Background & Aim. Non alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries. Population studies have demonstrated that men and posmenopausal women have higher prevalence of NAFLD. The aim was to investigate the prevalence of NAFLD in premenopausal, posmenopausal and polycystic ovary syndrome (PCOS) women.
Methods. A cross sectional study carried out at University Hospital in Mexico City from January 2009 to November 2009. One hundred ninety seven women who agreed to participate were divided into groups, comprising 93 with NAFLD and without NAFLD. Anthropometric, metabolic and biochemical variables were measured. Serum estradiol and cortisol concentrations were determined and compared between the groups.
Results. Of the 197 patients, 93(47.2%) had NAFLD and 104 (52.8%) did not have NAFLD. The prevalence of NAFLD in premenopausal, postmenopausal and PCOS patients was 32.2, 57.9, and 62%, respectively. Age, BMI, hip to waist ratio, fasting glucose, HOMA-IR, and insulin were significantly higher in NAFLD patients. Women without NAFLD had significantly higher levels of serum estradiol (100 ± 95.4) compared with NAFLD patients (55.5 ± 66.6) p = 0.001. By group with and without NAFLD: premenopausal (55.44±93.3 vs. 128.56 ± 109.22), posmenopausal (44.98 ± 51.41 vs. 42.72 ± 51.48) and PCOS women (64.9 ± 53.3 vs. 101.36 ± 80.89) had significantly different hormone profile.
Conclusion. These results suggest that NAFLD is more prevalent in postmenopausal and women with PCOS than those preme-nopausal ones. The estrogens may have a protective effect of against NAFLD in women.
NAFLD is currently recognized as the most common form of chronic liver disease in the United States and in many parts of the world. Some data suggest that Mexican Americans are more likely to have NAFLD and blacks are less likely compared with non-Hispanic whites.1 NAFLD is considered a nonspecific term encompassing several clinicopathologic entities (steatosis alone, steatonecrosis, steato-hepatitis and histologic alcoholic-like hepatitis) that are similar to alcoholic liver diseases2 in the absence of significant alcohol abuse. Simple hepatic steatosis and hepatic steatosis with nonspecific inflammation are believed to have a generally benign course, whereas nonalcoholic steatohepatitis (NASH) can progress to cirrhosis, leading to liver failure and hepatocellular carcinoma (HCC).3,4 Furthermore, it has been proposed that NAFLD is associated with metabolic syndrome and varying degrees of insulin resistance.5-7 Although insulin resistance and hyperinsulinemia are frequently found in obese subjects with NAFLD, both are also noted in lean subjects with fatty liver disease and normal glucose tolerance.
A two-hit theory best describes the progression from simple steatosis to NASH, fibrosis, or cirrhosis. These two hits consist of the accumulation of excessive hepatic fat primarily owing to insulin resistance, and oxidative stress owing to reactive oxygen species (ROS). Mitochondria are the major cellular source of ROS in cases of NASH. Antioxidants such as vitamin E, N-acetylcysteine, betaine, and others may be beneficial in the treatment of NASH.8,9
Interestingly, early descriptions by those who studied NAFLD suggested a female preponderance of the condition. More recent studies, however, including population-based studies, show that NAFLD affects both sexes equally or a higher proportion of men.10,11 In a prospective study, the incidence of NAFLD was higher in men than in women. Age was an independent predictor of the development of NAFLD in Japanese women but not men.12 The differences between the sexes in regards to the influence of age on the incidence and prevalence of NAFLD, are attributed to the putative protective effects of estrogen.
The aim of this study was to investigate the prevalence of NAFLD in premenopausal and postmeno-pausal women and women with PCOS.
MethodsPatient populationWe conducted a cross-sectional study in the check-up unit of the Diagnostic Clinic at the Medica Sur Clinic & Foundation (University Hospital) between February 2009 and December 2009. This hospital provides care for mainly middle-and high-income individuals from Mexico City and surrounding metropolitan areas. Our sample population of premenopausal and postmenopausal patients was formed from a series of consecutive asymptomatic subjects who were referred to the check-up unit by their companies as an annual employment requirement, not for symptomatic disease. Menopause was defined as one year or more without menses. All patients who were under hormonal treatment were excluded. Patients with PCOS were invited separately only if they had a previous diagnosis. Other exclusion criteria were an alcohol intake of more than 20 g/d, known liver disease, or current use of medication. Regarding liver disease, participants who tested positive for hepatitis B antigen or hepatitis C antibody and those who reported a history of known liver disease, including viral, genetic, autoimmune, and drug-induced liver disease, were also excluded. The study included 197 female subjects who agreed to participate and they were divided into six groups, comprising 93 women with NAFLD (29 premeno-pausal women, 33 postmenopausal women and 31 women with PCOS) and 104 women without NAFLD (61 premenopausal women, 24 postmenopausal women and 19 women with PCOS). The study was approved by the Human Subjects Committee at the Medica Sur Clinic & Foundation and conformed to the ethical guidelines of the 1983 Declaration of Helsinki. Written informed consent was obtained from all participants before entry into the study.
NAFLD diagnosisThe diagnosis of NAFLD was based on the presence of a bright liver at ultrasound scanning. Real-time ultrasonographic studies were performed while the subjects were fasting. A 3.5-MHz transducer was used to obtain the following images: sagital view of the right lobe of the liver and right kidney, transverse view of the left lateral segment of the liver and spleen, transverse view of the liver and pancreas, and any focal areas of altered echotexture (Elegra; Siemens Medical Systems, Mountain Grove, CA).
Metabolic syndromeBody weight was measured, in light clothing and without shoes, to the nearest 0.10 kg. Height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Overweight was defined as a BMI ranging from 25 to 29.9 kg/m2 and obesity when BMI was > 30 kg/m2. Waist circumference to the nearest 0.1 cm was measured at the midpoint between the lower border of the rib cage and the iliac crest, and hip circumference was similarly obtained at the widest point between hip and buttock. Body fat percentage was measured by bioelectrical impedance (Omron body fat analyzer model HBF-306INT). Metabolic syndrome (MS) criteria were considered according to the Third Report of the National Cholesterol Expert Prevention, Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III (ATPIII)).13
Blood pressure was measured in both arms on two different occasions and the mean value was used for the study. A complete physical examination was carried out to exclude other diseases including Cus-hing syndrome and congenital adrenal hyperplasia.
Analytical proceduresInsulin concentrations were measured using an immunoenzymometric assay (MEIA; Abbott Diagnostics), with inter-and intra-assay coefficients of variation less than 3%. Plasma glucose in the fasting state was measured in duplicate with an automated analyzer. The coefficient of variation for a single determination was 1.5%. Total cholesterol, high density lipoprotein (HDL-C) and triglyceride concentrations were measured by enzymatic colori-metric methods, using CHOL, HDL-C plus (second generation) and TG assays (Roche Diagnostics Co., Indianapolis, IN), respectively. Low density lipopro-tein cholesterol (LDL-C) concentrations were calculated using the Friedewald formula. Assessment of Insulin Resistance (IR) was made using the Homeos-tasis Model Assessment (HOMA-IR) originally described by Matthews et al.: HOMA-IR = ((fasting insulin (U/L) x fasting glucose (mmol/L))/22.5).14 Serum estradiol, testosterone and cortisol levels were measured by radioimmunoassay (RIA)(Beckman-Coulter).
Statistical analysisMean values and their standard deviations (SD) were used to resume the distribution of continuous variables comparing all groups. Chi Square test was used to compare prevalences of NAFLD and metabolic syndrome features among the groups. The non-parametric Kruskal Wallis test (KW) was used to make comparisons between all groups, also according to the presence of NAFLD and according to the menopausal state. Differences were considered significant with p values of < 0.05.
Unconditional univariate logistic regression analysis was conducted to estimate the probability of NAFLD associated with the menopausal status. All the analysis was carried out with the statistic program SPSS/PC v 16.0 (Chicago IL).
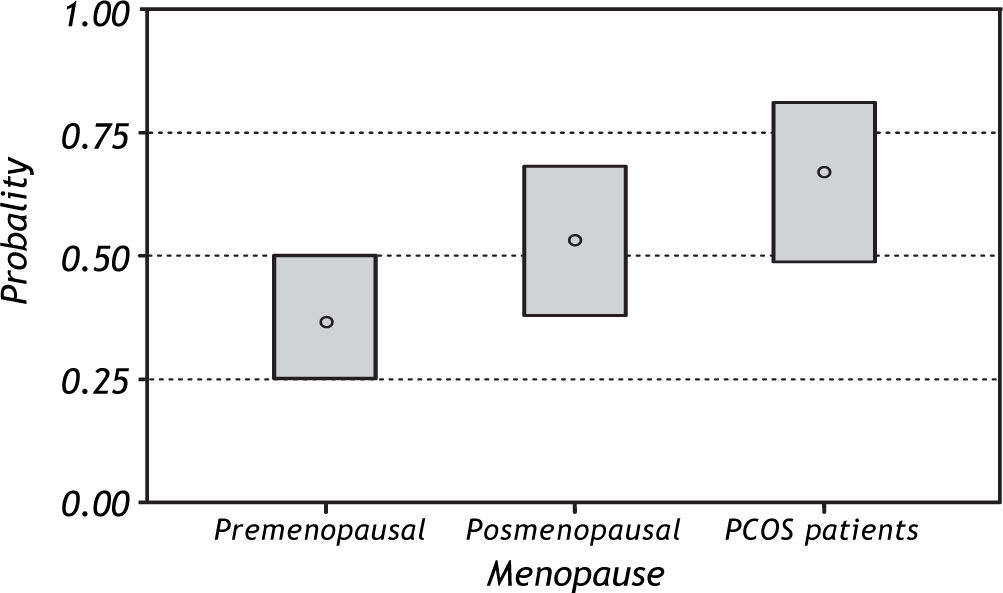
ResultsOf the 197 patients, 93(47.2%) had NAFLD and 104 (52.8%) did not have NAFLD. There were 90 premenopausal patients (46.2%), 57 postmenopausal patients (29.4%) and 50 PCOS patients (25.4%). The prevalence of NAFLD in premenopausal, postmeno-pausal and PCOS patients was 32.2%, 57.9%, and 62%, respectively.
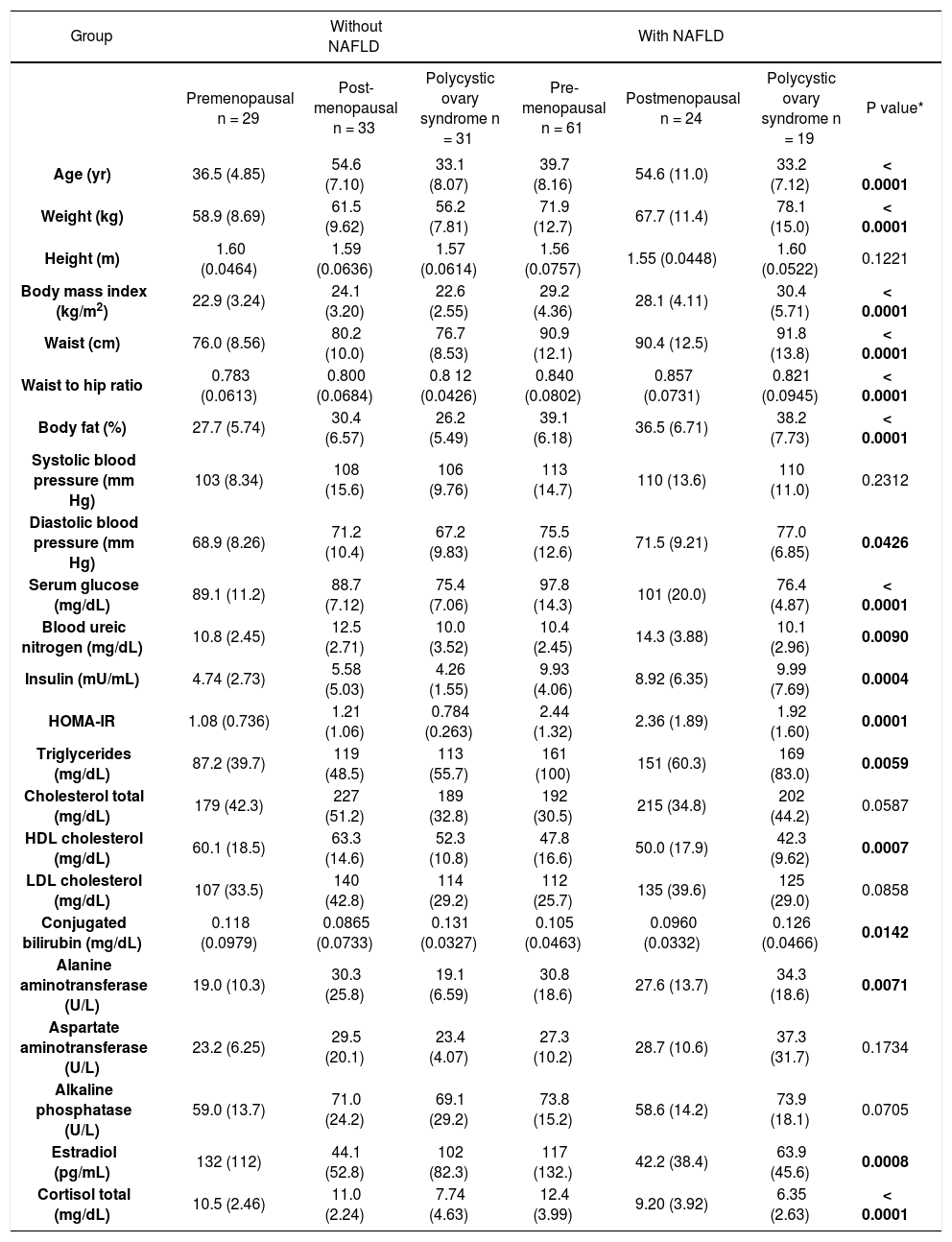
The comparisons of menopausal status and NA-FLD were statistically significant at the p = 0.05 level by the KW test for the following variables: age, anthropometric variables (waist, waist to hip ratio, BMI, percentage of body fat and diastolic blood pressure), HOMA-IR, liver enzymes (alanine amino-transferase (ALT), with a positive trend for aspartate aminotransferase (AST) and alkaline phosphatase (AP), serum glucose, blood ureic nitrogen (BUN), creatinine, total cholesterol, HDL-C, LDL-C, conjugated bilirubin, and serum estradiol and cortisol concentrations (Table 1).
Anthropometric, biochemical and metabolic characteristics of the patient groups.
| Group | Without NAFLD | With NAFLD | |||||
|---|---|---|---|---|---|---|---|
| Premenopausal n = 29 | Post-menopausal n = 33 | Polycystic ovary syndrome n = 31 | Pre-menopausal n = 61 | Postmenopausal n = 24 | Polycystic ovary syndrome n = 19 | P value* | |
| Age (yr) | 36.5 (4.85) | 54.6 (7.10) | 33.1 (8.07) | 39.7 (8.16) | 54.6 (11.0) | 33.2 (7.12) | < 0.0001 |
| Weight (kg) | 58.9 (8.69) | 61.5 (9.62) | 56.2 (7.81) | 71.9 (12.7) | 67.7 (11.4) | 78.1 (15.0) | < 0.0001 |
| Height (m) | 1.60 (0.0464) | 1.59 (0.0636) | 1.57 (0.0614) | 1.56 (0.0757) | 1.55 (0.0448) | 1.60 (0.0522) | 0.1221 |
| Body mass index (kg/m2) | 22.9 (3.24) | 24.1 (3.20) | 22.6 (2.55) | 29.2 (4.36) | 28.1 (4.11) | 30.4 (5.71) | < 0.0001 |
| Waist (cm) | 76.0 (8.56) | 80.2 (10.0) | 76.7 (8.53) | 90.9 (12.1) | 90.4 (12.5) | 91.8 (13.8) | < 0.0001 |
| Waist to hip ratio | 0.783 (0.0613) | 0.800 (0.0684) | 0.8 12 (0.0426) | 0.840 (0.0802) | 0.857 (0.0731) | 0.821 (0.0945) | < 0.0001 |
| Body fat (%) | 27.7 (5.74) | 30.4 (6.57) | 26.2 (5.49) | 39.1 (6.18) | 36.5 (6.71) | 38.2 (7.73) | < 0.0001 |
| Systolic blood pressure (mm Hg) | 103 (8.34) | 108 (15.6) | 106 (9.76) | 113 (14.7) | 110 (13.6) | 110 (11.0) | 0.2312 |
| Diastolic blood pressure (mm Hg) | 68.9 (8.26) | 71.2 (10.4) | 67.2 (9.83) | 75.5 (12.6) | 71.5 (9.21) | 77.0 (6.85) | 0.0426 |
| Serum glucose (mg/dL) | 89.1 (11.2) | 88.7 (7.12) | 75.4 (7.06) | 97.8 (14.3) | 101 (20.0) | 76.4 (4.87) | < 0.0001 |
| Blood ureic nitrogen (mg/dL) | 10.8 (2.45) | 12.5 (2.71) | 10.0 (3.52) | 10.4 (2.45) | 14.3 (3.88) | 10.1 (2.96) | 0.0090 |
| Insulin (mU/mL) | 4.74 (2.73) | 5.58 (5.03) | 4.26 (1.55) | 9.93 (4.06) | 8.92 (6.35) | 9.99 (7.69) | 0.0004 |
| HOMA-IR | 1.08 (0.736) | 1.21 (1.06) | 0.784 (0.263) | 2.44 (1.32) | 2.36 (1.89) | 1.92 (1.60) | 0.0001 |
| Triglycerides (mg/dL) | 87.2 (39.7) | 119 (48.5) | 113 (55.7) | 161 (100) | 151 (60.3) | 169 (83.0) | 0.0059 |
| Cholesterol total (mg/dL) | 179 (42.3) | 227 (51.2) | 189 (32.8) | 192 (30.5) | 215 (34.8) | 202 (44.2) | 0.0587 |
| HDL cholesterol (mg/dL) | 60.1 (18.5) | 63.3 (14.6) | 52.3 (10.8) | 47.8 (16.6) | 50.0 (17.9) | 42.3 (9.62) | 0.0007 |
| LDL cholesterol (mg/dL) | 107 (33.5) | 140 (42.8) | 114 (29.2) | 112 (25.7) | 135 (39.6) | 125 (29.0) | 0.0858 |
| Conjugated bilirubin (mg/dL) | 0.118 (0.0979) | 0.0865 (0.0733) | 0.131 (0.0327) | 0.105 (0.0463) | 0.0960 (0.0332) | 0.126 (0.0466) | 0.0142 |
| Alanine aminotransferase (U/L) | 19.0 (10.3) | 30.3 (25.8) | 19.1 (6.59) | 30.8 (18.6) | 27.6 (13.7) | 34.3 (18.6) | 0.0071 |
| Aspartate aminotransferase (U/L) | 23.2 (6.25) | 29.5 (20.1) | 23.4 (4.07) | 27.3 (10.2) | 28.7 (10.6) | 37.3 (31.7) | 0.1734 |
| Alkaline phosphatase (U/L) | 59.0 (13.7) | 71.0 (24.2) | 69.1 (29.2) | 73.8 (15.2) | 58.6 (14.2) | 73.9 (18.1) | 0.0705 |
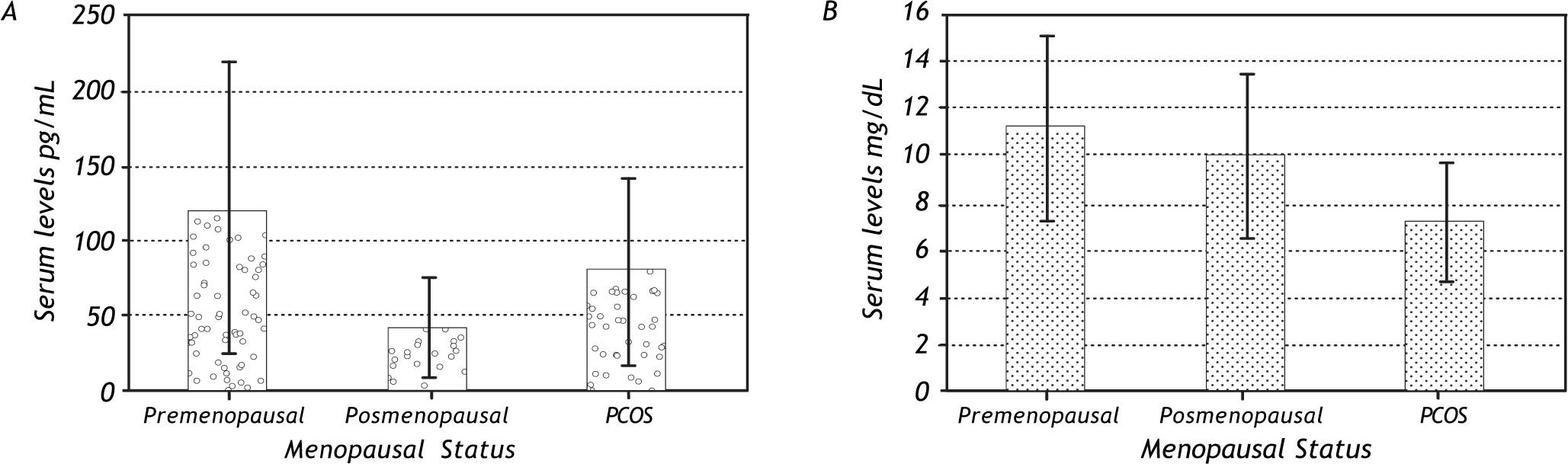
| Estradiol (pg/mL) | 132 (112) | 44.1 (52.8) | 102 (82.3) | 117 (132.) | 42.2 (38.4) | 63.9 (45.6) | 0.0008 |
| Cortisol total (mg/dL) | 10.5 (2.46) | 11.0 (2.24) | 7.74 (4.63) | 12.4 (3.99) | 9.20 (3.92) | 6.35 (2.63) | < 0.0001 |
* Kruskal-Wallis Test; P < 0.05 was considered significant.
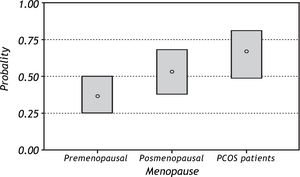
In general, patients with NAFLD were older, had greater BMI, central obesity and IR. With regard to MS, 11 (22%) patients with PCOS, 7 (7.7%) preme-nopausal and 13 (24.5%) postmenopausal patients fulfilled the criteria of metabolic syndrome (MS). The prevalence of MS was higher in postmenopausal 39%) and PCOS (36%) patients with NAFLD compared with the other groups. (Table 2) Figure 1 shows the predicted probability of NAFLD by meno-pausal status. Premenopausal women show a lower probability of NAFLD, which is significantly different from the probability of the other two groups. MS analysis of weight, height, BMI, waist, waist to hip ratio and percentage of body fat shows an increase in the probability of NAFLD with increasing values. In other words, women with high values of weight, BMI, waist, waist to hip ratio, and percentage of body fat have the highest probability of having NAFLD. We found a similar pattern after correcting for the effect of menopause, although the difference between postmenopausal women and women with PCOS was not significant.
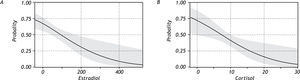
With regard to hormonal levels, we observed that premenopausal women had higher serum levels of estradiol and cortisol (Figure 2) and that premeno-pausal women with NAFLD had significantly higher levels of estradiol than the other groups. Postmeno-pausal women with or without NAFLD had no significant difference in cortisol and estradiol levels, but had the lowest serum values of estradiol (but not of cortisol) of all the groups. In the PCOS group, there was a significant difference in the values for estradiol and cortisol between patients with NAFLD and those without the disease, with patients without NAFLD having higher levels of these hormones.
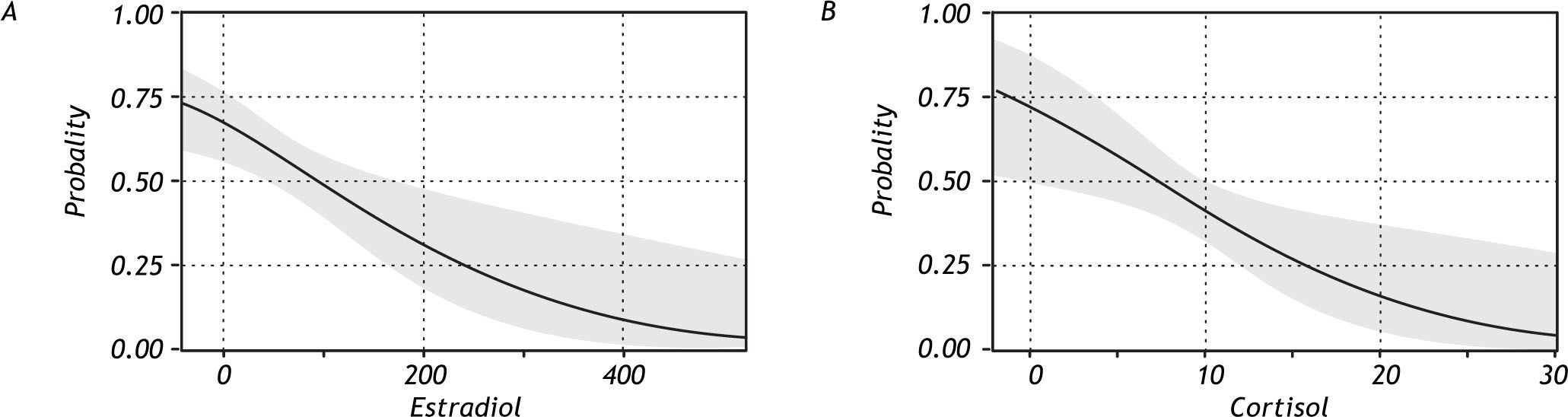
When the association between levels of estradiol and cortisol with the presence of NAFLD was analyzed, the results indicated a higher probability of developing NAFLD with decreasing serum levels of these hormones (Figure 3).
DiscussionNAFLD is a chronic condition in patients who do not have a history of excessive alcohol consumption and is characterized by more than 5% of hepatocytes affected with lipid accumulation. Caldwell, et al., in 1999 suggested a relationship between NAFLD and cryptogenic cirrhosis, and several other studies have shown that NAFLD patients are at higher risk of developing a wide range of progressive liver diseases including hepatocellular carcinoma.15,16
In previous studies, a possible role for estrogens in fatty liver disease has been suggested since Nguyen et al. demonstrated in 2001 that female patients with breast cancer who were receiving treatment with tamoxifen, a nonsteroidal antiestro-genic drug had a higher prevalence of intra-abdomi-nal fat accumulation and incidence of NAFLD.17
As has been reported in many studies,3,4 we observed large differences in BMI and abdominal obesity between the groups, especially between patients with and without NAFLD. Similar differences were observed with biochemical variables, particularly insulin resistance, which is the most common characteristic of subjects with NAFLD. The highest incidence of MS was found in patients with PCOS and NAFLD, whereas none of the patients with PCOS and without NAFLD fulfilled the criteria for metabolic syndrome.18
As mentioned above, it has been suggested that NAFLD is a disease more prevalent in men than in women and more frequent after menopause than it is in premenopausal women, implying that estrogens might have a role in the pathophysiology of fatty liver. Clark, et al., and Fraser, et al., have recently demonstrated in their epidemiological studies that the prevalence is higher in men than in women.10,19 An-gulo et al. in their study of predictors of fibrosis suggested that women with NAFLD had a higher association with fibrosis progression as they aged.20 In this study, the prevalence of NAFLD that we observed was clearly different according to menopausal status. We observed that the postmenopausal women had a higher prevalence of NAFLD than did the pre-menopausal women, but the highest incidence of NA-FLD was present in the patients with PCOS. It has been suggested that several physiological changes occur in postmenopausal women, as a result of the low serum levels of estrogen. One of the most important alterations is fat redistribution and its metabolic consequences including dyslipidemia and glucose intole-rance.21 These physiological and hormonal changes are probably the cause of the alterations of lipid metabolism in postmenopausal women who, as a consequence, have NAFLD. Kojima et al. found in their epidemiological study a higher prevalence of NAFLD in postmenopausal female patients than in men.22 In this study, we found a prevalence of NAFLD of nearly 60% in postmenopausal patients.
PCOS is a common disease and was first described by Stein and Leventhal in 1935. It affects 5-11% of women of reproductive age23 and is recognized as a major factor in alterations of the metabolic, cardiovascular and reproductive systems.24 The most important pathophysiologic feature of PCOS is IR, which is also the main characteristic of MS. Gamba-rin-Gelwan et al. found a prevalence of 55% of NA-FLD in female patients with PCOS,16 furthermore Cerda et al. observed a prevalence of 63% of PCOS patients with biochemical alterations and a prevalence of NAFLD of 41%.18 Moreover Setji et al. described several biochemical alterations, especially in liver enzymes, in patients with PCOS and when those patients underwent liver biopsy all of them had NASH with fibrosis.25 In the present study, we found that women with PCOS had a higher prevalence of NAFLD (62%) than did the other groups. This might have two pathogenic components: firstly, the altered hormonal profile and lower levels of estradiol in these patients and, secondly, the persistent state of hyperinsulinemia and insulin resistance typical of this disease.
Interestingly, we observed higher serum estradiol concentrations in patients without NAFLD, especially in the premenopausal women without NAFLD group, whereas postmenopausal women with NA-
FLD had the lowest cortisol and estradiol levels. Despite these findings, the group with highest prevalence of NAFLD was the PCOS group, which may be related to the two pathogenic components suggested above.
What possible mechanisms could explain the lower prevalence of NAFLD in premenopausal women? Interestingly, an animal study showed that hepatic steatosis becomes evident spontaneously in aromatase deficient mice, which lack the ability to produce estrogen and are impaired with respect to hepatocellular fatty acid β-oxidation. Estradiol replacement reduces hepatic steatosis and restores the impairment in mitochondrial and peroxisomal fatty acid β-oxidation to the wild-type level.26
Estradiol is a strong endogenous antioxidant that suppresses liver fibrosis in animal models and attenuates induction of oxidation-reduction sensitive transcription factors, hepatocyte apoptosis and stellate cell activation by avoiding ROS generation in cultures.27-30
One of the most important activities of estradiol is its effect on serum lipid concentration that results from estrogen-mediated effects on the hepatic expression of apoprotein genes. These effects decrease the concentration of LDL and increase the concentration of triglycerides, total cholesterol and HDL.31 We propose that another mechanism is the antioxi-dant effect that estradiol seems to have in several studies, which may be due to changes mediated by estrogen receptors in the expression of genes that regulate the production of superoxide.31,32 In hepatic steatosis, there is an increase in hepatocyte lipope-roxidation, which has as its consequence the activation of hepatic stellate cells. These are the principal target of inflammatory and oxidative stimulation and produce extracellular matrix components that cause triglyceride accumulation in hepatocytes and finally fibrosis.3
On the other hand when alcohol is involved, in vivo studies had concluded that the sensitivity of rat liver to alcohol-induced injury is directly related to estrogen, which increases endotoxin in the blood and CD14 expression in the liver, leading to increased TNF-alpha production.33 In studies of rats chronically treated with ethanol, toremefene, an antiestrogen agent, showed a partial protection of liver injury through significantly alleviated both ethanol induction of the pro-oxidant enzyme CYP2E1 and ethanol reduction of the oxidant-pro-tective enzyme Se-glutathione peroxidase. In humans, Kotoh et al., 2007 demonstrated that estrogen may exacerbate nonalcoholic steatohepati-tis in women with insulin resistance.34
As in the case of estradiol, here we found that plasma cortisol levels were higher in premenopause women, suggesting an unexpected protector effect of this hormone against NAFLD, interestingly cortisol metabolism may be altered in individuals with a metabolic profile such as the one present in patients with NAFLD.35
Some limitations of our study are, firstly, the fact that it is a cross-sectional study. For that reason, the patients were not followed up and we were not able to see the progression of the liver disease. There needs to be further research, such as a cohort study that could follow a group of patients from premenopause through time to determine es-tradiol and cortisol levels and the progression of fatty liver disease. This monitoring should also be performed in a group of PCOS patients from the diagnosis of this endocrinal pathology. Another important limitation of our study is that the fatty liver disease was diagnosed by hepatic ultrasound, whereas it has been demonstrated that liver biopsy is the gold standard for the diagnosis of NAFLD. Another limitation is the fact that the value of ga-mma-glutamyl transpeptidase (GGT), a very important feature that must be determined in all patients with liver disease, was not measured in all patients.
ConclusionThese results suggest that NAFLD is more prevalent in postmenopausal and women with PCOS than those premenopausal ones. The estrogens may have a protective effect of against NAFLD in women. However, future studies should focus on the role of estrogen in other populations and the mechanisms that explain this association between the NAFLD and estrogens are needed.
AcknowledgmentsThis study was supported by Medica Sur Clinic & Foundation.
Financial DisclosureThe authors have no financial arrangements with a company whose product figures significantly in the submitted.
Conflict of InterestNone.
Disclosure StatementThe authors have nothing to disclose.
Abbreviations- •
NAFLD: Nonalcoholic fatty liver disease.
- •
PCOS: Polycystic ovary syndrome.
- •
BMI: Body mass index.
- •
HOMA-IR: Homeostasis model assessment of insulin resistance.
- •
NASH: Nonalcoholic steatohepatitis.
- •
HCC: Hepatocellular carcinoma.
- •
ROS: Reactive oxygen species.
- •
LDL: Low density lipoprotein.
- •
HDL: High density lipoprotein.
- •
ALT: Alanine aminotransferase.
- •
AST: Aspartate aminotransferase.
- •
AP: Alkaline phosphatase.
- •
BUN: Blood ureic nitrogen.
- •
SD: Standard deviations.
- •
KW: Kruskal Wallis test.
- •
MS: Metabolic syndrome.
Kruskal-Wallis Test; P < 0.05 was considered significant.
This paper was presented in part at the Annual Meeting of the American Gastroenterological Association, New Orleans, LA, in 2010 and published as an abstract in Gastroenterology 2010;138: S803.
The authors want to dedicate this article to the National Autonomous University of Mexico (UNAM) for the 100th anniversary of its foundation.