The outbreak of SARS-CoV-2, an etiologic agent of the COVID-19 pandemic disease in late December 2019 has left the whole world aghast with huge health and economic losses. Due to a lack of specific knowledge and understanding at the initial stages, an unprecedented rise in COVID-19 cases has been recorded globally. Various preventive measures and strategies were implemented, however, for the radical control of SARS-CoV-2 infections; it seems that the only effective way to control the ongoing infections is large-scale vaccination. So far, WHO has approved 11 vaccines for emergency use namely Pfizer/BioNTech, Oxford/Astra Zeneca, Johnson and Johnson, Moderna, Covilo, Novavax, Covovax, Spikevax, Can Sino, Comirnaty, and Coronavac while five other needs approval. The worldwide vaccination dataset reveals that 65.7% of the world population has received their first dose of the COVID-19 vaccine. As a consequence of the proactive implementation of India's vaccination program, a historical milestone of administering over 1.9 billion doses of COVID-19 vaccines have been achieved on 19th May 2022. This review summarizes the different types of traditional and modern vaccine designing strategies with an emphasis on COVID-19. Moreover, the review highlights the status of vaccines for COVID-19 approved in India which includes both indigenous and non-indigenous vaccines. The present article also encompasses vaccine designing and developmental strategies, efficacy, safety profile and usage among the population, and the efficacy of modern vaccines over traditional ones.
El brote de SARS-CoV-2, un agente etiológico de la enfermedad pandémica COVID-19, a fines de diciembre de 2019, ha dejado al mundo entero horrorizado con enormes pérdidas económicas y de salud. Debido a la falta de conocimiento y comprensión específicos en las etapas iniciales, se ha registrado un aumento sin precedentes en los casos de COVID-19 a nivel mundial. Sin embargo, se implementaron diversas medidas y estrategias preventivas para el control radical de las infecciones por SARS-CoV-2; parece que la única forma eficaz de controlar las infecciones en curso es la vacunación a gran escala. Hasta el momento, la OMS ha aprobado 11 vacunas para uso de urgencia Pfizer/BioNTech, Oxford/Astra Zeneca, Johnson and Johnson, Moderna, Covilo, Novavax, Covovax, Spikevax, Can Sino, Comirnaty y Coronavac, mientras que otras cinco necesitan aprobación. El conjunto de datos de vacunación mundial revela que el 65,7% de la población mundial ha recibido su primera dosis de la vacuna COVID-19. Como consecuencia de la implementación proactiva del programa de vacunación de la India, el 19 de mayo de 2022 se logró un hito histórico de administrar más de 1900 millones de dosis de vacunas contra el COVID-19. Esta revisión resume los diferentes tipos de estrategias de diseño de vacunas tradicionales y modernas con énfasis sobre COVID-19. Además, la revisión destaca el estado de las vacunas para COVID-19 aprobadas en India, que incluye vacunas tanto indígenas como no indígenas. El presente artículo también abarca estrategias de diseño y desarrollo de vacunas, eficacia, perfil de seguridad y uso entre la población, y la eficacia de las vacunas modernas sobre las tradicionales.
The first case of the SARS-CoV-2 (COVID-19) infection was reported in Wuhan, China in December 2019 from where this disease spread globally. In a setting of a strongly connected and integrated world, coupled with the high transmissibility of the viral infection,1,2 the disease rapidly spread and was declared a public health emergency of international concern (PHEIC) on January 30, 2020. The unprecedented rise in the number of cases resulted in a declaration of COVID-19 as a pandemic in March, 2020. The COVID-19 disease spread exponentially and at present exists in more than 230 countries and has resulted in more than 39 million confirmed cases (WHO). As on date 20th July, 2022 the number of COVID-19 cases across the globe reached 561,156,416 with 6,365,510 casualties (WHO dashboard).
The WHO together with the United States Centres for Disease Control and Prevention (CDC), European Centre for Disease Prevention and Control (EDC) issued several guidelines and health advisories to prevent the spread of the outbreak. WHO declares COVID-19 vaccines as safe and data strongly suggests that the risk of infection and severity of symptoms associated with the disease is low for vaccinated people. WHO recommends wearing a mask in indoor public places, people above the age of 2 years should wear a mask in case they are not vaccinated, or fully vaccinated in an area with high risk of infection. People should wear masks in crowded outdoor places and for working at places where others are not vaccinated. A distance of 6 ft is essential and people should avoid crowded places unless urgent. WHO recommends proper washing of hands as well as covering is essential while coughing and sneezing to avoid the spread of this disease.
Coronaviruses are positive-sense, enveloped, single-stranded RNA viruses that possess helical nucleocapsid. Belonging to the family Coronaviridae, the coronavirus envelope comprises of club-shaped glycoprotein projections.3 The diameter of SARS-CoV-2 ranges from 60–140 nm, often found in its pleomorphic form.4 SARS-CoV-2 is a beta coronavirus belonging to the same subgenus as MERS-CoV and SARS-CoV.5 As SARS-CoV-2 shares 89% nucleotide identity similar to bat SARS- like- CoVZXC21 and 82% similarity with human SARS-CoV, hence, named SARS-CoV-2 by the International Committee on Taxonomy of Viruses4 and its transmissibility could be measured by R0 value (Reproduction number) 6 where SARS-CoV-2 has an R0 value range of 2–3.1,4
This virus contains 4 different structural proteins; spike (S), envelope (E), membrane (M) and nucleocapsid (N) and other structural and accessory proteins (HE, 3a/b protein and 4a/b protein).7,8 The spike protein (S) is a trimeric envelope glycoprotein that plays a vital role in the transmission of infection as it undergoes cleavage into an amino (N)-terminal S1 subunit which helps the incorporation of the virus into the host cell, hence a major target for vaccines. This S1 subunit has a neutralizing response to antisera due to its binding with the host cell.5,9,10 E protein forms the viroporins (E channels) and is involved in viral replication cycle.11 N-protein constitutes the helical nucleocapsid and binds with the viral RNA genome.12 M-protein, the most abundantly expressed protein in virus particle is the main organizer of coronavirus assembly and is involved in the morphogenesis and assembly of SARS-CoV-2 by interacting with the essential structural proteins and is also linked with the process of apoptosis in the host cell.13
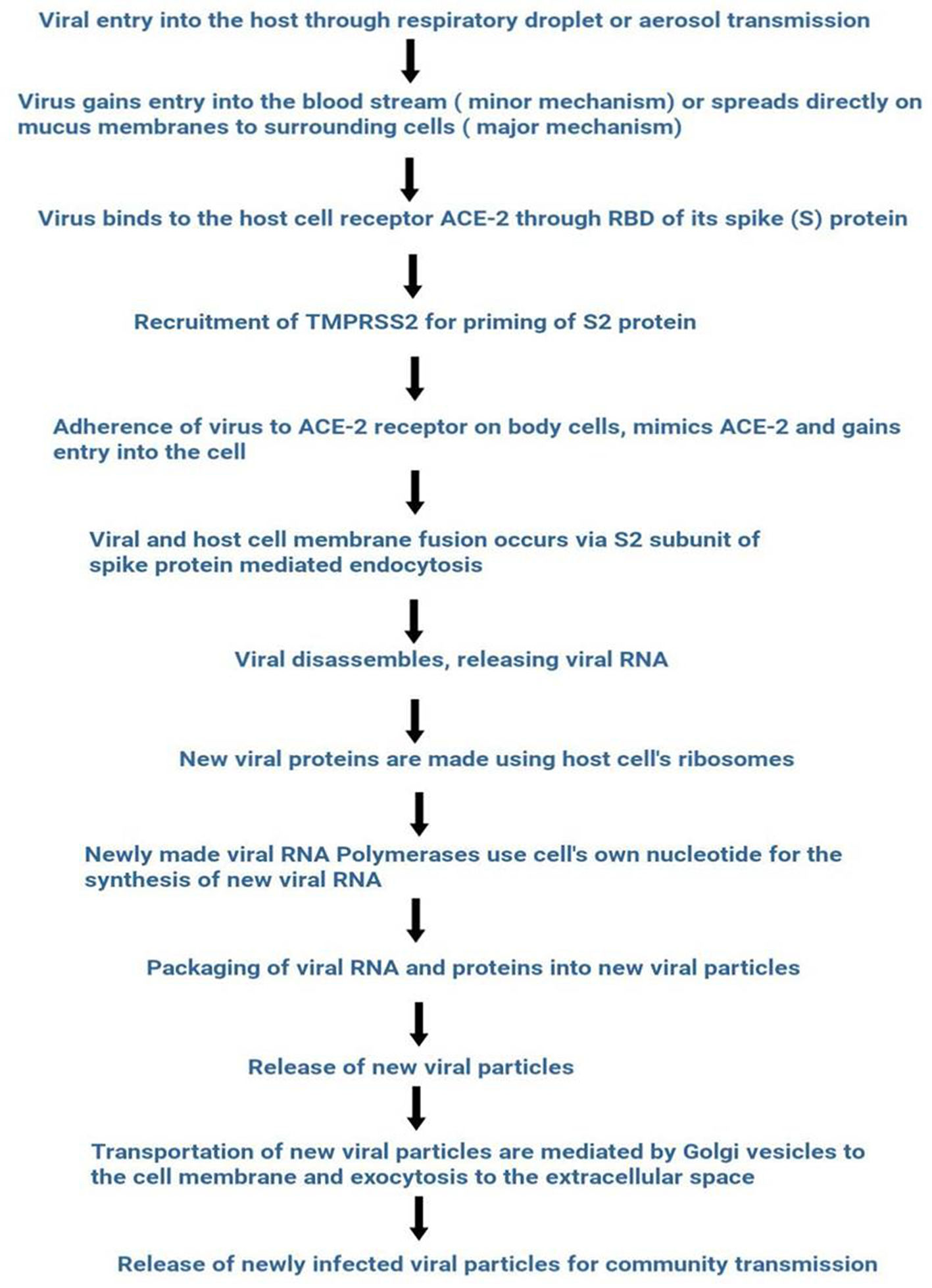
SARS-CoV-2 entry into the host's cell is facilitated by the spike RBD domain. This domain allows binding with the ACE-2 receptor abundantly present in respiratory epithelium and other organs such as the upper esophagus, proximal tubular cells of the kidney, myocardial cells, urothelial cells of bladder.14,15 SARS-CoV-2 captures the target cell by using human angiotensin-converting enzyme 2 (ACE-2) as the entry receptor through the spike glycoprotein (S-protein) and recruits the cellular serine protease TMPRSS2 (Type 2 transmembrane serine protease) for priming of S2 protein.16–21 ACE 2 and TMPRSS2 express in alveolar epithelial type II cells of host target cells.22,23 An overview of the life cycle of SARS-CoV-2 is shown in Fig. 1.
The incubation period of SARS-CoV-2 lies between 1–14 days and symptom manifestation starts around 5 days in most cases which correspond to the highest virus load in respiratory tract.24 The reported symptoms observed in COVID-19 patients are chest tightness/dyspnea, dry cough, fatigue, fever, gastrointestinal symptoms, headache, joint pain, loss of taste and smell, myalgia and sore throat. The principal target for SARS-CoV-2 is the respiratory system but can also affect other vital organs such as central nervous system (CNS), cardiovascular, renal, and gastrointestinal tract.4 SARS-CoV-2 mainly spreads through respiratory droplet transmission from person to person by a person's cough, sneeze or talk; or it may also occur through fomites, live viral particles adhering to inanimate objects.25,26
Previously, several articles have been published on the impact of COVID-19 on environmental factors, where multiple study showed a significant correlation between climatic indicators like humidity, temperature, due point, wind speed rainfall and SARS-CoV-2 induced fatality.27–31However, it has been evident from the previous research that temperature affects the COVID-19 transmissions, but have been found mixed i.e. positive, negative as well as insignificant on the transmissibility of COVID-19 infections.32–34 Another important indicator of environmental issues is air pollution which affects the COVID-19 transmission, morbidity and mortality rate.35–37 For instances, Northern Italy was severely affected by SARS-CoV-2 infections, where the air pollution was found more polluted than the rest of the country. Significantly higher incidence of COVID-19 related casualties have been reported.36,38,39 Therefore, it may be concluded that COVID-19 influences the environmental factors and vice versa. The pandemic has brought major loss of human life globally, and severely affecting almost every aspects of human life including global economy, ecosystem, health sector, industrial operations etc.
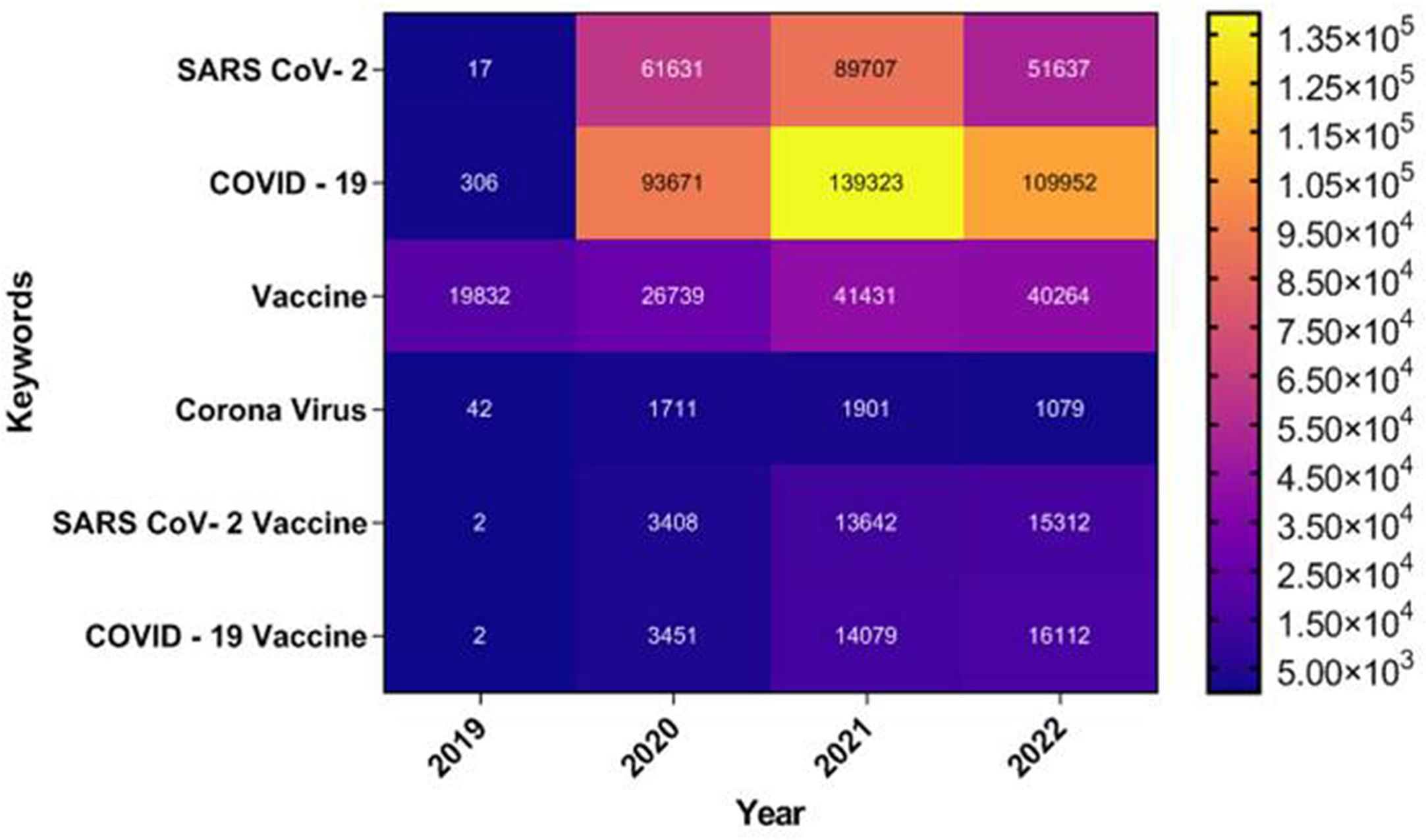
During the initial phases of viral outbreak no effective antiviral therapy was available, although several drugs (Hydroxychloroquine, Chloroquine, Remdesivir, Favipiravir, Azithromycin, Lopinavir), plasma therapy, oxygen treatment, antibiotics, convalescent plasma were being used in an emergency.26 But, the rapid spread and the urgency of the situation resulted in the development of COVID-19 vaccine in just 12 months of the onset of this pandemic. According to an official report from national public health agencies, as on 21st July 2022, 12.24 billion doses of COVID-19 vaccines have been administered worldwide. Today, India with its robust vaccine development program is not only manufacturing domestic COVID-19 vaccines but also distributing them in the global market.40 The present review highlights the different strategies used in traditional vaccine development. An emphasis was given on the strategies used by different institutes and companies for the development of COVID-19 vaccine. A thorough study was done on COVID-19 vaccine development strategy in Indian context. This review also highlights the challenges in the development of COVID-19 vaccine, the ethical concern and the future prospect of the vaccines. A detailed study has also been done on the linkage of COVID-19 with other diseases like cancer and autoimmunity. This study also includes the status of vaccination in different states of India, the status of approved and still under process vaccines. A futuristic model has also been suggested for the development of COVID-19 vaccine. This review is novel as it not only highlights the COVID-19 vaccine development strategies but links it with various diseases like autoimmunity and cancer and suggests the ethical concern, market size and future prospect of COVID-19 vaccine development in India. The step by step research done in this review is shown in Fig. 2.
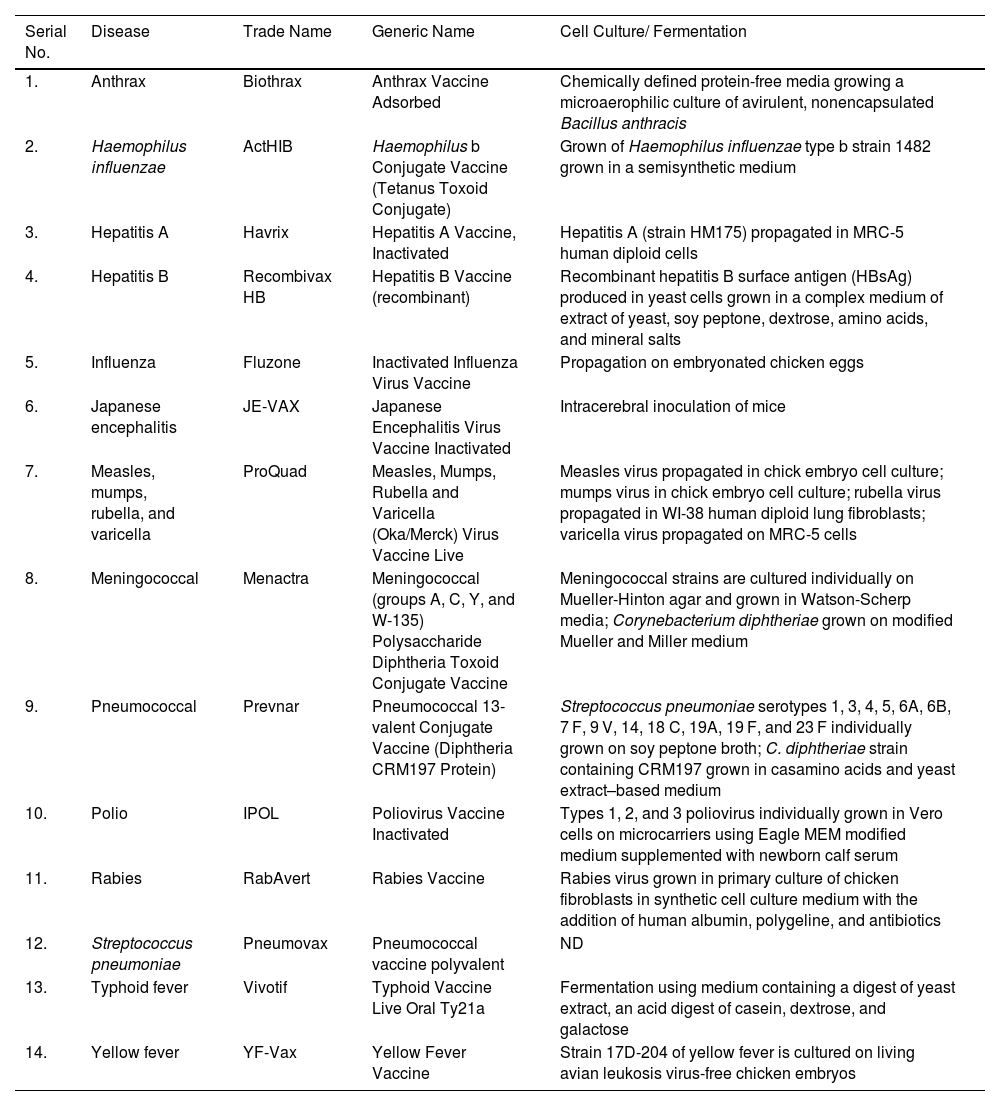
Traditional vaccine development strategiesVaccines are the biological molecules that induce an immune response against any infection or disease inside the body.41 Within the last few years, infectious diseases around the world have resulted in an increment of death rates and are known to be the major cause of mortality.42 Vaccination and immunization, the keystone of public health policy made a major contribution to global health. Development of a vaccine involves the use of microorganisms responsible for the disease development either in the killed or weakened form.43 Vaccine, the most effective prophylaxis has the potential to generate herd immunity in the communities thereby reducing the occurrence of disease and hence blocking its transmission.44 Vaccines help in producing memory cells and hence after subsequent infection with the same pathogen immunity develops faster.45 A safe vaccine does not produce any IgE mediated immune responses in the host's body and hence remains non- allergenic.46 Development of a successful vaccine generally requires 12–15 years including both public and private involvement. The fastest vaccine developed so far, before COVID-19 for mumps took 5 years.6 The live, attenuated mumps vaccine used nowadays in the United States, licensed in 1967 was developed by Maurice Hilleman using virus from his daughter. Several vaccines developed using traditional approach is shown in table 1.
Traditional vaccines and their licensed manufacturing processes.
| Serial No. | Disease | Trade Name | Generic Name | Cell Culture/ Fermentation |
|---|---|---|---|---|
| 1. | Anthrax | Biothrax | Anthrax Vaccine Adsorbed | Chemically defined protein-free media growing a microaerophilic culture of avirulent, nonencapsulated Bacillus anthracis |
| 2. | Haemophilus influenzae | ActHIB | Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate) | Grown of Haemophilus influenzae type b strain 1482 grown in a semisynthetic medium |
| 3. | Hepatitis A | Havrix | Hepatitis A Vaccine, Inactivated | Hepatitis A (strain HM175) propagated in MRC-5 human diploid cells |
| 4. | Hepatitis B | Recombivax HB | Hepatitis B Vaccine (recombinant) | Recombinant hepatitis B surface antigen (HBsAg) produced in yeast cells grown in a complex medium of extract of yeast, soy peptone, dextrose, amino acids, and mineral salts |
| 5. | Influenza | Fluzone | Inactivated Influenza Virus Vaccine | Propagation on embryonated chicken eggs |
| 6. | Japanese encephalitis | JE-VAX | Japanese Encephalitis Virus Vaccine Inactivated | Intracerebral inoculation of mice |
| 7. | Measles, mumps, rubella, and varicella | ProQuad | Measles, Mumps, Rubella and Varicella (Oka/Merck) Virus Vaccine Live | Measles virus propagated in chick embryo cell culture; mumps virus in chick embryo cell culture; rubella virus propagated in WI-38 human diploid lung fibroblasts; varicella virus propagated on MRC-5 cells |
| 8. | Meningococcal | Menactra | Meningococcal (groups A, C, Y, and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine | Meningococcal strains are cultured individually on Mueller-Hinton agar and grown in Watson-Scherp media; Corynebacterium diphtheriae grown on modified Mueller and Miller medium |
| 9. | Pneumococcal | Prevnar | Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein) | Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7 F, 9 V, 14, 18 C, 19A, 19 F, and 23 F individually grown on soy peptone broth; C. diphtheriae strain containing CRM197 grown in casamino acids and yeast extract–based medium |
| 10. | Polio | IPOL | Poliovirus Vaccine Inactivated | Types 1, 2, and 3 poliovirus individually grown in Vero cells on microcarriers using Eagle MEM modified medium supplemented with newborn calf serum |
| 11. | Rabies | RabAvert | Rabies Vaccine | Rabies virus grown in primary culture of chicken fibroblasts in synthetic cell culture medium with the addition of human albumin, polygeline, and antibiotics |
| 12. | Streptococcus pneumoniae | Pneumovax | Pneumococcal vaccine polyvalent | ND |
| 13. | Typhoid fever | Vivotif | Typhoid Vaccine Live Oral Ty21a | Fermentation using medium containing a digest of yeast extract, an acid digest of casein, dextrose, and galactose |
| 14. | Yellow fever | YF-Vax | Yellow Fever Vaccine | Strain 17D-204 of yellow fever is cultured on living avian leukosis virus-free chicken embryos |
ND – Not disclosed.
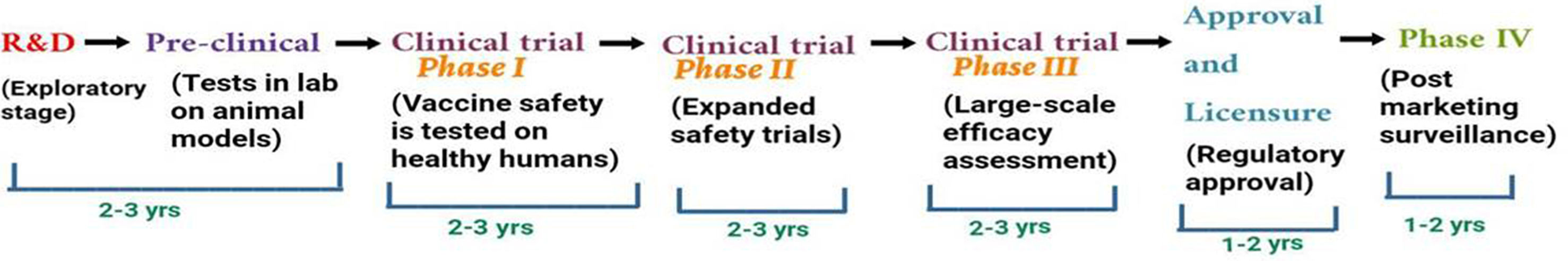
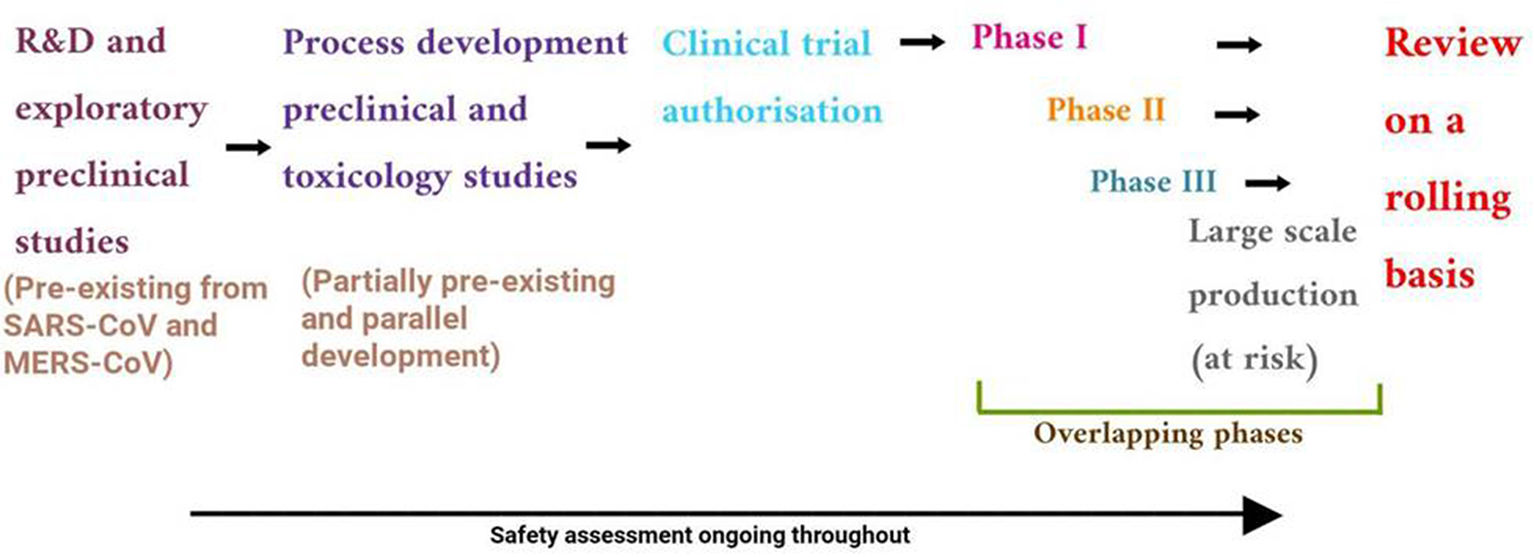
The development of successful vaccine safe for public use requires proper protocol development by regulatory agencies such as European Medicines Agency (EMA), the World Health Organization (WHO), the United States Food and Drug Administration (USFDA) and other bodies. The development process involves preclinical (in vitro and in vivo testing in animals) and clinical (clinical trials in human candidates) stages for the planning of clinical development path of novel vaccine candidate.47 The different stages of vaccine development involves exploratory, pre-clinical, clinical and post marketing stage as shown in Fig. 3.
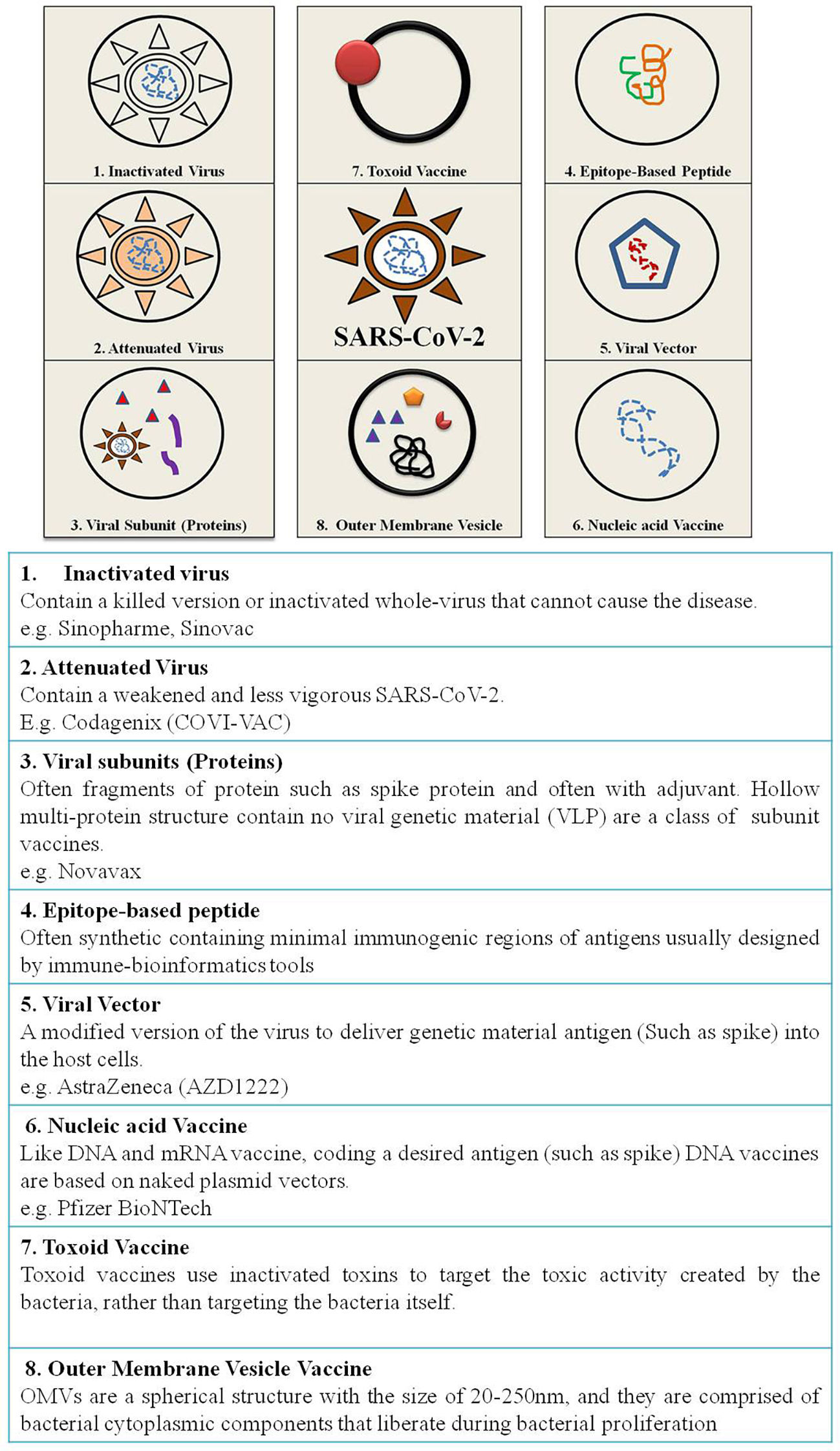
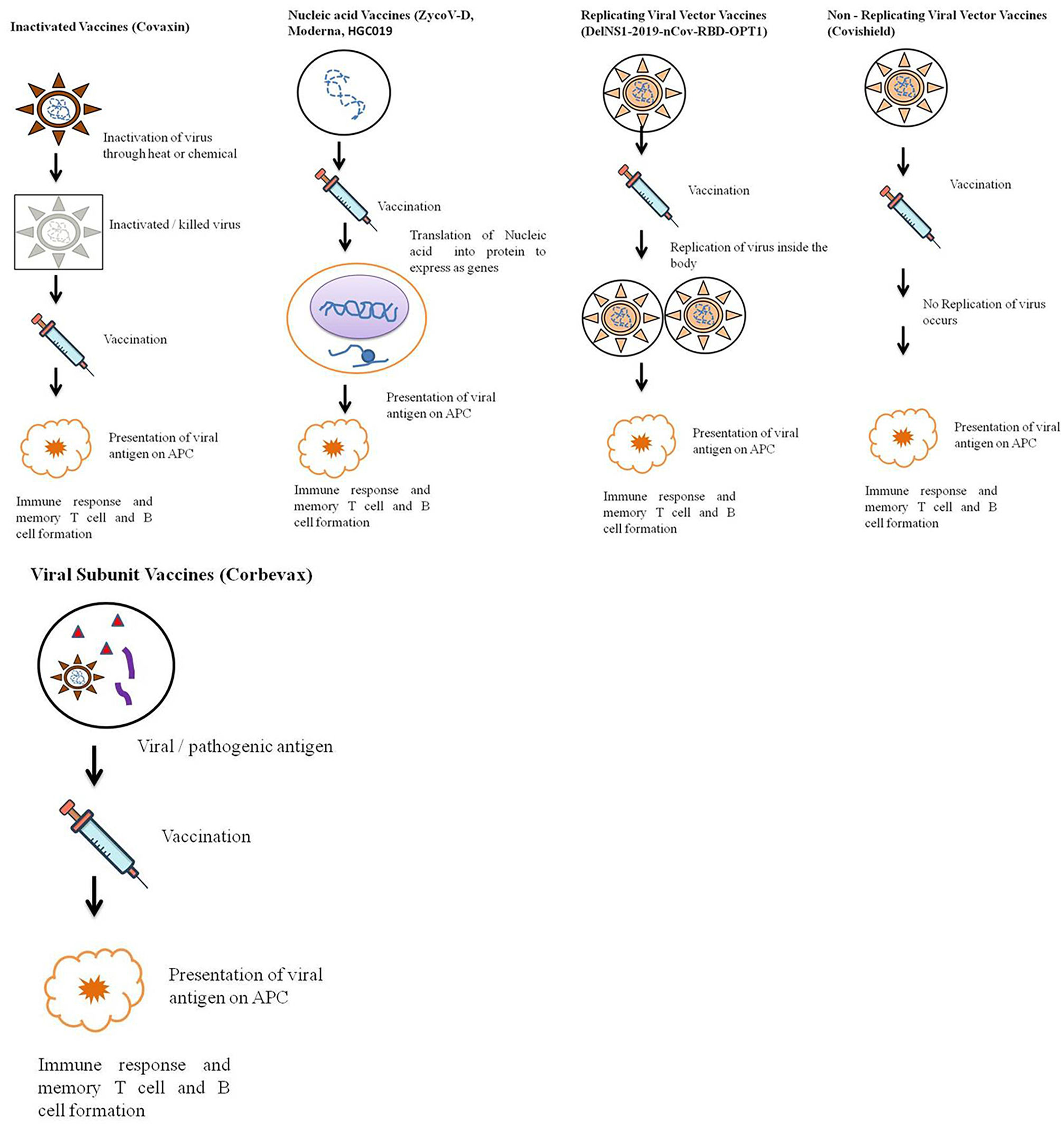
According to the candidate used for vaccine development vaccine can be live attenuated, inactivated, protein-based, nucleic acid based, viral vector based, toxoid, conjugate and outer membrane vesicles vaccine43 (Fig. 4).
Live attenuated vaccinesThis is the traditional method of vaccine development containing whole bacteria or virus in its weakened form so as to build up a protective immune response.48 Live attenuated vaccines can be obtained by transferring the disease causing organism through a series of cell cultures or embryos of animals (chick embryos).45 The weakened organism replicates same as a natural infection thereby causing a humoral and cell mediated immune response.44 The live attenuated vaccines induce immune system against specific viruses and often need only a single immunization without booster doses.45 However, live attenuated vaccines are not suitable for immunocompromised as well as weak people as the disease can develop. The vaccines developed using live attenuated form include BCG, smallpox, Polio (Albert Sabin's oral polio vaccine), measles, rubella, mumps, rotavirus vaccine, varicella vaccine, and yellow fever vaccine.44,45
Inactivated vaccinesThese vaccines do not contain any live ingredient as they are produced from pathogens deactivated by formaldehyde or heat.16 These chemicals impair the replicative ability of pathogens but they can still be recognized by the host's immune system. To induce more immunogenicity booster doses are required as these candidates are less immunogenic.49 The vaccines developed against Hepatitis A, rabies, Salk polio and influenza are some important inactivated vaccines.43,45,50
Protein based vaccinesProtein subunit vaccinesThese vaccines use antigenic components (like spike protein) generated in vitro.44 They include only epitopes that trigger the immune system.49 This vaccine does not contain whole bacteria or virus so that immune response can target only a subset of pathogen protein with antigenic properties.45 Subunit vaccines do not create long lasting immune responses, hence require adjuvants and additional booster doses.43 Although peptide vaccines possess high efficacy and several advantages, yet they are not licensed for human use being less immunogenic. These vaccines require adjuvants to enhance immunogenicity and susceptible to enzymatic degradation due to short stretch of amino acids.41 Acellular pertussis vaccines and Influenza vaccines are some vaccines developed using protein subunit approach.
Virus-like particles (VLPs)These molecules closely resemble viruses but incapable of infecting the host cell as they don't contain viral genetic material. They are safe and stimulate strong immune responses but are difficult to manufacture.44 These virus particles selected are immunological as well as capable of eliciting both antibody and cell-mediated immune responses.51
These virus particles can be generated experimentally using recombinant viral proteins in the laboratory. These particles may be expressed in an array of expression systems including prokaryotic cells, yeasts, insect cell lines, plants and mammalian cell lines.51 The capsid proteins of HIV, HBV, Hepatitis C, bacteriophages are commonly used to develop vaccine.51–54
Nucleic acid vaccinesNucleic acid vaccines are quick and easy to develop using genetic material from a pathogen.
DNA vaccinesDNA vaccines are produced by inserting antigenic components of a pathogen into a plasmid stimulating humoral as well as cell mediated immune response. Electroporation is commonly used for administration of these plasmids resulting in production of high antibody titer.44 These vaccines also encode for adjuvants required for the stimulation of adaptive immune response.16 The design of the vaccines allows its translocation inside the nucleus of the host cell, where it is transcribed into a functional copy of mRNA.55
RNA vaccinesThese vaccines contain messenger RNA (mRNA) molecule inside a shell of lipid membrane which protects as well as facilitates the entry of vaccine by fusing with the cell membrane. The mRNA molecule of the vaccine encodes for a disease specific antigen which is displayed on the cell surface eliciting a proper immune response.56 These mRNA molecules are safe as they do not cause disease and results in Th2 cell skewed response.44 mRNA vaccine supports rapid vaccine development program as it mimics antigen structure and expression similar to natural infection.57
Viral vector vaccinesThese vaccines are produced by isolating an antigen from pathogen and integrating it into a bacterial or viral vector system.43 The bacteria or virus behaves as a vector that replicates and expresses pathogenic gene inside the cell.45 Viral vector vaccines are grown on cell lines hence easily and cheaply developed. As viral vector vaccines produce endogenous antigen hence, activate both humoral and cellular immune responses in single dose.56,57 Viral vector vaccines depending on the replicating ability can either be replicating and non-replicating.
Replicating viral vector vaccinesThis vaccine strategy engages innate immunity targeting mainly mucosal sites. These types of vaccines have the ability to make new viral particles besides delivering the vaccine antigen when used as a vaccine delivery platform. Separate virus like measles or adenovirus are genetically engineered and modified to express the desired gene of interest.1 Adenoviruses are the most commonly used virus for replicating viral vector vaccines. Subsequent booster doses are essential to acquire long term humoral immunity. Examples of replicating viral vector vaccines are: HPV, pertussis and Hepatitis B.
Non-replicating viral vector vaccinesIn this vaccine strategy, viral vectors are genetically modified to impair the replicating mechanism and hence become non-replicating virus. These makes the viral strain attenuated and a potent vaccine candidate as it only triggers immune response without replicating in human host. Adenoviruses are the most commonly exploited virus for vector vaccines. These vaccines only produce the vaccine antigen and are unable to make new viral particles. The inactivated gene from an unrelated virus (measles or adenovirus) is genetically engineered to express the gene of interest.43 The non-replicating vaccine induce pathogen specific host response which includes killed pathogens, synthetic pathogen structure or recombinant pathogen product as antigens. This type of vaccine creates strong humoral as well as CD8+ T-cell mediated immune response but the strength depends on the type of viral serotype being used. Non-replicating vaccines are safer over others as the risk of disease onset is quite low.
Toxoid vaccinesToxoid vaccines contain attenuated toxins inducing humoral immune response. The purification of bacterial toxins followed by their inactivation with formaldehyde leads to generation of a toxoid, routinely used to make diphtheria and tetanus toxins. The inoculation with a toxoid, results in release of anti-toxoid antibody that readily binds and neutralizes its toxicity. Toxoid vaccines do not provide prolonged immunity; hence require regular booster doses for effective protection. Toxoid vaccines provide protection against diphtheria and tetanus.
Conjugate vaccinesConjugate vaccines made by combining two distinct components, hence similar to recombinant vaccines. Bacterial coat fragments are coupled to a carrier protein that is utilized in vaccination. Conjugate vaccines elicit a stronger co-immune response whereas fragments of bacteria show less immunological response. This bacterial fragment does not cause disease, but provides protection against future infections when associated with carrier proteins. These vaccines are used in pneumococcal vaccinations to protect children against bacterial illnesses.58
Outer membrane vesicles vaccineThe outer membrane of Gram-negative bacteria, sometimes form extracellular vesicles (outer membrane vesicles, OMV) with a diameter of 20 to 300 nm. Since the driving factor for OMV development remained unclear for a long period, the establishment of OMVs still remains unclear.59,60 OMV production assumed to be a random stress response from bacterial cells 61.
In recent years, OMVs have attracted significant attraction in form of vaccine delivery system against bacterial infections.62 OMV investigated as a potential vaccination against Neisseria meningitidis serogroup B illness. Following the success of the MeNZB OMV-based vaccine in suppressing N. meningitidis B epidemic in New Zealand, subsequent research culminated in approval of Bexsero, against meningococcal B strains 63.
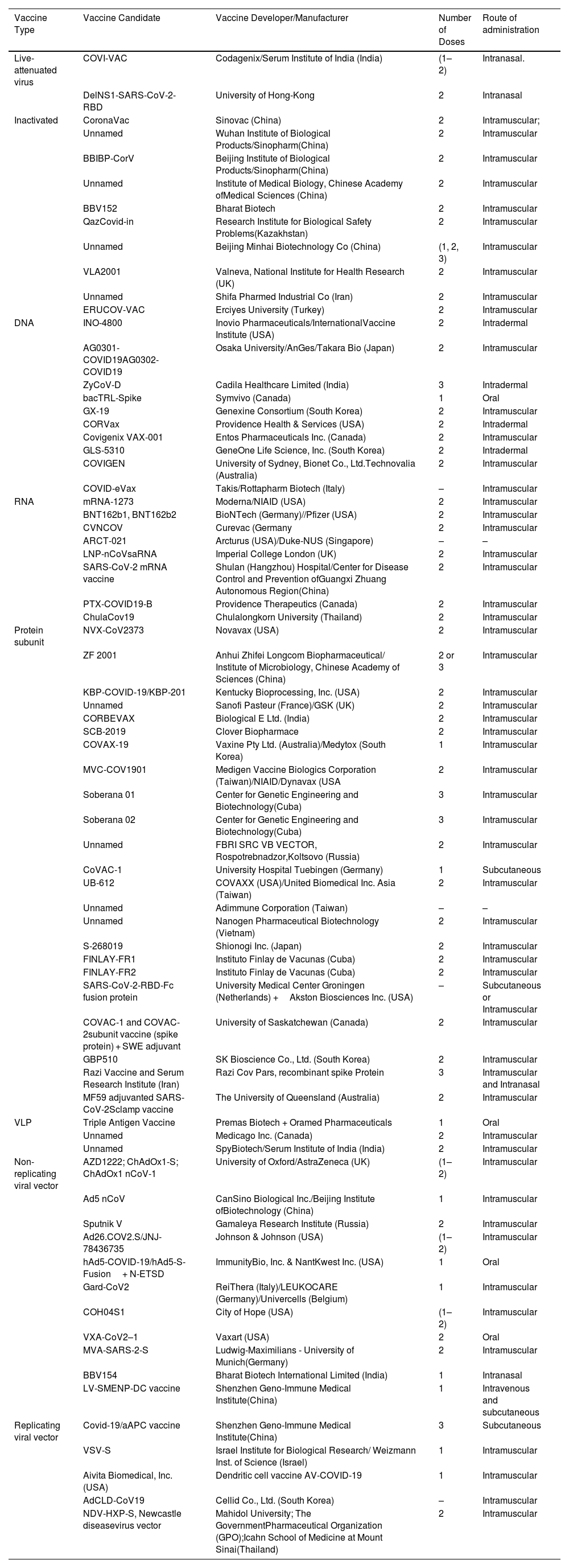
COVID-19 vaccine developmentVaccine development; a long and complex process which takes years and even decades for successful vaccine formulation. However, the COVID-19 vaccine development program took a year and was a miracle across the globe.60 COVID-19 vaccine development was a big challenge for the researchers as it was targeted to develop a vaccine in a span of 12–24 months.43 The rapid development of COVID-19 vaccine was facilitated by well-timed release of viral genome sequence, advancement and innovation in vaccine technology, active participation of global scientific community, robust regulatory framework, adequate funding by the government as well as huge market demand.61 By the start of December, 2020, the researchers around the globe announced excellent results in vaccine development (Table 2) and on 2nd December, 2020, vaccine developed by Pfizer with German biotech firm BioNTech, became the first fully tested vaccine to be approved for emergency use.62 Many vaccines were approved by several countries as shown in Table 3. The strategies used by the vaccines developed by several countries are shown in Table 4.
List of different types of COVID-19 Vaccines.
| Vaccine Type | Vaccine Candidate | Vaccine Developer/Manufacturer | Number of Doses | Route of administration |
|---|---|---|---|---|
| Live-attenuated virus | COVI-VAC | Codagenix/Serum Institute of India (India) | (1–2) | Intranasal. |
| DelNS1-SARS-CoV-2-RBD | University of Hong-Kong | 2 | Intranasal | |
| Inactivated | CoronaVac | Sinovac (China) | 2 | Intramuscular; |
| Unnamed | Wuhan Institute of Biological Products/Sinopharm(China) | 2 | Intramuscular | |
| BBIBP-CorV | Beijing Institute of Biological Products/Sinopharm(China) | 2 | Intramuscular | |
| Unnamed | Institute of Medical Biology, Chinese Academy ofMedical Sciences (China) | 2 | Intramuscular | |
| BBV152 | Bharat Biotech | 2 | Intramuscular | |
| QazCovid-in | Research Institute for Biological Safety Problems(Kazakhstan) | 2 | Intramuscular | |
| Unnamed | Beijing Minhai Biotechnology Co (China) | (1, 2, 3) | Intramuscular | |
| VLA2001 | Valneva, National Institute for Health Research (UK) | 2 | Intramuscular | |
| Unnamed | Shifa Pharmed Industrial Co (Iran) | 2 | Intramuscular | |
| ERUCOV-VAC | Erciyes University (Turkey) | 2 | Intramuscular | |
| DNA | INO-4800 | Inovio Pharmaceuticals/InternationalVaccine Institute (USA) | 2 | Intradermal |
| AG0301-COVID19AG0302-COVID19 | Osaka University/AnGes/Takara Bio (Japan) | 2 | Intramuscular | |
| ZyCoV-D | Cadila Healthcare Limited (India) | 3 | Intradermal | |
| bacTRL-Spike | Symvivo (Canada) | 1 | Oral | |
| GX-19 | Genexine Consortium (South Korea) | 2 | Intramuscular | |
| CORVax | Providence Health & Services (USA) | 2 | Intradermal | |
| Covigenix VAX-001 | Entos Pharmaceuticals Inc. (Canada) | 2 | Intramuscular | |
| GLS-5310 | GeneOne Life Science, Inc. (South Korea) | 2 | Intradermal | |
| COVIGEN | University of Sydney, Bionet Co., Ltd.Technovalia (Australia) | 2 | Intramuscular | |
| COVID-eVax | Takis/Rottapharm Biotech (Italy) | – | Intramuscular | |
| RNA | mRNA-1273 | Moderna/NIAID (USA) | 2 | Intramuscular |
| BNT162b1, BNT162b2 | BioNTech (Germany)//Pfizer (USA) | 2 | Intramuscular | |
| CVNCOV | Curevac (Germany | 2 | Intramuscular | |
| ARCT-021 | Arcturus (USA)/Duke-NUS (Singapore) | – | – | |
| LNP-nCoVsaRNA | Imperial College London (UK) | 2 | Intramuscular | |
| SARS-CoV-2 mRNA vaccine | Shulan (Hangzhou) Hospital/Center for Disease Control and Prevention ofGuangxi Zhuang Autonomous Region(China) | 2 | Intramuscular | |
| PTX-COVID19-B | Providence Therapeutics (Canada) | 2 | Intramuscular | |
| ChulaCov19 | Chulalongkorn University (Thailand) | 2 | Intramuscular | |
| Protein subunit | NVX-CoV2373 | Novavax (USA) | 2 | Intramuscular |
| ZF 2001 | Anhui Zhifei Longcom Biopharmaceutical/ Institute of Microbiology, Chinese Academy of Sciences (China) | 2 or 3 | Intramuscular | |
| KBP-COVID-19/KBP-201 | Kentucky Bioprocessing, Inc. (USA) | 2 | Intramuscular | |
| Unnamed | Sanofi Pasteur (France)/GSK (UK) | 2 | Intramuscular | |
| CORBEVAX | Biological E Ltd. (India) | 2 | Intramuscular | |
| SCB-2019 | Clover Biopharmace | 2 | Intramuscular | |
| COVAX-19 | Vaxine Pty Ltd. (Australia)/Medytox (South Korea) | 1 | Intramuscular | |
| MVC-COV1901 | Medigen Vaccine Biologics Corporation (Taiwan)/NIAID/Dynavax (USA | 2 | Intramuscular | |
| Soberana 01 | Center for Genetic Engineering and Biotechnology(Cuba) | 3 | Intramuscular | |
| Soberana 02 | Center for Genetic Engineering and Biotechnology(Cuba) | 3 | Intramuscular | |
| Unnamed | FBRI SRC VB VECTOR, Rospotrebnadzor,Koltsovo (Russia) | 2 | Intramuscular | |
| CoVAC-1 | University Hospital Tuebingen (Germany) | 1 | Subcutaneous | |
| UB-612 | COVAXX (USA)/United Biomedical Inc. Asia (Taiwan) | 2 | Intramuscular | |
| Unnamed | Adimmune Corporation (Taiwan) | – | – | |
| Unnamed | Nanogen Pharmaceutical Biotechnology (Vietnam) | 2 | Intramuscular | |
| S-268019 | Shionogi Inc. (Japan) | 2 | Intramuscular | |
| FINLAY-FR1 | Instituto Finlay de Vacunas (Cuba) | 2 | Intramuscular | |
| FINLAY-FR2 | Instituto Finlay de Vacunas (Cuba) | 2 | Intramuscular | |
| SARS-CoV-2-RBD-Fc fusion protein | University Medical Center Groningen (Netherlands) +Akston Biosciences Inc. (USA) | – | Subcutaneous or Intramuscular | |
| COVAC-1 and COVAC-2subunit vaccine (spike protein) + SWE adjuvant | University of Saskatchewan (Canada) | 2 | Intramuscular | |
| GBP510 | SK Bioscience Co., Ltd. (South Korea) | 2 | Intramuscular | |
| Razi Vaccine and Serum Research Institute (Iran) | Razi Cov Pars, recombinant spike Protein | 3 | Intramuscular and Intranasal | |
| MF59 adjuvanted SARS-CoV-2Sclamp vaccine | The University of Queensland (Australia) | 2 | Intramuscular | |
| VLP | Triple Antigen Vaccine | Premas Biotech + Oramed Pharmaceuticals | 1 | Oral |
| Unnamed | Medicago Inc. (Canada) | 2 | Intramuscular | |
| Unnamed | SpyBiotech/Serum Institute of India (India) | 2 | Intramuscular | |
| Non-replicating viral vector | AZD1222; ChAdOx1-S; ChAdOx1 nCoV-1 | University of Oxford/AstraZeneca (UK) | (1–2) | Intramuscular |
| Ad5 nCoV | CanSino Biological Inc./Beijing Institute ofBiotechnology (China) | 1 | Intramuscular | |
| Sputnik V | Gamaleya Research Institute (Russia) | 2 | Intramuscular | |
| Ad26.COV2.S/JNJ-78436735 | Johnson & Johnson (USA) | (1–2) | Intramuscular | |
| hAd5-COVID-19/hAd5-S-Fusion+ N-ETSD | ImmunityBio, Inc. & NantKwest Inc. (USA) | 1 | Oral | |
| Gard-CoV2 | ReiThera (Italy)/LEUKOCARE (Germany)/Univercells (Belgium) | 1 | Intramuscular | |
| COH04S1 | City of Hope (USA) | (1–2) | Intramuscular | |
| VXA-CoV2–1 | Vaxart (USA) | 2 | Oral | |
| MVA-SARS-2-S | Ludwig-Maximilians - University of Munich(Germany) | 2 | Intramuscular | |
| BBV154 | Bharat Biotech International Limited (India) | 1 | Intranasal | |
| LV-SMENP-DC vaccine | Shenzhen Geno-Immune Medical Institute(China) | 1 | Intravenous and subcutaneous | |
| Replicating viral vector | Covid-19/aAPC vaccine | Shenzhen Geno-Immune Medical Institute(China) | 3 | Subcutaneous |
| VSV-S | Israel Institute for Biological Research/ Weizmann Inst. of Science (Israel) | 1 | Intramuscular | |
| Aivita Biomedical, Inc. (USA) | Dendritic cell vaccine AV-COVID-19 | 1 | Intramuscular | |
| AdCLD-CoV19 | Cellid Co., Ltd. (South Korea) | – | Intramuscular | |
| NDV-HXP-S, Newcastle diseasevirus vector | Mahidol University; The GovernmentPharmaceutical Organization (GPO);Icahn School of Medicine at Mount Sinai(Thailand) | 2 | Intramuscular |
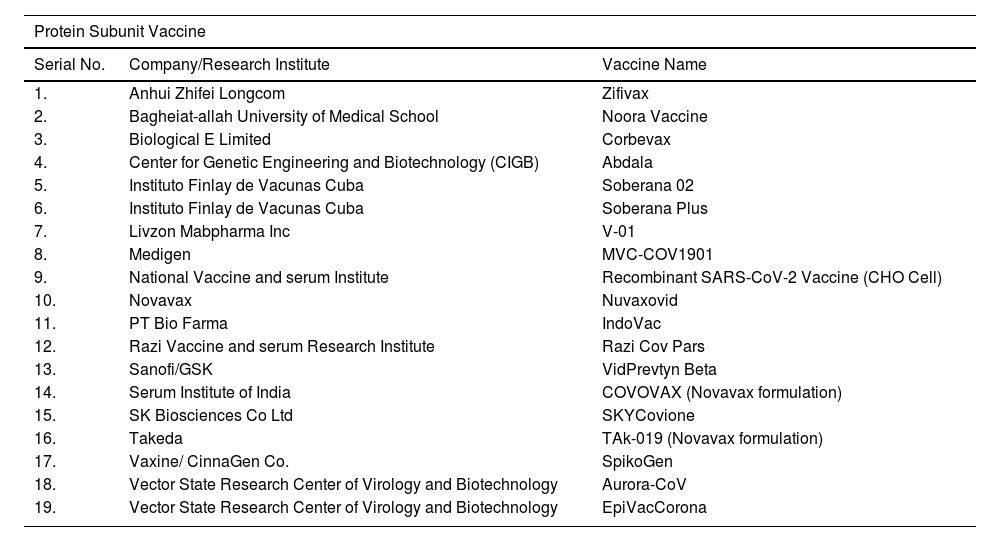
List of COVID-19 vaccines approved by at least one country.
| Protein Subunit Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Anhui Zhifei Longcom | Zifivax |
| 2. | Bagheiat-allah University of Medical School | Noora Vaccine |
| 3. | Biological E Limited | Corbevax |
| 4. | Center for Genetic Engineering and Biotechnology (CIGB) | Abdala |
| 5. | Instituto Finlay de Vacunas Cuba | Soberana 02 |
| 6. | Instituto Finlay de Vacunas Cuba | Soberana Plus |
| 7. | Livzon Mabpharma Inc | V-01 |
| 8. | Medigen | MVC-COV1901 |
| 9. | National Vaccine and serum Institute | Recombinant SARS-CoV-2 Vaccine (CHO Cell) |
| 10. | Novavax | Nuvaxovid |
| 11. | PT Bio Farma | IndoVac |
| 12. | Razi Vaccine and serum Research Institute | Razi Cov Pars |
| 13. | Sanofi/GSK | VidPrevtyn Beta |
| 14. | Serum Institute of India | COVOVAX (Novavax formulation) |
| 15. | SK Biosciences Co Ltd | SKYCovione |
| 16. | Takeda | TAk-019 (Novavax formulation) |
| 17. | Vaxine/ CinnaGen Co. | SpikoGen |
| 18. | Vector State Research Center of Virology and Biotechnology | Aurora-CoV |
| 19. | Vector State Research Center of Virology and Biotechnology | EpiVacCorona |
| Inactivated Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Bharat Biotech | Covaxin |
| 2. | Chumakov Center | KoviVac |
| 3. | Health Institutes of Turkey | Turkovac |
| 4. | Organization of Defensive Innovation and Research | FAKHRAVAC (MIVAC) |
| 5. | Research Institute for Biological Safety Problems (RIBSP) | QazVac |
| 6. | Shenzhen Kangtai Biological Products Co | KCONVAC |
| 7. | Shifi Pharmed Industrial Co | COVIran Barekat |
| 8. | Sinopharm (Beijing) | Covilo |
| 9. | Sinopharm (Wuhan) | Inactivated (Vero Cells) |
| 10. | Sinovac | CoronaVac |
| 11. | Valneva | VLA2001 |
| Non Replicating Viral Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Bharat Biotech | iNCOVACC |
| 2. | Cansino | Convidecia |
| 3. | Cansino | Convidecia Air |
| 4. | Gamaleya | Gam-COVID-Vac |
| 5. | Gamaleya | Sputnik Light |
| 6. | Gamaleya | Sputnik V |
| 7. | Janssen (Johnson & Johnson) | Jcovden |
| 8. | Oxford/AstraZeneca | Vaxzevria |
| 9. | Serum Institute of India | Covishield (Oxford/AstraZeneca formulation) |
| RNA Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Gennova Biopharmaceuticals Limited | GEMCOVAC-19 |
| 2. | Moderna | Spikevax |
| 3. | Moderna | Spikevax Bivalent Original/Omicron BA.1 |
| 4. | Moderna | Spikevax Bivalent Original/Omicron BA.4/ BA.5 |
| 5. | Pfizer/BioNTech | Comirnaty |
| 6. | Pfizer/BioNTech | Comirnaty Bivalent Original/Omicron BA.1 |
| 7. | Pfizer/BioNTech | Comirnaty Bivalent Original/Omicron BA.4/ BA.5 |
| 8. | Takeda | TAK-919 (Moderna formulation) |
| 9. | Walvax | AWcorna |
| VLP (Virus Like Particle) Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Medicago | Covifenz |
| DNA Vaccine | ||
|---|---|---|
| Serial No. | Company/Research Institute | Vaccine Name |
| 1. | Zydus Cadila | ZyCoV-D |
Types of COVID-19 Vaccine developed across the globe.
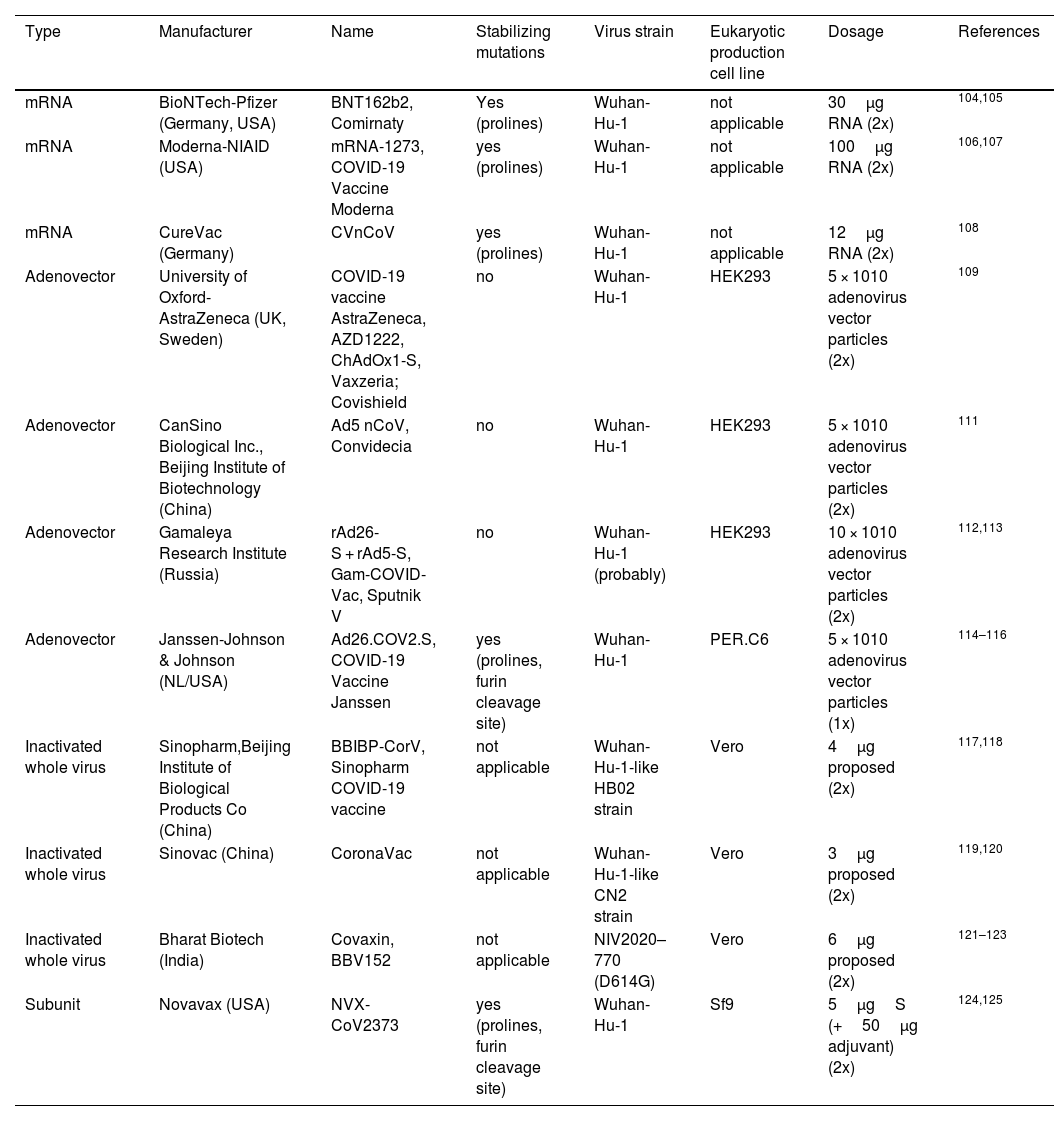
| Type | Manufacturer | Name | Stabilizing mutations | Virus strain | Eukaryotic production cell line | Dosage | References |
|---|---|---|---|---|---|---|---|
| mRNA | BioNTech-Pfizer (Germany, USA) | BNT162b2, Comirnaty | Yes (prolines) | Wuhan-Hu-1 | not applicable | 30μg RNA (2x) | 104,105 |
| mRNA | Moderna-NIAID (USA) | mRNA-1273, COVID-19 Vaccine Moderna | yes (prolines) | Wuhan-Hu-1 | not applicable | 100μg RNA (2x) | 106,107 |
| mRNA | CureVac (Germany) | CVnCoV | yes (prolines) | Wuhan-Hu-1 | not applicable | 12μg RNA (2x) | 108 |
| Adenovector | University of Oxford-AstraZeneca (UK, Sweden) | COVID-19 vaccine AstraZeneca, AZD1222, ChAdOx1-S, Vaxzeria; Covishield | no | Wuhan-Hu-1 | HEK293 | 5 × 1010 adenovirus vector particles (2x) | 109 |
| Adenovector | CanSino Biological Inc., Beijing Institute of Biotechnology (China) | Ad5 nCoV, Convidecia | no | Wuhan-Hu-1 | HEK293 | 5 × 1010 adenovirus vector particles (2x) | 111 |
| Adenovector | Gamaleya Research Institute (Russia) | rAd26-S + rAd5-S, Gam-COVID-Vac, Sputnik V | no | Wuhan-Hu-1 (probably) | HEK293 | 10 × 1010 adenovirus vector particles (2x) | 112,113 |
| Adenovector | Janssen-Johnson & Johnson (NL/USA) | Ad26.COV2.S, COVID-19 Vaccine Janssen | yes (prolines, furin cleavage site) | Wuhan-Hu-1 | PER.C6 | 5 × 1010 adenovirus vector particles (1x) | 114–116 |
| Inactivated whole virus | Sinopharm,Beijing Institute of Biological Products Co (China) | BBIBP-CorV, Sinopharm COVID-19 vaccine | not applicable | Wuhan-Hu-1-like HB02 strain | Vero | 4μg proposed (2x) | 117,118 |
| Inactivated whole virus | Sinovac (China) | CoronaVac | not applicable | Wuhan-Hu-1-like CN2 strain | Vero | 3μg proposed (2x) | 119,120 |
| Inactivated whole virus | Bharat Biotech (India) | Covaxin, BBV152 | not applicable | NIV2020–770 (D614G) | Vero | 6μg proposed (2x) | 121–123 |
| Subunit | Novavax (USA) | NVX-CoV2373 | yes (prolines, furin cleavage site) | Wuhan-Hu-1 | Sf9 | 5μgS (+50μg adjuvant) (2x) | 124,125 |
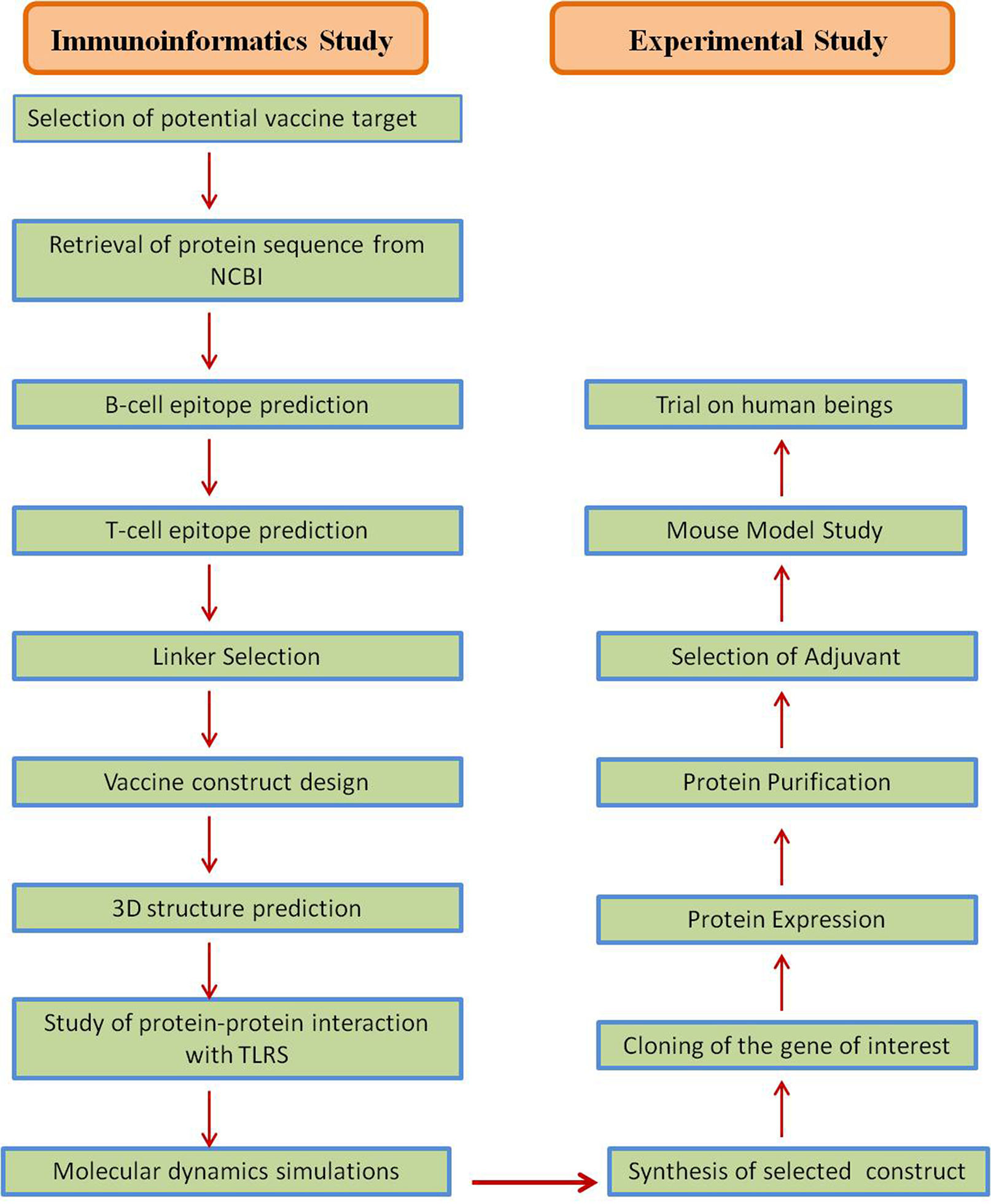
Several strategies were accepted in the development of Coronavirus vaccines (Table 4), and most of these strategies targeted the S-protein or the surface-exposed spike (S) as the major inducer of neutralizing antibodies.64 Jha et al., reported the antigenicity as well as allergenicity of different structural proteins of SARS-CoV-2 to design vaccines against SARS-CoV-2. This analysis showed that the envelope protein (E) to be highly antigenic having the antigenicity of 0.6025 followed by Membrane glycoprotein (M) having antigenicity of 0.5102, Nucleocapsid phosphoprotein having antigenicity of 0.5059 and Surface glycoprotein (S) with antigenicity of 0.4696.46 S protein plays a major role in the stimulation of protective immunity during infection with SARS-CoV-2 by evoking neutralizing antibodies and T-cell responses. Hence, the full length or partial protein of S-glycoprotein can be the most effective vaccine target against coronavirus.9,12,64,65 The vaccine designing strategy includes both immunoinformatics and experimental studies for getting a suitable candidate for vaccine candidate. The immunoinformatics study helps in selection of candidate whereas experimental studies confirm the candidate for vaccine production (Fig. 5).
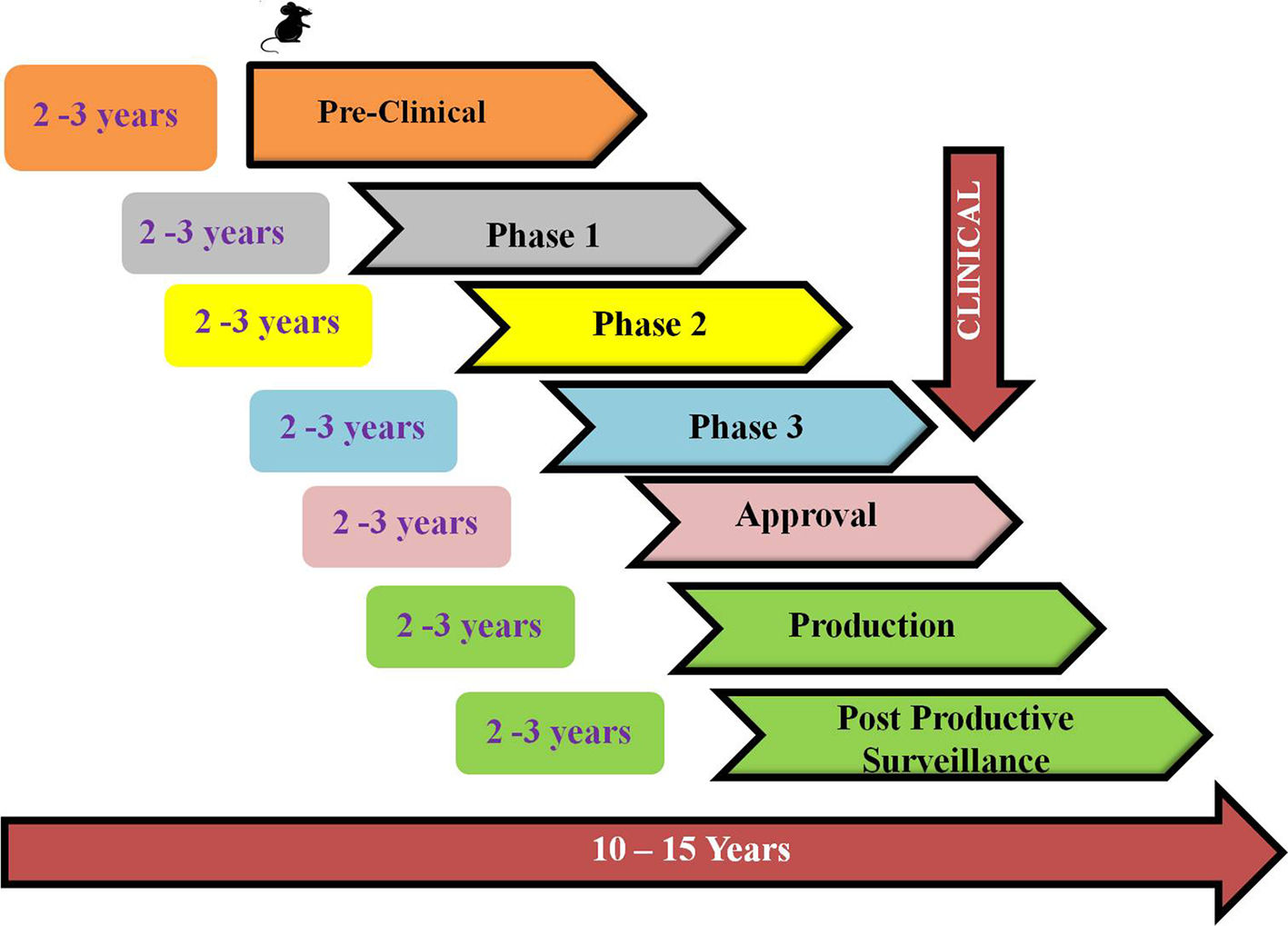
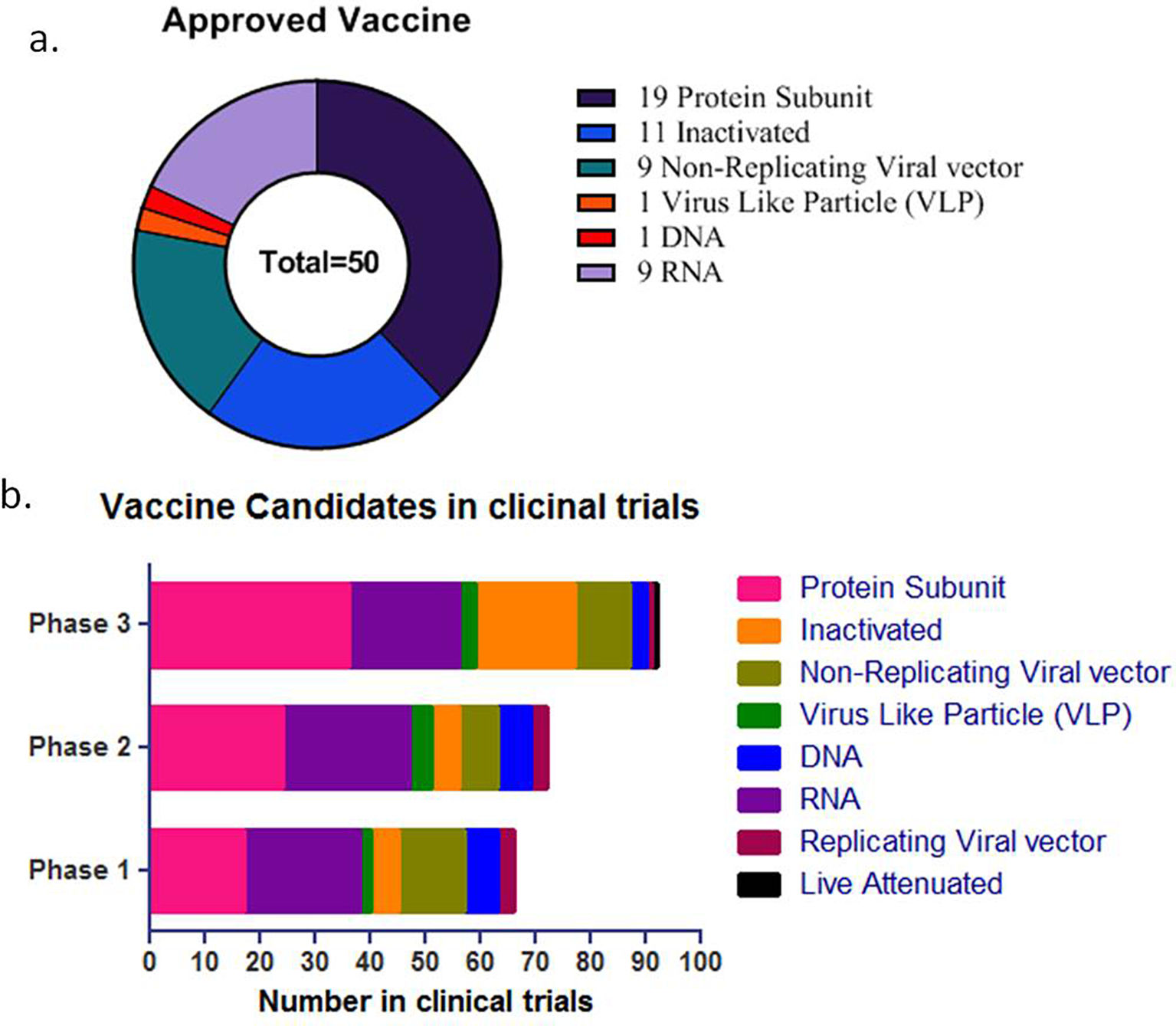
COVID-19 vaccine development process started with the genome sequence of SARS-CoV-2, first available on January, 11, 2020. (Fig. 6). The preclinical data for SARS-CoV and MERS saved considerable time eliminating the initial step of exploratory phase.43 Multiple clinical trials initiated to reduce the time horizon where one phase was immediately followed by second (Fig. 7).43 The different strategies used in COVID-19 vaccine development is shown in Fig. 8. A list of COVID-19 vaccines is shown in Table 5, while those approved by WHO is shown in Table 6.
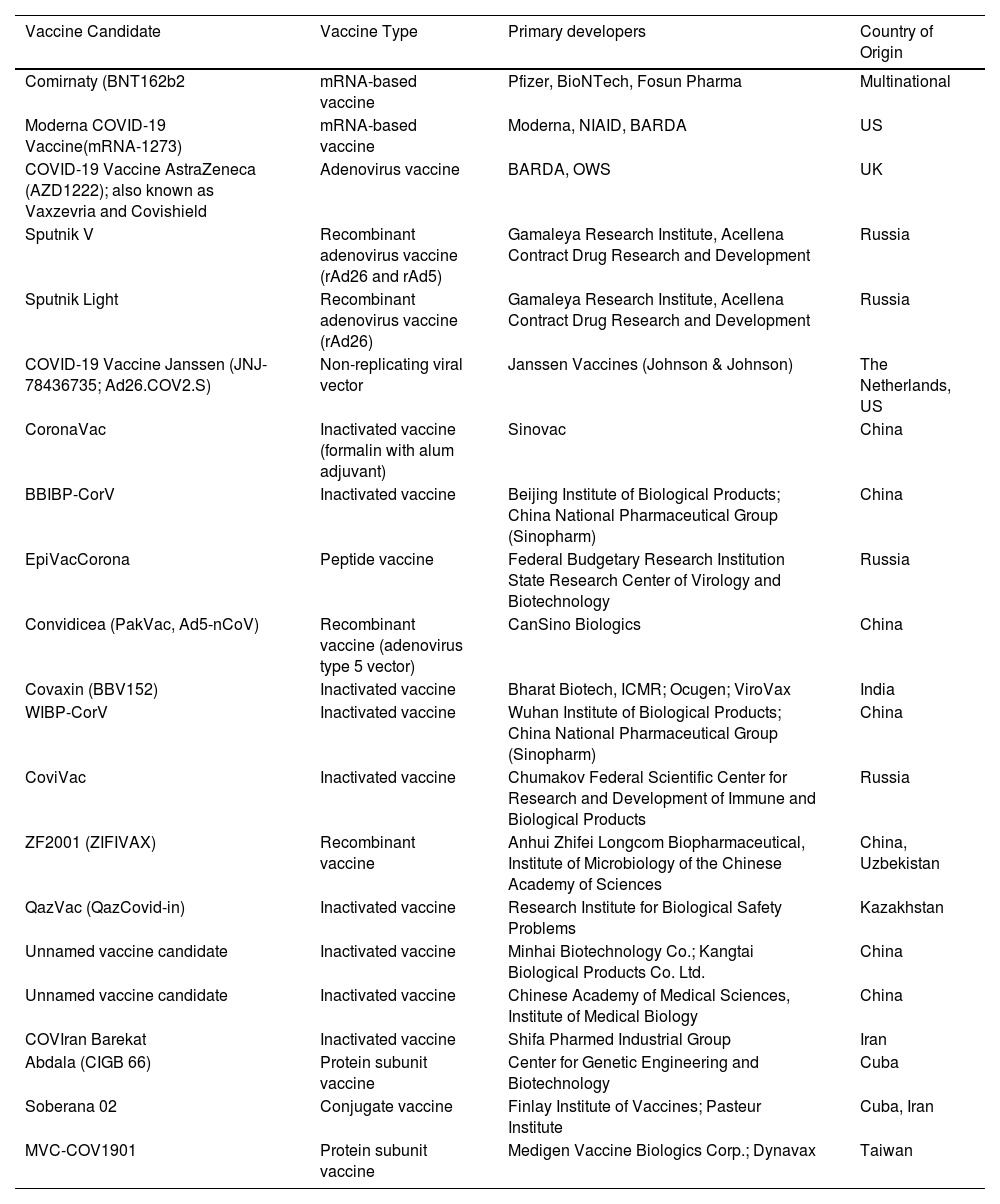
List of approved vaccines for COVID-19; data retrieved from https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker accessed on 23 August 2021.
| Vaccine Candidate | Vaccine Type | Primary developers | Country of Origin |
|---|---|---|---|
| Comirnaty (BNT162b2 | mRNA-based vaccine | Pfizer, BioNTech, Fosun Pharma | Multinational |
| Moderna COVID-19 Vaccine(mRNA-1273) | mRNA-based vaccine | Moderna, NIAID, BARDA | US |
| COVID-19 Vaccine AstraZeneca (AZD1222); also known as Vaxzevria and Covishield | Adenovirus vaccine | BARDA, OWS | UK |
| Sputnik V | Recombinant adenovirus vaccine (rAd26 and rAd5) | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia |
| Sputnik Light | Recombinant adenovirus vaccine (rAd26) | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia |
| COVID-19 Vaccine Janssen (JNJ-78436735; Ad26.COV2.S) | Non-replicating viral vector | Janssen Vaccines (Johnson & Johnson) | The Netherlands, US |
| CoronaVac | Inactivated vaccine (formalin with alum adjuvant) | Sinovac | China |
| BBIBP-CorV | Inactivated vaccine | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China |
| EpiVacCorona | Peptide vaccine | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | Russia |
| Convidicea (PakVac, Ad5-nCoV) | Recombinant vaccine (adenovirus type 5 vector) | CanSino Biologics | China |
| Covaxin (BBV152) | Inactivated vaccine | Bharat Biotech, ICMR; Ocugen; ViroVax | India |
| WIBP-CorV | Inactivated vaccine | Wuhan Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China |
| CoviVac | Inactivated vaccine | Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products | Russia |
| ZF2001 (ZIFIVAX) | Recombinant vaccine | Anhui Zhifei Longcom Biopharmaceutical, Institute of Microbiology of the Chinese Academy of Sciences | China, Uzbekistan |
| QazVac (QazCovid-in) | Inactivated vaccine | Research Institute for Biological Safety Problems | Kazakhstan |
| Unnamed vaccine candidate | Inactivated vaccine | Minhai Biotechnology Co.; Kangtai Biological Products Co. Ltd. | China |
| Unnamed vaccine candidate | Inactivated vaccine | Chinese Academy of Medical Sciences, Institute of Medical Biology | China |
| COVIran Barekat | Inactivated vaccine | Shifa Pharmed Industrial Group | Iran |
| Abdala (CIGB 66) | Protein subunit vaccine | Center for Genetic Engineering and Biotechnology | Cuba |
| Soberana 02 | Conjugate vaccine | Finlay Institute of Vaccines; Pasteur Institute | Cuba, Iran |
| MVC-COV1901 | Protein subunit vaccine | Medigen Vaccine Biologics Corp.; Dynavax | Taiwan |
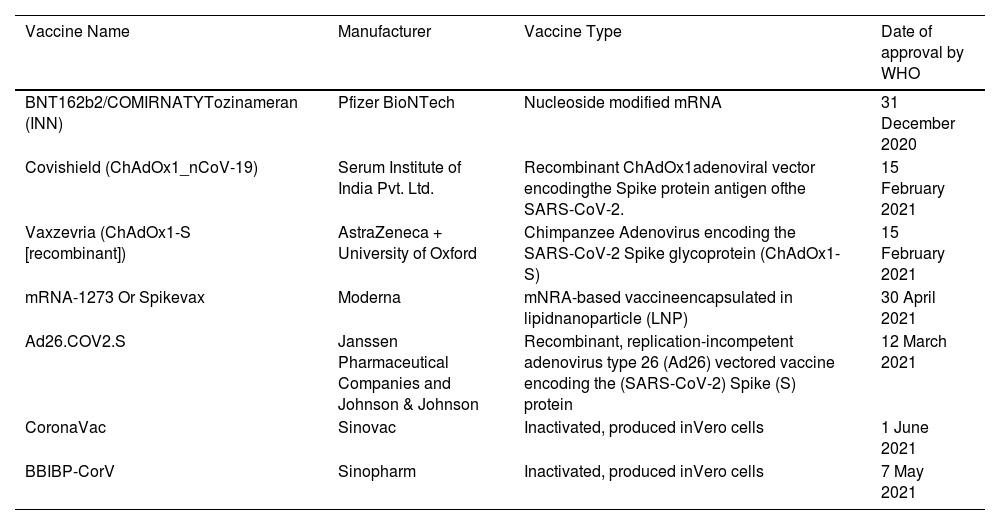
List of COVID-19 vaccines approved by World Health Organization (WHO).
| Vaccine Name | Manufacturer | Vaccine Type | Date of approval by WHO |
|---|---|---|---|
| BNT162b2/COMIRNATYTozinameran (INN) | Pfizer BioNTech | Nucleoside modified mRNA | 31 December 2020 |
| Covishield (ChAdOx1_nCoV-19) | Serum Institute of India Pvt. Ltd. | Recombinant ChAdOx1adenoviral vector encodingthe Spike protein antigen ofthe SARS-CoV-2. | 15 February 2021 |
| Vaxzevria (ChAdOx1-S [recombinant]) | AstraZeneca + University of Oxford | Chimpanzee Adenovirus encoding the SARS-CoV-2 Spike glycoprotein (ChAdOx1-S) | 15 February 2021 |
| mRNA-1273 Or Spikevax | Moderna | mNRA-based vaccineencapsulated in lipidnanoparticle (LNP) | 30 April 2021 |
| Ad26.COV2.S | Janssen Pharmaceutical Companies and Johnson & Johnson | Recombinant, replication-incompetent adenovirus type 26 (Ad26) vectored vaccine encoding the (SARS-CoV-2) Spike (S) protein | 12 March 2021 |
| CoronaVac | Sinovac | Inactivated, produced inVero cells | 1 June 2021 |
| BBIBP-CorV | Sinopharm | Inactivated, produced inVero cells | 7 May 2021 |
Vaccine based on live -attenuated SARS-CoV-2 virus
- •
DelNS1-SARS-CoV-2-RBD by University of Hong-Kong
DelNS1-SARS-CoV-2-RBD by University of Hong Kong is an example of vaccine based on attenuated or weakened SARS-CoV-2 virus. This vaccine uses flu vector to express a particular antigen to induce immunity targeting the critical element of Receptor Binding Domain (RBD) of SARS-CoV-2. DelNS1-SARS-CoV-2-RBD is one of the 5 vaccine technologies by China's Ministry of Science and Technology.66 This live attenuated vaccine (LAV), cultivated in the chick embryo or Madin Darby Canine Kidney Cells (MDCK cells) and administered intranasally.17
Vaccine based on inactivated SARS-CoV-2 virusSARS-CoV-2 is inactivated or killed by using different chemical techniques and the candidate vaccines under this group are injected intramuscularly.67
- •
CoronaVaC by Sinovac Biotech
It is a purified SARS-CoV-2, inactivated vaccine candidate which was previously known as PiCoVacc. It is designed by cultivating the SARS-CoV- 2 CN2 Strain inside Vero cells and in activating it with β-propiolactone. According to the preclinical trials it stimulates SARS-CoV-2 specific neutralizing antibodies (nAbs) in Rhesus Macque, rats and mice.68 On June 1, CoronaVaC vaccine was approved by WHO for emergency use listing (EUL) and finally approved for use in 26 countries on June 9, 2021. According to data from Brazilian trial this vaccine has an efficiency rate of 50.4% for prevention of symptomatic infection.
- •
Covaxin Bharat Biotech India
The research name of Covaxin is BBV152. It is India's indigenous vaccine developed in collaboration with ICMR (Indian Council of Medical Research) and NIV (National Institute of Virology). It has been approved by 9 countries which include India, Iran, Mauritius, Mexico, Nepal, Guyana, Paraguay, Zimbabwe and Philippines.
- •
BBIBP-CorV vaccine by Sinopharm
It is an inactivated vaccine developed by Beijing Bio-Institute of Biological products from strain.69 It is a Chinese state-owned company uses inactivated SARS-CoV-2 virus and the clinical trials showed that it had an efficacy rate of 79%. For the development of BBIBP-CorV, the researchers obtained three variants of coronavirus from patients, and the variants which multiplies fast in monkey kidney cells was selected. β-propiolactone is used to inactivate coronaviruses and hence it doesn't replicate inside the host. The proteins (including spike) of coronavirus remains intact hence, further mixed with a small amount of adjuvant (aluminum based compound) to boost response.70 This vaccine completed its phase III in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru and UAE and on 7th May, 2021, WHO listed Sinopharm COVID-19 vaccine for emergency use.
- •
Novavax (NVX-CoV2373)
The Novavax COVID-19 vaccine, NVX-CoV2373, a type of protein subunit vaccine uses nanoparticle based vaccine, designed by Novavax and Coalition for Epidemic Preparedness Innovations (CEPI). These vaccines are under trial in India under the brand name Covovax.71 This US based Novavax Inc. has a manufacturing contract with Serum Institute of India.
This vaccine is designed by creating an engineered baculovirus containing a gene for the modified SARS-CoV-2 spike protein. The S-protein was altered by the incorporation of two proline residues hence, stabilizing the pre-fusion form of protein. The baculovirus infects the culture of SF9 mother cells which forms the spike protein and displays it on the cell membranes. Spike proteins are cultivated and assembled onto a synthetic lipid nanoparticle purifying spike protein. Matrix M (based on a saponin) obtained from the soapbark tree (Quillaja saponaria), used as adjuvant in this vaccine.72 These vaccines can be stored and handled at above freezing temperature (35–46 °F) and administered as two intramuscular injections.
- •
Triple Antigen Vaccine by Premas Biotech or Orovax vaccine
Premas Biotech (India) in collaboration with Oramed Pharmaceuticals (Jerusalem) designed an oral vaccine to be swallowed as a pill instead of being injected. This is a VLP vaccine prototype which acts on 3 surface proteins of the SARS-CoV-2 virus; the spike, the membrane and the envelope protein. Premas known for developing recombinant proteins for vaccine development, such proteins are ‘difficult to express’ proteins (DTE-Ps). The triple proteins of SARS-CoV-2 have been co-expressed in an engineered Saccharomyces cerevisiae (D-crypt).17
- •
INO-4800
This vaccine was developed by Inovio Pharmaceuticals in partnership with Beijing Advaccine Biopharmaceuticals Suzhou. This strategy utilizes codon optimized S protein sequence of SARS-CoV-2 to which an IgE leader sequence is attached.17 The INO-4800 vaccine contains the plasmid pGX9501 encoding the entire length of spike glycoprotein of SARS-CoV-2. Inovio's proprietary platform uses CELLECTRA, a brief electrical pulse to open small pores in the cell reversibly to allow the plasmids to enter. INO-4800 can be injected intradermally with subsequent electroporation to transfer DNA plasmid directly into blood cells. According to the Lancet on December 23, 2020, INO-4800 showed excellent safety, tolerability as well as immunogenic in 100% of the vaccinated volunteers by stimulating either the cellular or humoral immune responses or both.
- •
mRNA − 1273 by Moderna
Spikevax, the brand name of mRNA-1273 vaccine, designed by Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). This vaccine comprises of synthetic mRNA enclosed in lipid nanoparticle (LNP) encoding the full length pre fusion stabilized spike protein (S) of SARS-CoV-2, therefore, stimulates a highly S-protein specific antiviral response (Fig. 9).17 This vaccine is designed on the basis of SARS and MERS and administered intramuscularly in deltoid muscle.1,73 The levels of nAb surpassed the levels found in convalescent sera after the administration of 250 μg dose levels.17,74 Initial efficacy assessment of Phase 3 CoV study of mRNA-1273 includes 30,000 subjects consisting of 196 cases of COVID-19 of which 30 cases being severe. The efficacy of this vaccine against COVID-19 was 100% and hence on 30th April, 2021, Moderna COVID-19 vaccine became the fifth vaccine to receive emergency validation from WHO.
- •
BNT162b1 (BioNTech|Fosum Pharma| Pfizer)
It is the first vaccine authorized by FDA Emergency Use Authorization (EUA) to prevent COVID-19 on December 11, 2020. On December 31, 2020, the WHO issued an EUL for BNT162b1 vaccine. It is a codon-optimized mRNA vaccine that encodes for the trimerized SARS-CoV-2 RBD.17 It is a liquid nanoparticle-formulated, nucleoside-modified RNA that encodes optimized SARS-CoV-2 full length spike protein.75 The vaccine displays an elicited immunogenicity as it uses additional T4 fibritin-derived fold on trimerization domain to the RBD antigen.17 The vaccine is based on Germany-baded BioNTech SE proprietary mRNA tech and was co-developed by BioNTech and Pfizer. BioNTech also collaborated with Fosun Pharma on March, 2020 and Fosun Pharma became the strategic partner of BioNTech in China.
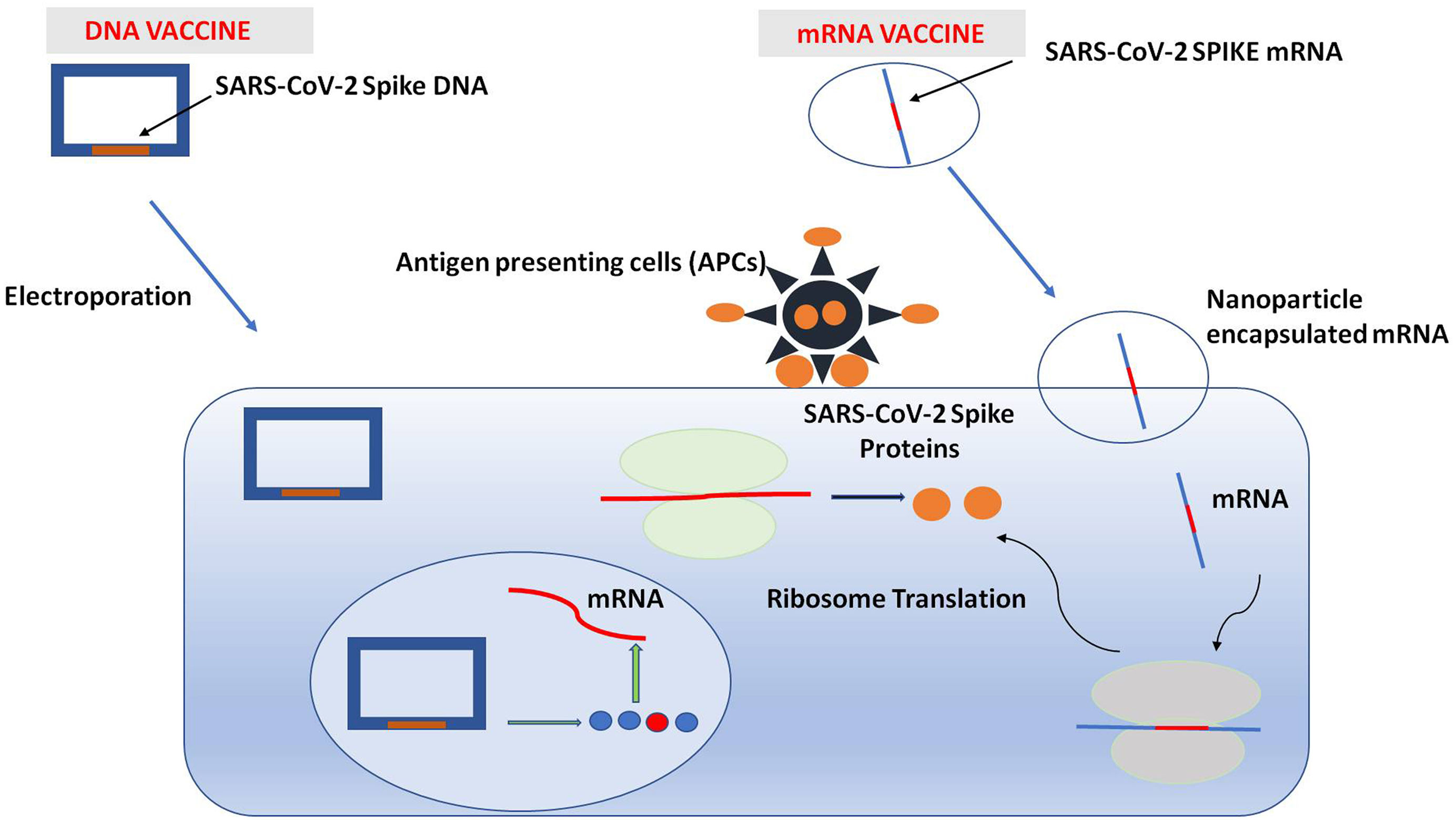
A pictorial comparison between DNA & mRNA vaccine with regard to their mechanism. DNA vaccine is in the form of circular DNA and contains the spike gene of SARS-COV-2. Electroporation enhances the permeability of plasma membrane which allows the entry of DNA into the cytoplasm and ultimately to nucleus. In nucleus, DNA is transcribed into mRNA which is translated into spike proteins of SARS-COV-2. These proteins are expressed on cell membrane. mRNA vaccines are encapsulated in nanoparticles and are integrated into cytoplasm. Spike proteins are produced which are expressed on cell membrane. These proteins are recognized by antigen presenting cells (APC) which triggers an immunological response.
- •
Ad5-nCoV (CanSino Biologics Inc | Beijing Institute of Biotechnology)
This is a non-replicating viral vector vaccine, administered with a single dose. This vaccine is based on CanSino BIO's adenovirus viral vector vaccine technology. The vaccine is designed by using the Admax system from the Microbix Biosystem.76 The CanSino Biologics Convidicea is a genetically engineered vaccine candidate with the replication defective adenovirus type 5 as the vector to express SARS-CoV-2 spike protein. The attenuated adenovirus infects human cell readily, incapable of causing disease and hence, delivers genetic material coding spike protein. Therefore, S proteins are produced which travels to the lymph nodes, where the immediate response produces antibodies.
- •
Ad26.COV2.S or Janssen COVID-19 vaccine or Johnson & Johnson COVID-19 vaccine
A non-replicating vaccine developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, subordinate of Johnson & Johnson, based on AdVac and PER.C6® technologies. Janssen's AdVac® vectors are genetically modified adenoviruses which mimics components of pathogens. Advac viral vector can induce long-term humoral as well as cellular immune responses. Janssen produces neutralizing antibodies against a range of SARS-CoV-2 variants such as Delta (B.1.617.2) variant, the partially neutralization-resistant Beta (B.1.351) variants, the Gamma (P.1) variants and others, including the Alpha (B.1.1.7), Epsilon (B.1.429), Kappa (B.1.617.1) and D614G variants, the original SARS-CoV-2 strain (WA1/2020) as well as on Omicron variant. This vaccine produces durable immune response for upto 8 months after vaccination.
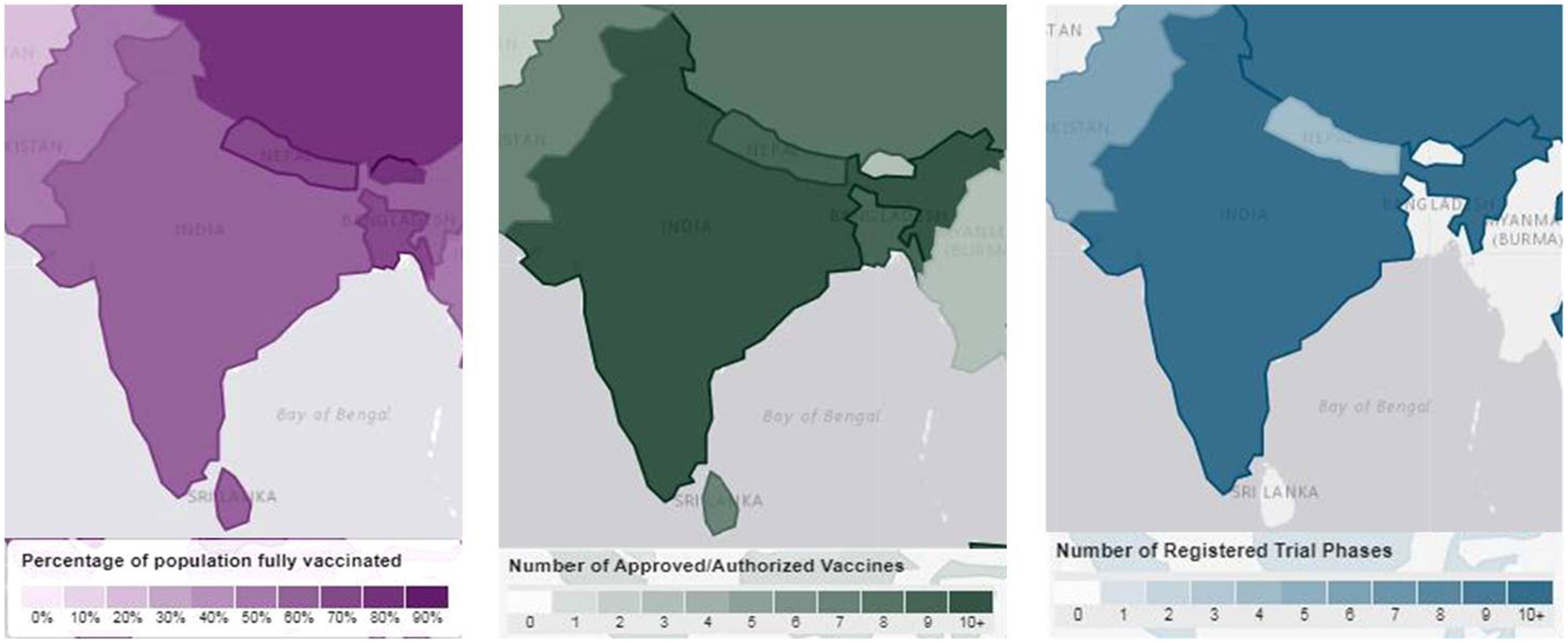
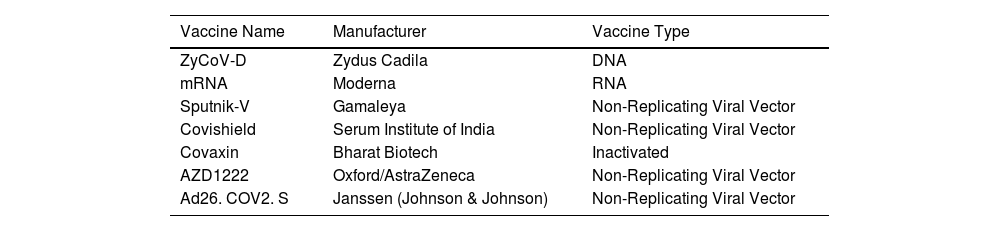
India, the second largest populated country in the world, with several pharmaceutical manufacturing bodies plays a central role in the COVID-19 vaccine development.77 India began administrating COVID-19 vaccines on 16th January, 2021 and the outcome of this vaccination drive reveals 94% of the Indian population has received at least first dose and 86% of the eligible Indian population has received both doses of vaccine.78 The list of COVID-19 vaccines approved in India is shown in Table 7 and in Fig. 10.
List of COVID-19 Vaccines approved in India.
| Vaccine Name | Manufacturer | Vaccine Type |
|---|---|---|
| ZyCoV-D | Zydus Cadila | DNA |
| mRNA | Moderna | RNA |
| Sputnik-V | Gamaleya | Non-Replicating Viral Vector |
| Covishield | Serum Institute of India | Non-Replicating Viral Vector |
| Covaxin | Bharat Biotech | Inactivated |
| AZD1222 | Oxford/AstraZeneca | Non-Replicating Viral Vector |
| Ad26. COV2. S | Janssen (Johnson & Johnson) | Non-Replicating Viral Vector |
India approved Oxford- AstraZeneca (manufactured under license by Serum Institute of India under the trade name Covishield and Covaxin (Tables 8 and 9). The list of individuals vaccinated throughout the country state-wise is shown in Table 10.
List of Indigenous Vaccines of India.
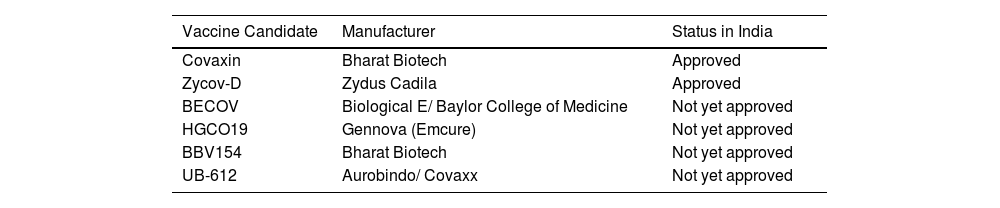
| Vaccine Candidate | Manufacturer | Status in India |
|---|---|---|
| Covaxin | Bharat Biotech | Approved |
| Zycov-D | Zydus Cadila | Approved |
| BECOV | Biological E/ Baylor College of Medicine | Not yet approved |
| HGCO19 | Gennova (Emcure) | Not yet approved |
| BBV154 | Bharat Biotech | Not yet approved |
| UB-612 | Aurobindo/ Covaxx | Not yet approved |
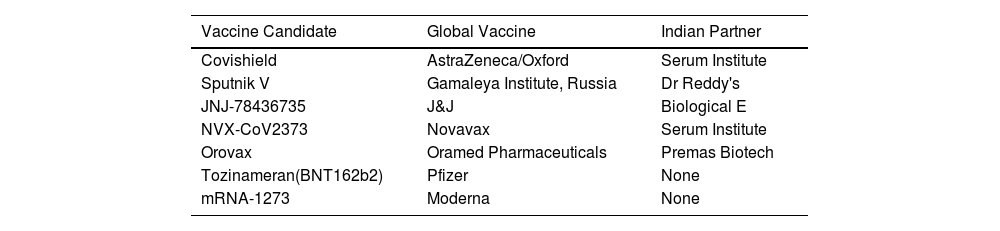
List of Non-Indigenous Vaccines of India or Global Vaccines with Indian Partners.
| Vaccine Candidate | Global Vaccine | Indian Partner |
|---|---|---|
| Covishield | AstraZeneca/Oxford | Serum Institute |
| Sputnik V | Gamaleya Institute, Russia | Dr Reddy's |
| JNJ-78436735 | J&J | Biological E |
| NVX-CoV2373 | Novavax | Serum Institute |
| Orovax | Oramed Pharmaceuticals | Premas Biotech |
| Tozinameran(BNT162b2) | Pfizer | None |
| mRNA-1273 | Moderna | None |
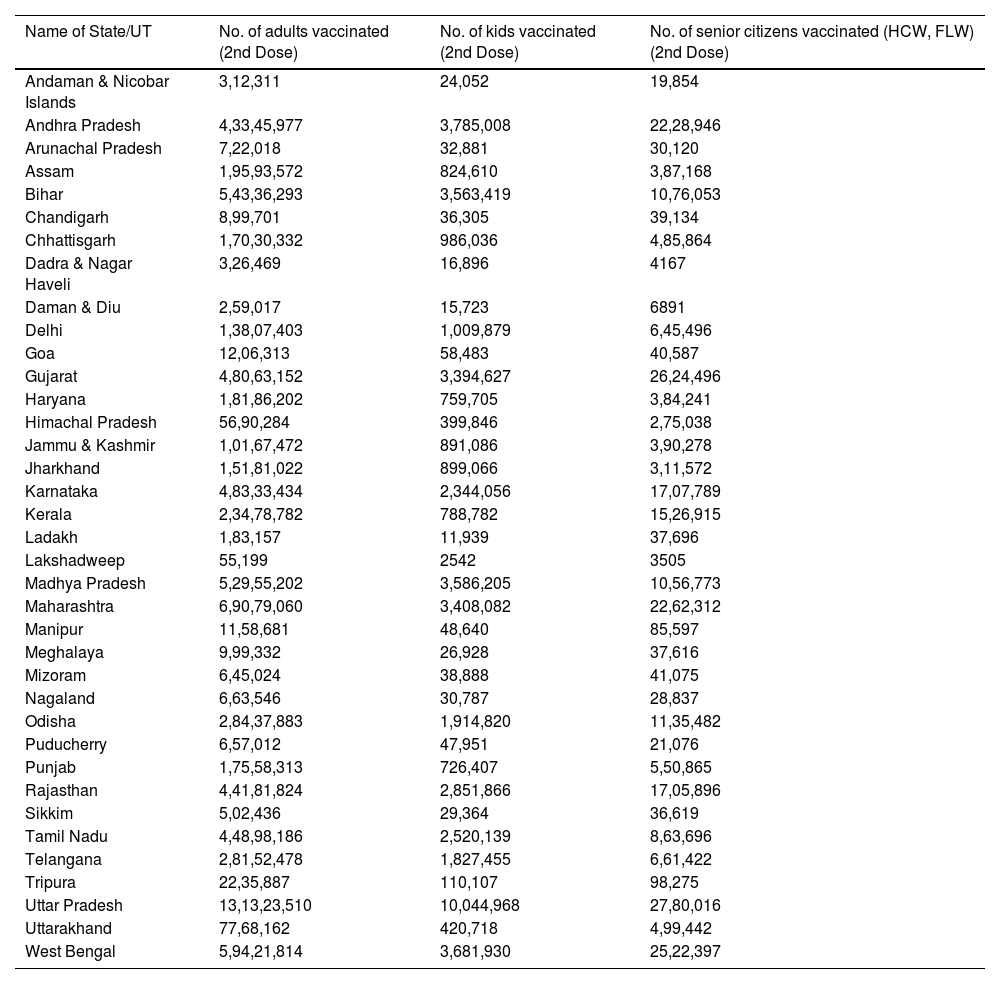
Cumulative coverage report of COVID-19 Vaccination (As on 5th May 2022) (Source: www.mohfw.gov.in).
| Name of State/UT | No. of adults vaccinated (2nd Dose) | No. of kids vaccinated (2nd Dose) | No. of senior citizens vaccinated (HCW, FLW) (2nd Dose) |
|---|---|---|---|
| Andaman & Nicobar Islands | 3,12,311 | 24,052 | 19,854 |
| Andhra Pradesh | 4,33,45,977 | 3,785,008 | 22,28,946 |
| Arunachal Pradesh | 7,22,018 | 32,881 | 30,120 |
| Assam | 1,95,93,572 | 824,610 | 3,87,168 |
| Bihar | 5,43,36,293 | 3,563,419 | 10,76,053 |
| Chandigarh | 8,99,701 | 36,305 | 39,134 |
| Chhattisgarh | 1,70,30,332 | 986,036 | 4,85,864 |
| Dadra & Nagar Haveli | 3,26,469 | 16,896 | 4167 |
| Daman & Diu | 2,59,017 | 15,723 | 6891 |
| Delhi | 1,38,07,403 | 1,009,879 | 6,45,496 |
| Goa | 12,06,313 | 58,483 | 40,587 |
| Gujarat | 4,80,63,152 | 3,394,627 | 26,24,496 |
| Haryana | 1,81,86,202 | 759,705 | 3,84,241 |
| Himachal Pradesh | 56,90,284 | 399,846 | 2,75,038 |
| Jammu & Kashmir | 1,01,67,472 | 891,086 | 3,90,278 |
| Jharkhand | 1,51,81,022 | 899,066 | 3,11,572 |
| Karnataka | 4,83,33,434 | 2,344,056 | 17,07,789 |
| Kerala | 2,34,78,782 | 788,782 | 15,26,915 |
| Ladakh | 1,83,157 | 11,939 | 37,696 |
| Lakshadweep | 55,199 | 2542 | 3505 |
| Madhya Pradesh | 5,29,55,202 | 3,586,205 | 10,56,773 |
| Maharashtra | 6,90,79,060 | 3,408,082 | 22,62,312 |
| Manipur | 11,58,681 | 48,640 | 85,597 |
| Meghalaya | 9,99,332 | 26,928 | 37,616 |
| Mizoram | 6,45,024 | 38,888 | 41,075 |
| Nagaland | 6,63,546 | 30,787 | 28,837 |
| Odisha | 2,84,37,883 | 1,914,820 | 11,35,482 |
| Puducherry | 6,57,012 | 47,951 | 21,076 |
| Punjab | 1,75,58,313 | 726,407 | 5,50,865 |
| Rajasthan | 4,41,81,824 | 2,851,866 | 17,05,896 |
| Sikkim | 5,02,436 | 29,364 | 36,619 |
| Tamil Nadu | 4,48,98,186 | 2,520,139 | 8,63,696 |
| Telangana | 2,81,52,478 | 1,827,455 | 6,61,422 |
| Tripura | 22,35,887 | 110,107 | 98,275 |
| Uttar Pradesh | 13,13,23,510 | 10,044,968 | 27,80,016 |
| Uttarakhand | 77,68,162 | 420,718 | 4,99,442 |
| West Bengal | 5,94,21,814 | 3,681,930 | 25,22,397 |
Covaxin (BBV152), developed by Indian pharmaceutical company Bharat Biotech in collaboration with Indian Council of Research (ICMR) and National Institute of Virology (NIV) is an inactivated vaccine.79 The vaccine designed using whole virion inactivated Vero cell derived technology with a toll- like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG).80,81 The NIV-2020-770 strain isolated from ‘Vero CCL-81’ cells with sequence in GISAID (EPI_ISL_420545).82,83 It uses a complete infective SARS-CoV-2 viral particle with RNA surrounded by a protein shell but contain dead virus incapable of infection.84 The vaccine was assigned for Phase I and Phase II human clinical trials by DCGI in July, 2020 with two doses in 28 days.80
Covaxin consists of 6 μg of whole virion inactivated SARS-CoV-2 antigen, inactive ingredients such as aluminum hydroxide gel (250 μg), TLR 7/8 agonist (imidazoquinoline) 15 μg, 2-phenoxyethanol 2.5 mg and phosphate buffer saline up to 0.5 ml. The sticks of coronaviruses were produced and soaked with beta-propiolactone. This compound disabled the replicating ability of coronaviruses but their proteins including spike remained intact. Once an individual is being vaccinated some of the inactivated viruses are engulfed by antigen presenting cells. The APCs process and present the coronavirus on its surface for recognition by T-helper cells. When B cell surface proteins latch onto the coronavirus, B cell locks on and pull part all of the virus inside and present coronavirus fragments on its surface. As the B cells get activated it proliferates and produces antibodies that can target the spike proteins.65
In Phase I clinical trial against hCoV-19/India/2020770 (homologous), and two heterologous strains from the unclassified cluster, namely, hCoV-19/India/2020Q111 and hCoV-19/India/2020Q100, BBV152 elicited a remarkable neutralizing antibody response.83 In Phase II clinical trial, with 6 and 3 antigen in imidazoquinoline (TLR7/TLR8 agonist adsorbed on aluminum hydroxide gel), the vaccine exhibited significant results in plaque reduction neutralization test (PRNT50)-based assay.81 According to the press release on 3rd March, 2021 of the Indian Council of Medical Research (ICMR), Phase III results of the Covaxin has shown an interim vaccine efficacy of 81%.
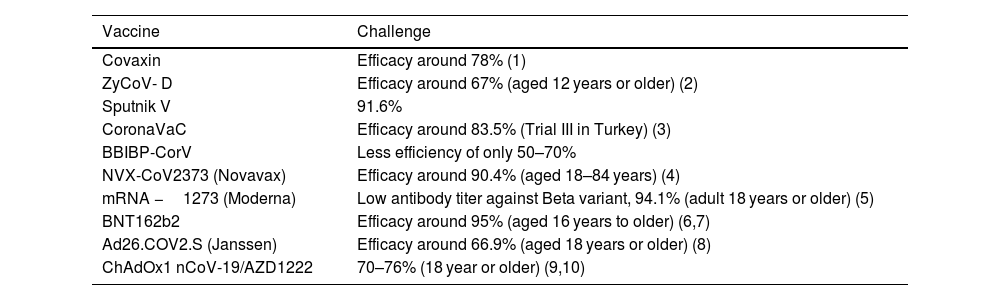
The formulation containing the toll like receptor 7/8 agonist also induced T helper cell 1 based antibody response with elevated levels of SARS-CoV-2 specific interferon gamma and CD4 cells. Covaxin has shown its neutralizing property against the variants- B.1.1.7 (Alpha), P.1- B.1.1.28 (Gamma) & P.2 – B.1.1.28 (Zeta), B.1.617 (Kappa), B.1.351 and B.1.617.2 (Beta & Delta). The efficacy data of Covaxin signifies 65.2% protection against the SARS-CoV-2, B.1.617.2 Delta variant and is 93% effective against severe disease.80
COVISHIELDCovishield (AZD1222) developed at the Jenner Institute, University of Oxford in UK and licensed from British pharmaceutical company AstraZeneca is a non-replicating viral vaccine. Covishield got first approval for restricted use on January 3, 2021. The AZD1222 (ChAdOx1 nCoV- 19 Corona Virus Vaccine (Recombinant), designed at Oxford University consists of a replication deficient chimpanzee adenoviral vector ChAdOx1, with SARS-CoV-2 surface glycoprotein gene.85
The Covisheld vaccine prepared using L-Histidine, L-Histidine hydrochloride monohydrate, Magnesium chloride hexahydrate, Polysorbate 80, Ethanol, Sucrose, Sodium chloride, EDTA and water for injection. The Oxford-AstraZeneca team used a modified version of chimpanzee adenovirus (ChAdOx1) which can enter cells but can't replicate inside them. The vaccine can be refrigerated at 38–46 °F for the vaccine to last at least for 6 months. After the vaccine injection, the adenovirus encounters the cells and affix onto proteins on the cell's surface. The cell swallows up the virus in a bubble and pulls it inside; once the virus gains entry inside, it leaves the bubble and moves to the nucleus, where the cell's DNA is stored. The adenovirus pushes its DNA into the nucleus but it can't replicate itself whereas the coronavirus spike protein copies itself. These protruding spikes and spike protein fragments acts as recognition particles for the immune system. When the vaccinated cell dies, the broken cells contain spike proteins and fragments that can be taken up by antigen presenting cells. B cells may collide with the coronavirus spikes and get activated by helper T cells resulting in antibody formation and prevent infection by blocking the spikes from attaching to other cells.65
The phase 3 trial confirms that the group which received a low first dose vaccination followed by a standard second dose demonstrated 90.0% efficacy while the group which received a standard dose followed by a booster dose showed 62.1% efficacy; the overall efficacy at least 2 weeks after the second dose of vaccine was therefore calculated to be 70.4%.
ZyCoV- DThis is a DNA based vaccine, designed by Ahmedabad based Zydus Cadila in collaboration with National Biopharma Mission (NBM) and the Department of Biotech, Government of India. It is the India's first indigenously developed DNA vaccine. This vaccine has been approved for emergency use in India by DCGI for adults and children above the age of 12 years. This is India's first needle free COVID-19 vaccine which is administered with a disposable needle -free injector. The vaccine contains plasmid that has gene encoding the spike protein of SARS-CoV-2.86 The expression and localization of S-protein expressed by ZyCoV-D were assessed using immunofluorescence assay by Dey et al. The immunofluorescence studies with rabbit anti S1 antibody depicted a strong signal in the Vero cells transfected with ZyCoV-D. This study demonstrates the ability of the ZyCoV-D vaccine to express efficiently in mammalian cells and can induce antibodies production.86 ZyCoV-D was assessed in vivo in different animal models and has showed the potential to elicit immune responses against SARS-CoV-2 S antigen. ZyCoV-D can also induce secondary immune response as the serum IgG levels against spike protein detected even after three months after the last dose.86 According to an interim study, the three doses of ZyCoV-D vaccine prevents symptomatic disease in 66% of vaccinated individuals. According to Momin et al., ZyCoV-D vaccine was found to be safe, well tolerated and immunogenic in phase 1 trial.87
HGC019HGC019, India's first mRNA vaccine made by Pune- based Gennova biopharmaceuticals in collaboration with Seattle-based HDT Biotech Corporation uses bits of genetic code to stimulate an immune response. This mRNA based vaccine candidate contains a short, synthetic version encoding the spike (antigen) protein of SARS-CoV-2. Upon injection into the person's body, the synthetic mRNA taken to muscle cells, where multiple copies of antigen is formed. The mRNA is associated with the lipid inorganic nanoparticle (LION™) which acts as mRNA vaccine delivery system equilibrating the mRNA and also acts as adjuvant. The vaccine is unique as it uses the most prominent mutant of spike protein, D614G. The vaccine is stable at 2–8 °C and uses the absorption chemistry to attach mRNA to the nano-lipid carrier's surface to intensify the release kinetics of mRNA within the cells compared to the encapsulation chemistry. On 24 August, 2021, DCGI gave a nod for phase II/ III trials to HGC019 vaccine after its positive results of Phase I trial.
CORBEVAXCorbevax (BioE COVID-29 or BECOV2D), a protein subunit vaccine developed by Biological E Limited (BioE) an Indian biopharmaceutical company, American company Dynavax Technologies (DVAX), the Baylor College of Medicine in Houston, US. It is a recombinant protein subunit vaccine which uses spike RBD, adsorbed to the adjuvant alum. Four compounds with these components are currently being assessed in Phase I/ II clinical study in India to select the final vaccine candidate to be examined in subsequent Phase III trials. In preclinical models, combination of Alum with Dynavax Technologies Corporation's CpG with N1C1 antigen evoked a highly synergistic, balanced immune response.88
SPUTNIK VSputnik V (Gam-COVID-Vac), non-replicating vector vaccine designed by Gamaleya National Research Centre for Epidemiology and Microbiology of the Ministry of Health of the Russian Federation, Moscow, Russia. Scientists adjoined the gene for coronavirus spike protein to two types of adenovirus i.e., Ad26 and Ad5 and modified them so that they could penetrate cells but could not replicate. The use of two varying serotypes is a unique technique that provides effective long term immunity and boosts the immune response. The first dose (based on Ad26) is administered and injected on the first day and the second dose (based on Ad5) is administered on the second day to boost immune response.89,90
The vaccine trial from Moscow reported an efficacy of 91.6% after the second dose for all age groups with no unusual side effects.88 Sputnik V's efficacy against the Delta variant was found to be 83.1% and also showed a six times reduction in infection risk as confirmed by Russian Ministry of Health on July, 2021. According to the results of Phase III clinical trials of Sputnik V, after getting the vaccine 98% of people developed humoral immune response and 100% of people developed cellular immune response. Serum Institute of India got the authorization on June 4, 2021 by DCGI to manufacture Sputnik V COVID-19 at its Hadapsar facility.
Vaccination in India is an arduous task with a country having a population that covers 1.3 billion people. Free vaccination to everyone in India is being provided to all above the age of 18 from June, 21 as per the announcement by Prime Minister Shri Narendra Modi. While the vaccine program is gaining momentum in India, trust in vaccine by the people and their acceptances among the citizens are key determinants of the success of any vaccination program. Across the globe there are sections of people who are facing vaccine hesitancy with myths and misinformation's circulating on different platforms. Government of India is already running several awareness campaigns to establish trust for vaccination among people and dispel hesitancy. To streamline the process of vaccination, Indian government developed a digital platform Co-Win where one could book an appointment for vaccination, get the trusted information and check the status of vaccines.89
Effect of SARS-CoV-2 vaccine on Variant of Concern (Omicron)Omicron represents a highly mutated version of SARS-CoV-2.90 The Technical Advisory Group on SARS-CoV-2 (TAG-VE) of WHO classified the B.1.1.529 strain known as omicron as a Variant of Concern (VOC). Omicron was first detected in South Africa in November, 2021.91 As per data of Global Science and Primary Sources (GISAID), 4992 omicron sequences have been reported by 57 countries till December, 2021. Omicron is worrisome variant as it has more than 50 mutations, out of which 30 are spike protein mutations. 15 mutated sites are located within the receptor-binding domain (RBD), the portion which interacts with host cells before entry inside the cell and hence an increase in transmission rate of infection is possible.92
Spike protein sequences analysis led to identification of two sub clades of omicron. Sub-Clade 1 has a lower sequence frequency and has mutation at three sites, namely 417 K, 440 N and 440 G. Sub-clade II has a high occurrence globally and has mutation sites at 417 N, 440 K and 446 S. Omicron shares several common mutation with delta variant of SARS-CoV-2, however many additional mutations can increase the infectivity rate of this variant. As per scientific data available till date, no evidence suggests that omicron has a greater severity than other VOCs. However, many issues such as increased transmission rate, virulence, elevated risk reinfection and possible decline in efficacy of vaccines and other therapeutics remain unresolved. A simulated study involving artificial intelligence (AI) tried to analyze the effect of RBD mutations on the infectivity of omicron and efficacy of available vaccines. The study showed that mutation involving N440K, T478K and N510Y might increase infectivity of omicron by 2–10 times in comparison to delta variant.93 Studies have also shown that omicron can cause reinfection at a greater rate.94,95
The idea that omicron variant can evade vaccine-mediated immunity remains unclear, however sudden spike in omicron positivity rate together with greater number of hospitalization in South Africa remain a major concern and further studies are required.96 In order to counter omicron variant, Pfizer and BioNtech are preparing to alter their mRNA vaccine shots.27,38,86 Pfizer aims to identify escape variant in omicron in order to design omicron specific vaccine. However, lack of scientific data suggests that it will be too early to make any conclusions regarding efficacy of existing vaccines against omicron variant.
Current landscape and market size for vaccine development in IndiaA lot of struggle around the globe has resulted in development of vaccines in last 60 years. World Health Organization (WHO) helped the world with its outreach programme to bring vaccines to poor population also. These strategies helped in prevention of spread of many contagious diseases. India, being the world's largest supplier of vaccines produces 62% of global demand. India's pharmaceutical industry started growing significantly since 2012. Earlier, India's pharmaceutical market share was less than desirable at $ 500 million which reached to $ 1.3 billion in 2019. This growth in share was due to the increase in vaccine development and outreach programmes.
India, the largest vaccine producer, currently exports two-thirds of its production while rest one-third is used domestically. Since growth in production has increased significantly, the Indian pharmaceutical industry is expected to reach 65 billion USD by 2024. The low cost production as well as the research and development in vaccines as well as pharmaceuticals contribute towards country's cost. The vaccines and pharmaceuticals produced in India are not only effective but 33% less costly than other markets. This cost effectiveness results in high export as well as its reach ability to the poor section of the country.
India's vaccine industry can grow upto $4 billion from $2 billion due to its growing vaccine as well as pharmaceutical industry as per the report by global consulting firm Kearney, in collaboration with the Confederation of Indian Industry (CII). India needs to accelerate its momentum in using sound biotech strategies in investments, research and developments as well as start-ups so as to reach its goal. This needs support from private, academia as well as government sector.
Ethical concern in COVID-19 vaccine developmentThe urgent demand for the production of effective vaccine against COVID-19 arises question on the efficacy and safety of the vaccine. While a lot more research is still needed to actually check the immunogenicity of the vaccine candidates many vaccines are approved for humans. The experts and scientists working in this field are giving their best to produce effective vaccine against COVID-19 as many vaccines are under trial and many approved. To meet the demands of vaccine there is a rush and this can compromise with the effectiveness of the vaccine and any loophole can result in loss of trust in vaccines.
To combat the risk of COVID-19 infection, the only way is acquiring herd immunity among population. Hence, to meet the demand it is required for everyone to get vaccinated, first the more vulnerable group followed by rest of the population. In India, major population has been vaccinated with all the doses of vaccine and administers should ensure for rest of the boosters doses to administered on time.
The emergence of different variants of SARS-CoV-2 is now a major concern for the effectiveness of the vaccine, as vaccines developed so far used earlier variants of SARS-CoV-2. The researchers and scientists still believe the earlier developed vaccines will still work on new variants may be with less efficacy. Several studies suggested BNT162b2 to be effective against new variants but with less efficacy. Also, the mRNA-1273 vaccine proved to be more effective for beta variant but with short span of immune response.
The major concern in vaccine development is the proper distribution of vaccines to every citizen of the country with proper trials. India, has tried to vaccinate every individual with requisite doses on priority basis, as health care workers and aged people were vaccinated first followed by the young people. Several volunteers worked to educate about the importance of vaccines and for equal distribution in all parts of India.
COVID-19 and autoimmune diseasesCOVID-19 is linked with autoimmune disorders raising concern about the long term effect on human health. Hence, a good understanding of the complications of COVID-19 in autoimmune disorders patients is urgently required to guide researchers and doctors in treating patients with systemic lupus erythematous (SLE), infammatory bowel disease (IBD), systemic sclerosis (SSc), rheumatoid arthritis (RA) and others. The main reason behind the concern of COVID-19 in autoimmune disease patients is the treatment with immunosuppressive drugs, which can increase the susceptibility to COVID-19. Several studies on patients with autoimmune disorders showed the impact of COVID-19 on these patients.97,98 Tan et al. 2021 reported that majority of autoimmune disorder patients died within 30 days of hospitalization with COVID-19. Autoantibodies were recovered in some patients with COVID-19 and some people developed autoimmune disorders after suffering from COVID-19.99 To reduce the COVID-19 infections, vaccination is the only possible treatment. Some studies reported the occurrence of autoimmune diseases like immune thrombotic thrombocytopenia, autoimmune liver diseases, Guillain-Barré syndrome, IgA nephropathy, rheumatoid arthritis and systemic lupus erythematosus in persons getting COVID-19 vaccine.100 As per WHO guidelines regarding COVID-19 vaccine, few people can get minor side effects including autoimmune disorders upon vaccination. These autoimmune disorders meet the criteria for diagnóstic of Adjuvant-Induced Autoimmune Syndrome (ASIA syndrome).101 The probable reason behind these autoimmune disorders is the molecular mimicry and hence formation of autoantibodies. There is still scanty information on whether the autoimmune disorders are caused by COVID-19 vaccines or mere a chance effect and studies need to be done in this regard. Geisen et al. reported the safety and efficacy of COVID-19 mRNA vaccines as safe with no considerable side effects.102 These studies open new avenues for searching a relationship between immune system, COVID-19, its vaccine and autoimmune disorders.
COVID-19 and cancerCOVID-19 becomes a serious concern for not autoimmune disease patients but more chronic for cancer patients. Cancer patients are at higher risk of complications due to COVID-19 and it increases more in aged people. In this pandemic era, cancer patients are more susceptible to infection as they are immunocompromised and also due to their treatment. The immunosuppression in cancer patients leads to serious complications as they are more prone to infections and may require early treatment if diagnosed with COVID-19. Cancer patients are nearly 3.5 fold more prone to COVID-19 infections with increased risk of hospitalization and ventilation.103 Cancer treatment during the COVID period is also a major concern as chances of infection increase and several events can occur like septic shock, myocardial infarction, respiratory distress syndrome and others. The risk of nosocomial infection also increases in cancer patients due to hospitalization. The development of COVID-19 vaccines and their randomized trials resulted in high level of safety and efficacy but the trial did not included immunocompromised individuals like cancer patients. As the cancer patients are more prone to infections, many countries prioritize vaccines for them. These patients show reduced cellular as well as humoral immune responses as treated with chemotherapy and immunosuppressive treatments.104 There are very less clinical studies on the impact of these vaccines on cancer patients. A report by National COVID Cohort Collaborative suggested that individuals with cancer and vaccinated are more prone to severe infection than individuals without cancer and vaccinated.105 The infections were more serious in individuals with hematological malignancy as well as those getting immunosupressive therapies. Earlier several vaccination studies in patients with cancer against diseases like influenza, hepatitis B and others proved these patients require more than one dose of vaccine, being immunocompromised. For instance, study on influenza vaccine showed two doses to be more effective in cancer patients rather than one showing more immunogenicity.106 Similar studies showed two doses to be effective against hepatitis B infection in cancer patients. These studies suggest increasing the number of COVID-19 vaccine doses in cancer patients as they are less immunocompetent. mRNA vaccines show more effective response than adenovirus vector based vaccines.107 Hence, regular interval of vaccination is an useful tool against COVID-19 in cancer patients.
DiscussionSudden emergence of COVID-19 disease has become a serious concern of human health worldwide. Hence, development of antiviral therapeutics are exigent to combat COVID-19 infection. Major symptoms of SARS CoV-2 infection include severe respiratory distress, sore throat, dyspnea, fever, cough etc. Infection of this disease starts within 2 days and it may continue upto 14 days. Generally the transmission of COVID-19 spread through human to human contact or by inanimate matter that have been infected to SARS CoV-2 (based on the recommendation of centre for disease control and prevention).108,109,111
Earlier studies have shown that hesitancy towards vaccine creates a major threat to the public health globally, as the resurgence of measles and pertussis.112–116 The crucial stage in development of vaccine is not only in developing the vaccine but its efficacy and safety. Previously, traditional ways of vaccine development was a labour intensive process because immunological correlation such as identification and investigation of immunogenic agent were needed.117,118 The vaccine development using modern techniques requires enormous testing regarding safety and efficacy of the vaccine. Several scientific community and agencies are working for the development of efficient coronavirus vaccine around the globe. Agencies like Moderna and the Vaccine Research Centre are together developing an mRNA-based vaccine candidate, where mRNA is coated with lipid vesicle for easy incorporation and also Codagenix is working in collaboration with the Serum Institute of India to develop live attenuated viral vaccine. The companies like Novavax, Sichuan Clover Biopharmaceuticals, iBio, and the University of Queensland are in process of developing S glycoprotein targeted vaccine. Several strategies like the viral vector-based vaccines, targeting the S glycoprotein are still under process to be developed into efficient vaccine for COVID-19.
Several vaccine candidates are now in Phase 3 clinical trials, including AstraZeneca/AZD1222, Oxford's Moderna's mRNA1273, and Sinovac's CoronaVac vaccines. With many expecting a COVID-19 vaccine to be available by the end of 2020 or early 2021, it is still too seen that how the vaccine distribution takes place how national interests will play out, or whether the vaccine will ultimately prove to be effective and safe when injected to the global population at large.117
Six biotech enterprises in India alone, including Serum Institute of India, Zydus Cadila, Biological E, Indian Immunologicals, Bharat Biotech, and Mynvax, are collaborating with several worldwide vaccine makers. They are developing DNA vaccines, live attenuated recombinant measles vaccines, inactivated viral vaccines, subunit vaccines, and vaccines created using codon optimization (Coronavirus, 2020). Furthermore, academic institutions such as the National Institute of Immunology (NII), the Indian Institute of Science (IISc), the International Centre for Genetic Engineering and Biotechnology (ICGEB) New Delhi, the Translational Health Science and Technology Institute (THSTI), and others are working to develop vaccines, therapies, and SARS-CoV-2 animal models in order to halt the pandemic as soon as possible.119 A detailed map showing the vaccination rate, number of approved and in trial vaccines from India is shown in Fig. 12.
The current research focus has been on the design and manufacture of epitope-based peptide vaccines using various immunoinformatics prediction approaches. The traditional method of producing an effective vaccine needs extensive research, identification, and creation of an immunological link with SARS-CoV-2. In general, the development of peptide vaccines necessitates the identification of immunodominant B-cell and T-cell epitopes capable of producing specific immunological responses. Furthermore, for a peptide vaccine to be highly immunogenic, a target molecule's B-cell epitope must be paired with a T-cell epitope. T-cell epitopes are typically made up of 8–20 amino acids (small peptide fragments) and have been found to be more desirable, resulting in a long-lasting immune response mediated by CD8+ T-cells,120–123 whereas B-cell epitopes are made up of a lineal chain of amino acids that can be a protein.122–125 Several articles are published each year on vaccines against different viruses as shown in Fig. 13.
Finally, a vaccine needs to be properly tested and without knowing the effectiveness of the vaccine candidate and associated risk of its side effect, it can be dangerous. The durability and efficacy of a vaccine should be first target in developing a vaccine against COVID-19.
Current challenges and future prospectIn this current pandemic situation several agencies accelerated their vaccine development program in 6–12 months which usually takes 10–15 years. This reduces the trial period for the vaccine as phase trials are done on smaller groups, which itself is a great challenge in vaccine development. The occurrence of side effects due to less trial phase especially in people with co-morbidity is a major challenge but the vaccines developed in India till date did not produce any side effects. The major challenges posed by some of the COVID-19 vaccines is shown in Table 11.
Some challenges faced by COVID-19 vaccines.
| Vaccine | Challenge |
|---|---|
| Covaxin | Efficacy around 78% (1) |
| ZyCoV- D | Efficacy around 67% (aged 12 years or older) (2) |
| Sputnik V | 91.6% |
| CoronaVaC | Efficacy around 83.5% (Trial III in Turkey) (3) |
| BBIBP-CorV | Less efficiency of only 50–70% |
| NVX-CoV2373 (Novavax) | Efficacy around 90.4% (aged 18–84 years) (4) |
| mRNA −1273 (Moderna) | Low antibody titer against Beta variant, 94.1% (adult 18 years or older) (5) |
| BNT162b2 | Efficacy around 95% (aged 16 years to older) (6,7) |
| Ad26.COV2.S (Janssen) | Efficacy around 66.9% (aged 18 years or older) (8) |
| ChAdOx1 nCoV-19/AZD1222 | 70–76% (18 year or older) (9,10) |
In this current situation, the occurrence of different variants of SARS-CoV-2 like alpha, delta and omicron with high infectivity rate is a matter of concern. The vaccines developed till date were against the earlier variants of SARS-CoV-2, but still researchers and scientists across the globe suggest the vaccines to be equally effective against the new variants too. The emergence of new variants over time will always be a matter of concern on the effectiveness of vaccines.
There is also a concern of discrepancy in distribution of vaccines equally to developed and developing nations. However, several organizations including WHO is trying to lower this discrepancy still it is matter of concern. The developed countries preordered the vaccine doses but developing nations could not order and hence till date developed nations are completely vaccinated whereas developing nations still need more vaccines. So, this discrepancy in vaccination can lead to further spread of this virus.
The proper vaccination of children is also a major challenge as majority of the vaccines have been tried on adults itself. But according to many organizations including WHO, vaccination of children is also important to stop the spread of this pandemic. Many vaccines are under trial for children and in future can be developed to vaccinate the children and hence break the chain of spread of this virus. The ongoing large scale vaccination programme produces huge amount of biomedical and plastic wastes worldwide and, therefore, triggering adverse effect on the environment. The main objective of the mass vaccination campaign is to exit the emergency situation arising as a result of COVID-19 pandemic. However, the vaccination approach created an intense knock-on-effects on the environment. The mass vaccination waste of used discarded vials has thimerosal-mercury based preservative which is found to be hazardous to aquatic ecosystem as well as humans when released improperly in the water bodies. The reckless use of PPE as a preventive measure to COVID-19 has significantly added to the microplastic fibers in the environment.126,127 On the other hand, positive impact of COVID- 19 induced lockdown has also been reported in the recent studies that have shown that significant reduction in many pollutants around the world during the period of lockdown. The lockdown exhibited the better solutions for the nature's rejuvenation by means of natural resource preservation which is prerequisite for sustainable development.128,129
Although COVID-19 vaccines have reduced the chances of infection and hence mortality still certain problems needs to be resolved in the future. The development of mix and match vaccine and the development of new vaccines such as nanoparticle vaccines, adding different adjuvants to improve vaccine and changing the vaccine administration route are some points to be resolved in future. The safety of vaccine for infants, pregnant women and immunocompromised individuals is a matter of future concern to curb this disease completely. The variant of concerns are always emerging and hence a major concern in the future. These mutations are common and problematic and needs to be considered prior to any vaccine development. So, it is essential to consider these mutations while administering vaccines as several vaccines have shown fewer efficacies against certain SARS-CoV-2 variants. Although the COVID-19 vaccines have proved efficient in both animal studies and clinical trials, and several vaccines have been authorized for emergency use by the WHO, adverse effects including pain at the injection site and fever, and some major complications such as coagulation dysfunction, myocarditis, immune disorders, nervous disorder and lymphatic system diseases caused by COVID-19 vaccine, calls for concerns about vaccine safety. The future prospect to be followed and monitored is shown in Fig. 11. The rate of these complications is low as it occurs mainly in individuals with underlying diseases. So to reduce these risks healthcare workers should ensure the lives of the patients by the timely treatment of complications.
COVID-19, a transmissible disease has become a major threat to mankind worldwide. The world witnessed a tremendous collapse of economy, burdened healthcare workers, rising unemployment and a major health catastrophe. The development of COVID-19 vaccine is a major challenge and only definitive solution to curb the viral infections. The catastrophic impact of COVID-19 catalyzed the vaccine development around the globe and the exceptional advancement in vaccine development has brought the hope throughout the world that the battle against this deadly virus can be won in the near future. India's COVID-19 vaccination drive started on January 16, 2021 and today India has become the fastest country in the vaccination program with global mass COVID-19 vaccination campaigns. It is also important to monitor the long-term efficiency and safety of the authorized vaccines as well as analyze different strategies of combining vaccines that might elicit levels of neutralizing antibodies.
Moreover, not only vaccine candidate rather the mechanism of virus infection to the host needs to be explored to completely curb this infection from the world. A trans-multi-disciplinary workforce is required to reduce, understand and hence stop this viral infection. As all across the globe first line of vaccine designing has been done and many are in progress, it is necessary to reduce the rate of virus infection and to stop the reoccurrence of this infection. It is also necessary to understand the immunopathology and other important aspects of this virus like its host specific impact and vulnerability of aged people.
Meanwhile, many vaccine candidates are being tried and several vaccines are under trial to reduce the infection of coronavirus. This review sheds light on the different vaccine strategies being used nowadays. Also, this review tried to compare the traditional and the modern vaccine strategies. Vaccine design is an important aspect and antigen candidate needs to be explored along with vaccine delivery system before massive production of the vaccine. To provide proper immunization, on one side the vaccine developed so far needs to be produced massively and different grades of vaccine needs to be tried for their immunogenicity on various populations.
None.