onset revealed an incipient acute inflammatory demyelinating polyradiculoneuropathy (Fig. 2).
(A) Sensory electroneurography shows the absence of bilateral median nerve response and low-amplitude responses in bilateral ulnar nerves with a normal sural nerve. (B) Motor neurography shows responses of the external sciatic popliteal, internal sciatic popliteal, bilateral median, and right ulnar nerves with lengthened distal motor latency. (C) F waves study shows tibial nerve and common peroneal nerve without response. (D) Electromyography shows an absence of acute denervation. These findings are suggestive of incipient sensory-motor demyelinating polyradiculoneuropathy. The examination was carried out at 15 days of onset.
Clinical manifestations, neurophysiological, and laboratory findings were consistent with Guillain-Barre Syndrome (GBS). The patient underwent plasmapheresis, leading to progressive symptom improvement.
At the one-month follow-up, the patient continued to show clinical improvement with residual weakness at hip flexion, generalized hyporeflexia, and the ability to walk without support, with resolution of all PRES-related symptoms. At the fourth-month follow-up, the EMG demonstrated improvement in latency, amplitude, and conduction velocity, and the F wave reappeared (Fig. 3G).
The final diagnosis was GBS with dysautonomia as an initial manifestation triggered by COVID-19 infection.
DiscussionThis case adds to the increasing evidence linking COVID-19 infection to GBS1. The patient exhibited unusual symptoms of autonomic dysfunction, specifically posterior reversible encephalopathy syndrome (PRES), caused by blood pressure fluctuations and inappropriate antidiuretic hormone syndrome.
Autonomic nervous system neuropathy carries a high mortality rate (6% in dysautonomia patients versus 2% in non-dysautonomia patients) and results in poorer functional outcomes.2 This clinical case highlights the autonomic dysfunction that patients with COVID-19-associated Guillain-Barré Syndrome (GBS) may exhibit and emphasizes the importance of recognizing this dysfunction during the COVID-19 parainfectious phase (between 10 and 21 days after infection) for early intervention.3
A lack of awareness can lead to underdiagnosis and potential harm, often requiring intensive care. Dysautonomia should be considered in the differential diagnosis of GBS, especially with an infectious trigger. The appearance of PRES as a result of autonomic dysregulation in post-COVID-19 GBS patients is particularly concerning.2,4 Early diagnosis and treatment of PRES with blood pressure management and supportive care are crucial.
The exact pathophysiology of GBS related to COVID-19 is unclear, but it is believed that an immune-mediated response triggered by the virus plays a significant role. SARS-CoV-2 infection can cause an abnormal immune response damaging the peripheral nervous system, including the autonomic nervous system.1
It is essential to consider SARS-CoV-2 infection in patients with dysautonomia, as early recognition can improve management and prognosis. COVID-19 may trigger GBS through mechanisms like molecular mimicry and systemic inflammation, highlighting the need for vigilance and a high index of suspicion in patients with autonomic symptoms after SARS-CoV-2 infection. Close monitoring of GBS patients for autonomic instability, including uncommon neurological presentations like PRES, is critical.
In summary, this case highlights the importance of recognizing the link between COVID-19 and dysautonomia in GBS, particularly the potential for PRES. Understanding this association is crucial for an early diagnosis and treatment, improving outcomes and reducing mortality.
Conflicts of interestAuthors declare no conflict of interest.