Vacunas COVID-19: desarrollo y práctica - COVID-19 vaccines: development and practice
Más datosThe ongoing COVID-19 pandemic has imposed a series of challenges on the scientific community. One of the biggest was the development of safe and effective vaccines in record time, which could be achieved through a global effort. A topic of great discussion has been the technology surrounding these vaccines: ranging from the well-known inactivated virus vaccines to the latest RNA vaccines. As vaccines became available, another point also came into question: their efficacy and effectiveness against the original Wuhan strain and its variants. Among the numerous variants, 5 of them (Alpha, Beta, Gamma, Delta and, more recently, Omicron) gained greater prominence due to their epidemiological relevance. In this scenario, with numerous variants and several vaccine options, scientific information can often be mismatched. This review aims to provide an overview of the efficacy, effectiveness, and safety of 11 vaccines in use or under development against the original Wuhan strain and the variants of concern identified by the World Health Organisation (WHO). Simultaneously, we aim to explore possible scenarios that can be expected shortly regarding new variants and vaccines. Overall, COVID-19 vaccines have satisfactory efficacy and loss of effectiveness against SARS-CoV-2 variants, especially the Omicron strain.
La pandemia de COVID-19 en curso ha impuesto una serie de dificultades a la comunidad científica. Una de las mayores ha sido el desarrollo de vacunas seguras y efectivas en tiempo récord, lo cual ha podido lograrse mediante un esfuerzo global. Una cuestión objeto de gran discusión ha sido la tecnología que rodea a estas vacunas, que fluctúa entre las bien conocidas que contienen virus inactivados, a las últimas vacunas basadas en ARN. A medida que se fue disponiendo de vacunas, también surgió otro punto en cuestión: su eficacia y efectividad contra la cepa originaria de Wuhan y sus variantes. Entre estas, cinco de ellas (Alfa, Beta, Gama, Delta y, más recientemente Ómicron) ganaron mayor importancia debido a su relevancia epidemiológica. En este escenario, con numerosas variantes y diversas opciones de vacunas, la información científica puede verse a menudo desfasada. El objetivo de esta revisión fue aportar una visión general sobre la eficacia, efectividad y seguridad de once vacunas en uso o en desarrollo contra la cepa originaria de Wuhan y las variantes preocupantes identificadas por la Organización Mundial de la Salud (OMS). De manera simultánea, otro objetivo es explorar los posibles escenarios que pueden preverse a corto plazo en cuanto a las nuevas variantes y vacunas. En general, las vacunas contra la COVID-19 tienen una eficacia y una pérdida de efectividad satisfactorias contra las variantes del SARS-CoV-2, especialmente la cepa Ómicron.
COVID-19 is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged in Wuhan, China, in December 2019.1 Patients with COVID-19 generally develop cough, fever, chills, dyspnea, muscle aches, diarrhoea, headache, nausea, stuffiness, nasal, and rhinorrhea. However, this disease can also lead to respiratory failure, heart muscle damage, nervous system problems, kidney failure, and death.2 Some patients have mild or no symptoms but can still spread the virus. These symptoms have higher severity in older males with underlying health conditions.3
On 11 March 2020, the World Health Organisation (WHO) declared COVID-19 as a pandemic. To this date, there have been over 386 million cases and 5.7 million deaths from COVID-19 worldwide.4 Recommended preventive strategies to avoid the widespread dissemination of the new coronavirus are as follows: wear a mask over nose and mouth in public places, stay 2 m away from others, avoid crowds and poorly ventilated spaces, wash hands often with soap for at least 20 s, clean and disinfect tables, doorknobs, light switches, and other frequently touched surfaces.5 Nevertheless, the best way for curbing the pandemic is vaccination.6
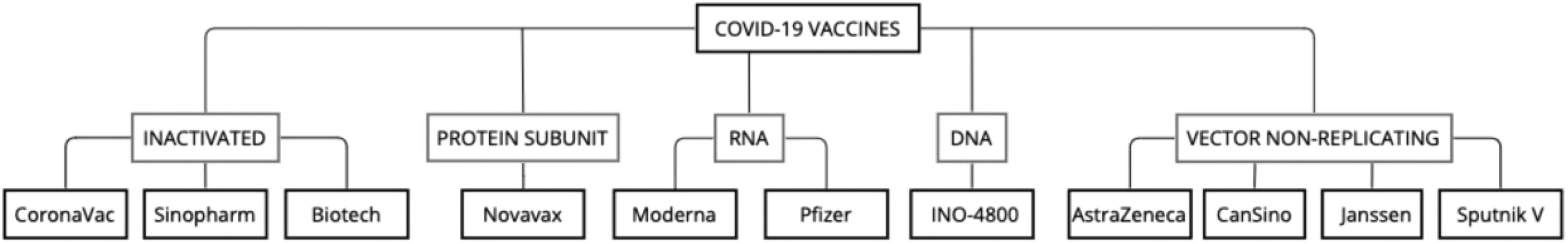
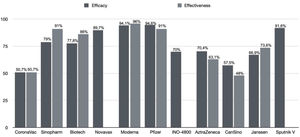
Therefore, there was a global effort to develop a vaccine against COVID-19 with unprecedented fast results. The effectiveness of this preventive measure can be noticed in countries that already vaccinated a large part of their population. In this review, 11 COVID-19 vaccines (Fig. 1) are approached with details of their trials, safety, efficacy, effectiveness, and update of effectiveness against SARS-CoV-2 variants.
SARS-COV-19 and its variantsThe SARS-CoV-2 is the seventh coronavirus known to infect human beings and the third large-scale outbreak of this family of viruses. SARS-CoV-2 is a single-stranded RNA virus with a structural protein denominated spike (S) responsible for mediating the virus's entry into host cells. This protein has a receptor-binding domain (RBD) that connects to the angiotensin-converting enzyme 2 (ACE2), a cellular receptor mostly present in the respiratory tract.7,8
Viruses can constantly undergo mutations, which can change their characteristics, such as increased transmissibility, virulence, and reduced effectiveness of vaccines.9 From the original Wuhan-Hu-1 strain, novel SARS-CoV-2 variants began to be documented worldwide during the pandemic. Some of them were classified either as variants of concern (VOCs) or variants of interest (VOIs). VOIs are those with relevant epidemiological impacts and critical genetic changes that can affect the disease severity, transmissibility, diagnosis, or other vital aspects. In turn, VOCs are VOIs that were assessed to have a global public health significance in terms of transmissibility, change in clinical disease presentation, or resistance against current therapeutic measures.10
There are not currently circulating VOIs. Alpha, Beta, Gamma, Delta, and Omicron are the 5 VOCs designated by WHO, but just Delta and Omicron are currently circulating. Alpha, Beta, and Gamma have demonstrated to no longer pose a major added risk to global public health compared to Delta and Omicron. Therefore, they are now designated as previously circulating VOCs. The VOCs have a mutation called D614G in common, but each contains varying Spike protein RBD substitutions and different key attributes.10,11
The Alpha variant was confirmed as a VOC in December 2020, and its earliest samples were documented in the United Kingdom in September 2020. This variant is also known as B.1.1.7 according to its Pango nomenclature—a system utilised for naming SARS-CoV-2 genetic lineages. The Alpha variant has 17 mutations in its genome, of which 8 are in the Spike protein.12–14 The mortality hazard ratio associated with this variant was 1.64 in positive-tested patients.15 Studies have also shown that B.1.1.7 have a 43%–100% increase in transmissibility over other variants.16,17
Initially found in South Africa in May 2020, the Beta variant (Pangolin B.1.351, which includes its descendent lineages B.1.351.2 and B.1.351.3) was designated as a VOC in December 2020.12,13,18,19 Studies have shown that this variant is more resistant to neutralisation by some monoclonal antibodies, convalescent plasma (9.4 fold), and vaccinee sera (10.3–12.4-fold).20
Gamma was a variant first found in Brazil in November 2020 and declared a VOC in December of the same year. Its original Pango nomenclature was B.1.1.28.1 but later gained the alias P.1 due to its length.12,13,18,19 Similar to the Beta variant, P.1 is resistant to monoclonal antibodies, neutralisation by convalescent plasma (6.5-fold) and vaccinee sera (2.2–2.8-fold). This similarity lies in the fact that both Beta and Gamma share the E484K mutation.21
The Delta variant (B.1.617.2) was first registered in India in October 2020 and later designated a VOC in May 2021.10,18 It has a record of having even greater transmissibility than the Alpha variant (OR = 1.64, 95% CI 1.26–2.13).22
More recently, Omicron (B.1.1.529) had its earliest documented samples in November 2021 and was designated a VOC in the same month. This variant has 37 amino acid substitutions in its Spike protein, 16 of which are RBD substitutions.10,18 As a note of concern, research has shown that Omicron is completely or partially resistant to the majority of SARS-CoV-2 neutralising antibodies, which can be challenging for public health services.23 Vaccine sera examined also seems to generally provide reduced neutralisation for Omicron variant.24 Some of the most relevant aspects of these variants are shown in Table 1.25–31
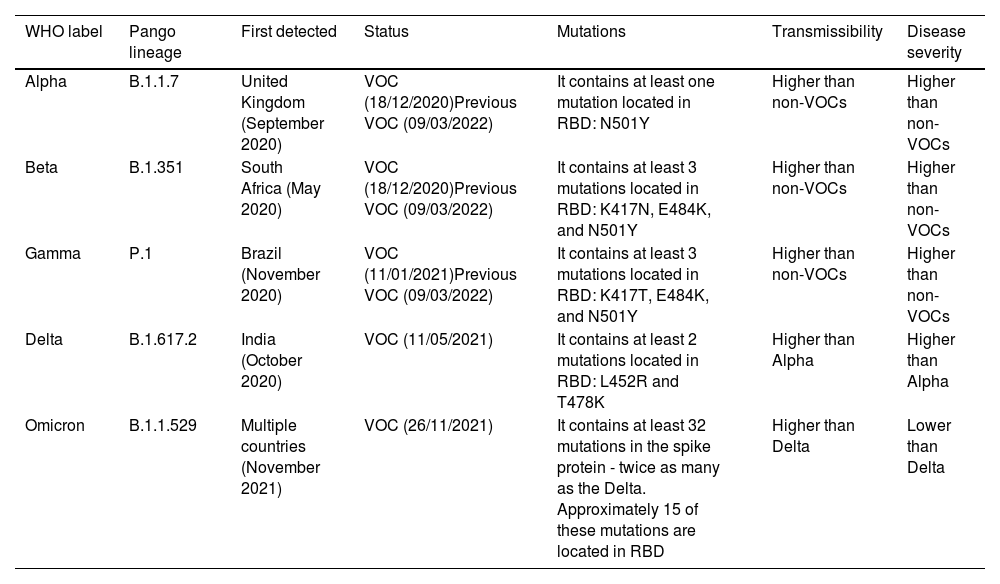
Characteristics of SARS-CoV-2 variants.
| WHO label | Pango lineage | First detected | Status | Mutations | Transmissibility | Disease severity |
|---|---|---|---|---|---|---|
| Alpha | B.1.1.7 | United Kingdom (September 2020) | VOC (18/12/2020)Previous VOC (09/03/2022) | It contains at least one mutation located in RBD: N501Y | Higher than non-VOCs | Higher than non-VOCs |
| Beta | B.1.351 | South Africa (May 2020) | VOC (18/12/2020)Previous VOC (09/03/2022) | It contains at least 3 mutations located in RBD: K417N, E484K, and N501Y | Higher than non-VOCs | Higher than non-VOCs |
| Gamma | P.1 | Brazil (November 2020) | VOC (11/01/2021)Previous VOC (09/03/2022) | It contains at least 3 mutations located in RBD: K417T, E484K, and N501Y | Higher than non-VOCs | Higher than non-VOCs |
| Delta | B.1.617.2 | India (October 2020) | VOC (11/05/2021) | It contains at least 2 mutations located in RBD: L452R and T478K | Higher than Alpha | Higher than Alpha |
| Omicron | B.1.1.529 | Multiple countries (November 2021) | VOC (26/11/2021) | It contains at least 32 mutations in the spike protein - twice as many as the Delta. Approximately 15 of these mutations are located in RBD | Higher than Delta | Lower than Delta |
RBD: Receptor-Binding Domain, VOC: Variant of Concern.
According to data collected on 4 February 2022, 141 vaccines are in clinical development, and 194 are in pre-clinical development. Multiple platforms have been used to create these vaccines, including protein subunit (34% of the candidates in the clinical phase), RNA (16%), non-replicating viral vector (14%), inactivated virus (14%), DNA (11%), and replicating viral vector vaccines (3%).32
As of 23 December 2021, there are 10 vaccines approved by the WHO- Emergency Use Listing Procedure [EUL]: COMIRNATY® (tozinameran or BNT162b2) by Pfizer-BioNTech; Ad26.COV2.S by Janssen–Cilag International NV; SPIKEVAX (mRNA-1273) by Moderna Biotech; SARS-CoV-2 Vaccine (Vero Cell), Inactivated (lnCoV) by Sinopharm-Beijing Institute of Biological Products [BIBP]; CoronaVac by Sinovac Life Sciences Co., Ltd.; COVAXIN® by Bharat Biotech; COVOVAX™ by Serum Institute of India Pvt. Ltd.; NUVAXOVID™ by Novavax CZ a.s.; VAXZEVRIA (AZD1222) by Oxford-AstraZeneca; and COVISHIELD™ by Serum Institute of India Pvt. Ltd.32
It is essential to understand that VAXZEVRIA and COVISHIELD™ are ChAdOx1-S nCoV-19 Corona Virus vaccines.33 Notwithstanding the WHO - EUL procedure, many countries approved phase II and III trials for other vaccines. Notable examples include INO-4800, Convidecia, and Sputnik V.
These vaccines can be classified by their designs, each with different mechanisms of action, limitations, and advantages. INO-4800 is a DNA vaccine. Moderna and Pfizer-BioNTech developed mRNA vaccines. CoronaVac, Sinopharm, and Covaxin are inactivated virus vaccines, while Novavax is a protein subunit vaccine. Viral vector vaccines include the ones created by AstraZeneca, CanSino, Johnson & Johnson, and Sputnik.34
Inactivated and live attenuated, and nucleoid vaccines are easy and quick to mass-produce. However, inactivated and live attenuated vaccines do not confer long-term host immunogenicity like nucleoid vaccines. Recombinant vaccines also may induce weak immunogenicity, whereas viral vector vaccines can induce strong humoral and cellular immunity.35 It is important to note that, while widely effective, viral vector and mRNA-based vaccines present problems with cold chain supply and vaccine wastage.36
Moreover, other novel therapies aside from vaccination are used to treat COVID-19. Monoclonal antibodies, such as Bamlanivimab-Etesevimab and Casirivimab-Imdevimab, have faced clinical trials with positive results. They have demonstrated efficacy in reducing severe disease and hospitalisation and treating severe cases.37,38
Inactivated vaccinesInactivated vaccines are non-live vaccines that aim to boost humoral immunity and cell-mediated response by inactivating preparations of the whole virus. They cannot reactivate since they do not contain infectious or living particles. Therefore, they are usually safe to use in immunocompromised individuals. An existing limitation of this type of vaccine is that, generally, its protection tends to be less than those seen in live vaccines. For this reason, it requires adjuvants or several doses to improve its efficacy. Adjuvants are substances frequently utilised in non-live vaccines to enhance the pathogen's immunogenicity.39 Overall, Sinovac, Sinopharm, and Bharat vaccines reported similar humoral responses.40
CoronaVac by Sinovac Life Sciences Co., LtdCoronaVac is an inactivated vaccine that contains the dead COVID-19 virus. This type of vaccine is very safe since it does not use live components. However, an immune response might not be achieved at the first dose needing booster doses for a long-lasting efficacy.41,42
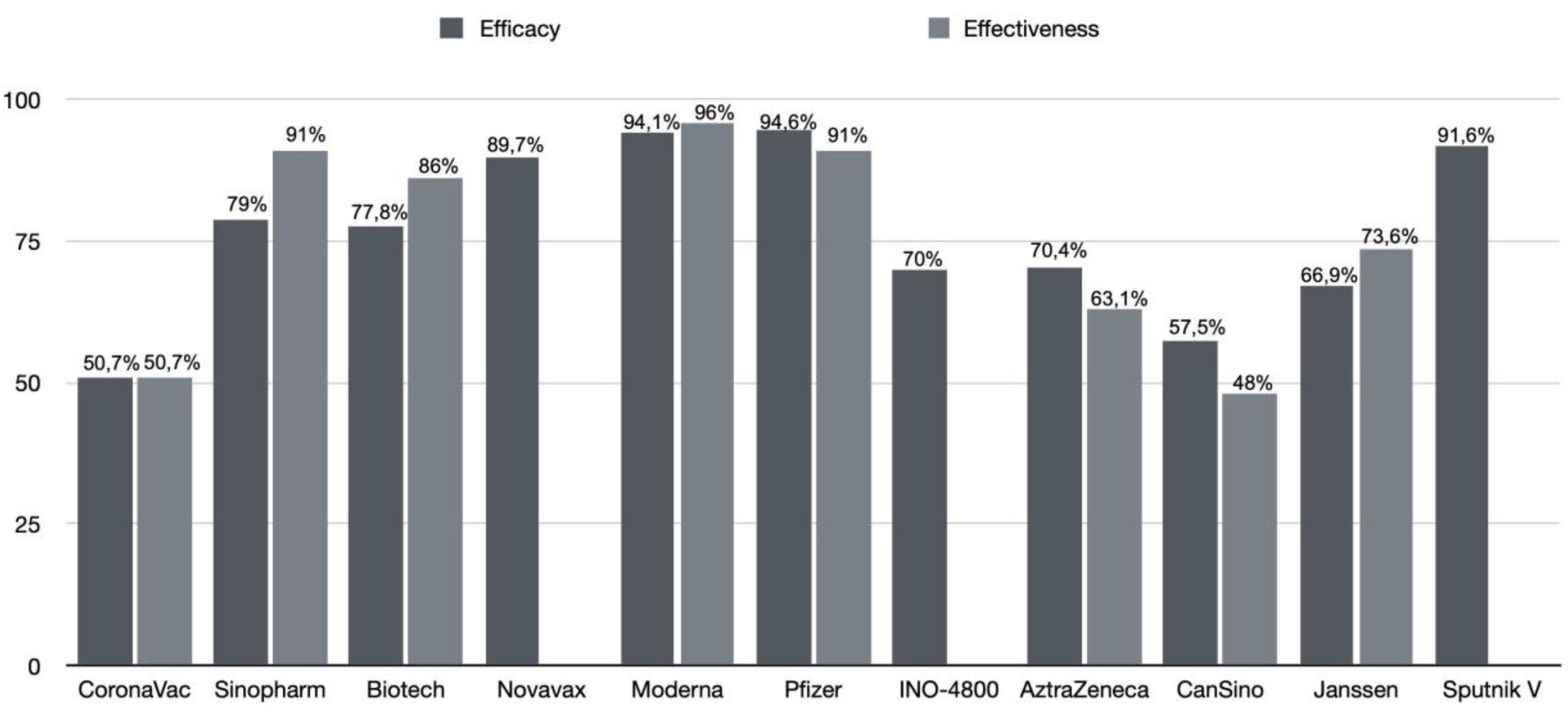
Phase III trial comprised 75,3% white participants, 16,8% multiracial, and 5,2% black or African. Of them, 22,5% were obese, 12,6% had cardiovascular disease, and 3,4% diabetes. Overall, the main adverse reactions were pain at the injection site (46,4%), headache (34,6%), and fatigue (15,4%) reported 0–28 days after both doses. Efficacy against symptomatic COVID-19 was 50.7%, and 83.7% for moderate cases, and 100% for severe cases. Meanwhile, the efficacy of underlying diseases was 74,9% for obese people, 39,5% for cardiovascular disease, and 48,6% for diabetes. Moreover, seroconversion against variants of concern was 95,5% for B.1.1.28 and P.2 and 73.3% for P.1. However, in elderly participants, seroconversion decreased to 47,8% in B.1.1.28 variant, 65,2% P.2, and 60.9% P.1.43
A double-blind, randomised, placebo-controlled phase 3 trial performed in Turkey with volunteers aged 18–59 years showed vaccine efficacy of 83.5% against symptomatic COVID-19, 14 days or more after the second dose, and a good safety and tolerability profile.44
To estimate CoronaVac effectiveness between vaccinated health care workers, reported cases in São Paulo and a prediction model were compared. Overall, 2 weeks after the second dose, effectiveness was 50,7%, increasing to 73,8% in week 5. Furthermore, vaccinated individuals who tested positive for COVID-19 were randomly selected to identify variants of concern. In 40,1% and 3,5% of participants were detected P.1 and B.1.1.7. variants, respectively.45
Effectiveness was also estimated in an elderly population during a P.1 variant epidemic. The effectiveness after 0–13 days and 14 days or more post-second dose was 18.2% and 41.6%, respectively, revealing lesser effectiveness among the elderly. Effectiveness was 61.8% in participants of 70–74 years, 48.9% in participants of 75–79 years, and 28% in 80 years or older participants. Furthermore, a single dose of CoronaVac did not reduce the chances of testing positive for COVID-19.46
A study created pseudoviruses carrying the spike protein of both VOCs and VOIs to evaluate CoronaVac's effectiveness. The plasma samples analysed were collected 14 days after individuals received the second dose of CoronaVac. There was a 2.9-, 5.5-, 4.3-, 3.4-, 12.5-, 3.2-, and 6.4-fold reduction in neutralising Alpha, Beta, Gamma, Delta, Omicron, Lambda, and Mu variants, respectively. For comparison, the neutralisation assay with convalescent plasma demonstrated 2.2-, 5.4-, 4.8-, 2.6-, 10.5-, 1.9-, and 7.5-fold reduction in neutralising Alpha, Beta, Gamma, Delta, Omicron, Lambda, and Mu variants, in the same order.47
BBIBP-CorV (Vero Cells) by SinopharmBBIBP-CorV is also an inactivated vaccine that uses the whole SARS-CoV-2 virus killed by chemicals. This inactivated virus is mixed with the adjuvant aluminium hydroxide to improve immune responses. Later, it is applied to the human body, where it reaches immune cells that produce antibodies to protect from further infections. Initially, scientists obtained the most common strains of COVID-19 in China. After that, they chose one variant and applied a chemical that disabled replication, but the proteins (spikes) in the virus were maintained.48,49
In phase I, participants were 18–80 years old with a mean age of 53,7 years, and 47% were male. Elderly participants had fewer reports of adverse effects between them; only 16,2% had any adverse reaction compared with 40% of participants aged 18–59 years. Overall, the most reported adverse effect was pain at the injection site noted by 13% of participants followed by fever (1%) and fatigue (1%). Seroconversion was seen in 100% of participants aged 18–59 years after 28 days after both doses and 92,8% in elderly participants.49
Another study with 40 382 participants who received a 2-dose inactivated vaccine developed from WIVO4 or HBO2 strain or an aluminium hydroxide-only control had 121 days follow up. Symptomatic COVID-19 was identified in 95 participants in alum-only, 26 participants in WIOV, and 21 in the HBO2 group, representing a 72.8% and 78.1% efficacy, respectively, compared with control.50
On 7 May 2021, WHO approved Sinopharm for emergency use and recommended its use for people aged 18 years old or older with a 2-dose regimen 3–4 weeks interval. Based on available evidence, it also revealed a 79% efficacy for symptomatic and hospitalised COVID-19 cases.51
A study was conducted in Sri Lanka with 282 participants vaccinated with BBIBP-CorV. The data gathered suggests that the vaccine has a similar level of protection against the Delta and Beta variants as those previously infected. Compared with reference strain, there was a 1.38-fold reduction in neutralising antibody titers against B.1.617.2, while there was a 10-fold reduction against Beta.52
COVAXIN® (BBV152) by Bharat BiotechAnother type of inactivated SARS-CoV-2 vaccine is BBV152 or Covaxin, created by Bharat Biotech International. It utilises the Algel-IMDG adjuvant, which is an imidazoquinoline class molecule adsorbed to aluminium.36,40
A double-blind, randomised phase II clinical trial was conducted with patients aged 12–65 years. Of the 380 participants, 190 (50%) received the 3 μg vaccine formulation with Algel-IMDG, of which 26% were females, and 74% were males. The other 50% of the patients received 6 μg with Algel-IMDG; 24% were females, while 76% were male. There was a 28-day interval between the 2 administered doses. Overall, there were mild adverse reactions, being injection site pain the most common one (2,6% from the 3 μg dose group and 3,2% from the 6 μg group).40
In the placebo-controlled phase III trial, the selected formulation was the 6 μg vaccination dose. Among the participants who received the BBV152 vaccine, 32,7% were female, and 67,3% were male, with a mean age of 40,1 years. 22,2% of the patients had a coexisting chronic condition. The estimated efficacy of this vaccine is 77,8% for symptomatic COVID-19 and 93,4% for severe COVID-19. In individuals with a pre-existing chronic medical condition, its efficacy was 64,2% and 63,6% for asymptomatic COVID-19. Covaxin also has a 65,2% efficacy against B.1.617.2 (Delta), and 90,1% efficacy against B.1.617.1.36
The immunogenicity of the Covaxin vaccine was tested against B.1.1.7, no different from a hallmark strain.53 However, there was a significant reduction in neutralisation titre for Beta and Delta variants in comparison to B.1.54 A cross-sectional study demonstrated reduction in neutralising antibodies titre for Delta, Delta AY.1, and B.1.617.3 variants, compared with the B.1 strain.55
Another study analysed the reinfection rate among healthcare workers vaccinated with Covaxin in India. The data collected was during 2 waves of the pandemic, with the second of the B.1.617.2 variant. Among the participants, 55% were male, 32.7% were diagnosed with COVID-19, and their mean age was of 36.6 years. The reinfection incidence density is 7.26 per 100 person-years. Cases of moderate to severe disease were seen in a higher proportion in participants aged 45 years or older (13.2%) than those aged 44 years or younger (8.2%). Two doses of the vaccine have an effectiveness rate of 86%, while one dose does not confer significant effectiveness compared with the unvaccinated participants (12%).56
Protein subunitLike inactivated vaccines, subunit protein vaccines are also a type of non-live vaccine. As aforementioned, they are safer to use in immunocompromised people. Generally, subunit vaccines cause fewer adverse reactions than live or whole-organism vaccines, but their immune response can be less effective since they contain fewer antigens.39 However, this type of vaccine is relatively difficult to manufacture since it takes time to find the best antigen combination.57
NUVAXOVID™ (NVX-CoV-2373) by NovavaxNVX-CoV2373 vaccine was co-developed by Novavax and the Coalition for Epidemic Preparedness Innovations foundation. It is a recombinant protein subunit vaccine. This COVID-19 vaccine uses a trimeric full-length SARS-CoV-2 spike glycoprotein with an adjuvant called Matrix-M™. The adjuvant enhances activated T cell, B cell, and APC populations. As well as recruit and increase the frequency of CD4+ and CD8+ and neutralising antibodiesk.57–59
Participants were primarily white in phases I and II (78,6%), and 50,4% were male. The volunteers who received the adjuvant presented anti-Spike IgG in higher levels and more CD4+ proliferation. On average, 91,4% of participants had mild adverse events like pain, erythema or swelling and all of them seroconverted 14 days after the second dose.58
In phase III clinical trial, 15 187 participants underwent randomisation. A total of 48.4% were women, and 44.6% had coexisting risk factors for Covid-19. The median age was 56 years. After the first dose, vaccines reported systemic adverse events, such as headache (24.5%), fatigue (19.4%), and muscle pain (21.4%), which were also reported after the second dose (40%, 40.3%, and 40.3% respectively). The calculated vaccine efficacy was 89.7%. A post hoc analysis identified the Alpha variant in 66 participants and non-Alpha variant in 29 individuals. The vaccine showed an efficacy of 86.3% against the B.1.1.7 variant and 96.4% against non-B.1.1.7 variants.60
A randomised, observer-blinded, placebo-controlled trial was conducted in the United States and Mexico with 29 949 participants who underwent randomisation. 48.2% of the participants were female, 75.9% identified as white, 47.3% had coexisting conditions, and their mean age was 47 years. The calculated vaccine efficacy was 89.3%. Against the Alpha variant, the efficacy was 93.6%, and against any VOC or VOI was 92.6%.61
Efficacy was also evaluated in a trial with 4387 people, 30% HIV-positive. There was an outbreak of B.1.351 variant during this trial, representing 92,7% of COVID-19 cases. Overall, NVX-CoV2373 efficacy was 49,4% in seropositive participants and 60,1% in HIV-negative, representing lower protection against the South Africa strain.62
RNA vaccinesBoth Moderna and Pfizer created vaccines that utilise modified RNA to encode the SARS-CoV-2 Spike protein, employing a lipid nanoparticle (LNP) delivery system. They have a good safety profile since they do not utilise the actual pathogen or integrate it into host DNA. These vaccines have the disadvantage of complicated transportation and distribution for hot climates and underdeveloped countries, mostly because of their storage requirements. Pfizer vaccines vials must be stored between −80 °C and −60 °C, while Moderna vaccines are stored between −25° and −15 °C.63
SPIKEVAX (mRNA-1273) by Moderna BiotechmRNA-1273 is a lipid-nanoparticle (LNP) encapsulated mRNA vaccine encoding a pre-fusion stabilised form of the SARS-CoV-2 spike protein (S-2P).64 Like the RNA vaccines, mRNA-1273 vaccine works by teaching cells in our bodies how to produce a protein that initiates an immune response. First, mRNA enters immune cells and gives them instructions to produce a protein or a piece of it. Then, this protein is displayed on the cell surface and is recognised as an intruder initiating antibody production. Afterwards, the information on how to protect from this pathogen is stored, and the person will have a favourable response when attacked again.65,66
Moderna vaccine has 2-dose regimen 28 days apart, and all participants had mild to moderate local and systemic adverse events, which mainly occurred after the second dose. Phase II participants were 97% white, 29% male, aged 55–87 years old with a mean age of 64,3 years. After the second dose, they reported similar adverse effects between different age groups. For instance, pain was reported by 60% of participants aged 18–55 years and by 55% in people aged 55–87 years. As well, as headache 40% in the younger group and 39% between older participants and fatigue 43,6% and 49% in the same groups, respectively.64
Phase III was a randomised, observer-blind, placebo-controlled trial that included a more diverse sample: people with 18 up to 95 years old, an increased number of racial and ethnic minorities (although 79.2% were white), and people with other diseases (chronic lung disease, cardiac disease, obesity, diabetes, liver disease, and HIV). The main adverse events were pain, erythema, swelling, and lymphadenopathy in the injection site. Moreover, the main systemic adverse events were headache, fatigue, myalgia, arthralgia, nausea, and chill, predominant in the mRNA-1273 group compared with the placebo group. Seroconversion rates were 100% in all participants 14 days post-second dose. Furthermore, efficacy against symptomatic COVID-19 was 94,1% at least 14 days after the second dose and 100% against severe disease.67
In addition, mRNA-1273 effectiveness was tested in Canada. The study involved 324 033 symptomatic tested individuals with 16 years or more. Of these, 2222 received only 1 dose of mRNA-1273 vaccine. The vaccine effectiveness 14 days after the first dose was 73%, increasing to 96% after 7 days of the second dose. The results suggest that after the first dose of mRNA-1273 vaccine, the potential to transmit COVID-19 can be reduced.68
Immunogenicity data 90 days after the second vaccination of mRNA-1273 vaccine was described. The results show that the titers of binding and neutralising antibodies after 90 days can provide durable humoral immunity.69 However, another study also evaluated the durability of mRNA-1273 against COVID-19, and its variants were estimated by measuring the levels of binding antibodies 15 days posted the second dose when efficacy was peaking. The antibodies against COVID-19 decreased after 411 days. Similarly, the antibody against SARS-CoV-2 strains such as B.1.351 (South Africa Strain) reduced after 100 days, P.1 (Brazil strain) after 202 days, B.1.429 (California variant) after 258 days and B.1.1.7 after 309 days. This data suggests that a booster shot will be needed after a year to increase protection against a resistant variant.70
The vaccine effectiveness was also tested against COVID-19 variants. One dose of mRNA-1273 had 55.6%, 2 doses had 60.7%, and 3 doses had 94% effectiveness against the Delta variant. Against Omicron, the vaccine effectiveness was 20% for 1 dose, 42.8% for 2 doses, and 67.7% for 3. In immunocompromised individuals, the 3-dose vaccine effectiveness against Omicron was only 21.7%.71
COMIRNATY® (tozinameran or BNT162b2) by Pfizer/BioNTechBNT162 is an RNA-based vaccine developed by Pfizer and BioNTech. This vaccine, as previously described, is formulated with lipid nanoparticle technology that delivers the genetic information of the immunogen to antigen-presenting cells and generates potent immune responses.72 After analysing two candidates in previous studies: the RNA BNT162b1 that encodes a soluble and trimerised receptor-binding domain, and RNA BNT162b2 that encodes the full-length transmembrane spike glycoprotein, locked in its prefusion conformation, the RNA BNT162b2 was selected for the sequence of the clinical studies due to greater tolerability compared to BNT162b1, particularly in older adults.72,73
In phases II and III trials, 43 548 persons 16–91 years were involved. Of these, 51% of participants were male, 83% white, 35% obese, and 21% had a chronic condition. The predominant adverse effects were site pain felt by 66% of older-aged participants compared with 78% of younger volunteers post the second dose. Other adverse events were fatigue (59%/51%), headache (52%/39%), and fever (16%/11%). After the first dose, 121 cases of COVID-19 were identified, but only 39 of them were in the BNT162b2 vaccine group. This result shows a 52% efficacy after only 12 days post-vaccination. Similarly, 7 days after the second dose, 178 volunteers tested positive for COVID-19, and only 9 of them were in the vaccine group reaching a 94,6% efficacy against symptomatic COVID-19.74 Chung et al. showed BNT162b2 effectiveness of 59% against symptomatic COVID-19, 14 days or more after the first dose, and 91% 7 days or more after the second dose.68
Some studies show that the elderly population may need a booster dose to ensure robust protection and effectiveness against COVID-19. A study regarding BNT162b2 age-dependent immune responses with 2 groups, one of them aged below 60 years old and other 80 years or more, revealed that post-second dose 31.3% of the elderly group had no detectable neutralising antibodies while the younger group only 2.2% did not have neutralising antibodies.75 Equally, Collier et al. showed that neutralising antibody responses after the first vaccine dose diminished with increasing age, especially in over 80 years old.76
Pfizer vaccine efficacy against variants of concern (VOCs) was tested in a study with 140 participants with a median age of 72 years old, and 51% of them were women. Overall, after the first dose, neutralising antibodies decreased, especially in people over 80 years old. Likewise, lower neutralisation rates against B.1.1.7 (UK variant), P.1 (Brazil variant), and B.1.351 (South Africa variant) were observed.76
One strategy to maximise speed in vaccination was to offer the first dose of Moderna or Pfizer to as many people as possible and delay the second dose. It was determined that delayed second doses increased efficacy when the vaccine efficacy was above 80%. Mortality levels from COVID-19 were analysed with standard vaccination and delayed the second dose per 100 000 people. The results were 226 (standard dose) vs 179 (delayed the second dose) in vaccines with 70% efficacy, 223 vs 207 in vaccines with 80% efficacy, and 235 vs 236 with 90% efficacy. In general, delaying second doses of mRNA vaccines may be ideal when their efficacy is at or above 80%.77
The United Kingdom government announced to delay the second dose of BNT162b2 for 12 weeks after the first dose to reduce the total number of severe COVID-19 cases. A study in Israel supported this strategy by estimating the efficacy of a single dose. Moreover, after 21 days, the estimated efficacy was 90%, indicating that the second dose can be delayed and offer a high level of protection.78
A study conducted with South African participants analysed the BNT162b2 vaccine effectiveness against the Omicron variant. The previously infected individuals who received vaccination had 73% protection for symptomatic disease, while those who were not infected and only received vaccination had 35% protection. However, both groups had a 22-fold escape from vaccine-elicited neutralisation. Against severe infection, Pfizer's efficacy is predicted to be 95% for previously infected and vaccinated people and 77% for only vaccinated.79
DNA vaccinesBoth DNA and mRNA vaccines employ genetic information to instruct cells to produce specific proteins rather than the entire virus. Prior to the SARS-CoV-2 outbreak, no gene-based vaccines had been approved.57
INO-4800 by Inovio PharmaceuticalsAs well as other DNA vaccines, INO-4800 have fragments of DNA encoding foreign proteins in a bacterial plasmid.80 This plasmid is delivered to human cells by CELLECTRA electroporation, which generates electrical pulses that create pores in the cell membrane, allow DNA passage, and generate an immune response inducing B and T cells. Since DNA vaccines have no live components, the risk of developing COVID-19 is low and they are easy to manufacture because scientists do not have to handle threatening microorganisms, just the sequenced genome of SARS-CoV-2.81,82
In phase I clinical trial, most of the participants were white, 82.5%, male 55%, and their mean age were 34.5 years. They reported mild adverse effects such as pain in the injection site (7,5%), erythema (5%), and nausea (2,5%). Overall, seroconversion was detected in 95% of participants 14 days after the second dose, and 81% had neutralising antibodies by live virus neutralisation.82
Furthermore, in phase II, 85% of participants were white, 47.4% male, and their mean age were 44.4 years. The primary reported adverse effects were pruritus (26,6%), headache (19,2%), pain at the injection site (18,9%), and erythema (15,2%). INO-4800 showed a 70% efficacy in preventing symptomatic COVID-19 cases.83
INO-4800 was tested against SARS-CoV-2 variants to promising results. While testing the B.1.1.7 and B.1.351 variants against the Wuhan strain, there was a 2.1- and 6.9-fold reduction, respectively. Surprisingly, there was no reduction in neutralising activity when comparing it to B.1.351 variant.11
Vector non-replicating vaccinesVectored vaccines can combine the benefits of both live and subunit vaccines. They are made from non-pathogenic infectious viruses that expresses a pathogen's antigenic protein genes.39
VAXZEVRIA (AZD1222 or ChAdOx1-S) by Oxford-AztraZenecaChAdOx1 nCoV-19 vaccine is a replication-deficient chimpanzee adenovirus vector that encodes the spike protein of SARS-CoV-2 and induces a robust humoral and cell-mediated response, predominantly type-1T helper cells.84
In phases I and II, participants were 18–55 years old, 50,2% were male, and 90,9% were white. Succeeding vaccination adverse effects were less reported by participants who took prophylactic paracetamol 24 h post-vaccination. 52,5% of participants reported pain, whereas 32% took paracetamol. This also happened with tenderness (70,5%/61,5%), fatigue (59%/43%), and headache (54,5%/49%) in the participants who did not take paracetamol and does who did, respectively.85
Antibodies against SARS-CoV-2 peaked after 28 days of vaccination with a medium of 157 ELISA units (EU) that represents the total IgG. On day 56, IgG levels stayed high at 119 EU. Volunteers who received a prime-boost had 639 EU on day 56. This result shows that a single dose can increase spike-specific antibodies, but the 2-dose regimen had more IgG in all participants. T-cell responses peaked on day 14 and were maintained at day 56. However, there was no evidence that the second dose increased cellular responses.85
ChAdOx1 showed the efficacy of 70,4% after 2 standard doses and 64,1% after 1 dose against symptomatic COVID-19. In subgroup analysis, the efficacy of participants who received a low dose followed by a standard dose was 90%. The booster dose was 6 or 12 weeks after the first dose, no significant difference in efficacy estimates between the 2 applied intervals.86
A case–control study in India analysed data from 2766 cases and 2377 controls to determine AZD1222's effectiveness. The median age in the case group was 35, and the participants were predominantly male (65.2%). The calculated total vaccine effectiveness was 63.1%. A single dose was 46.2% effective, and complete vaccination is 81.5% effective against the moderate-to-severe disease. The vaccine remained effective against the Delta variant since there was still spike-specific T-cell responses during the analysis.87
Moreover, after the occurrence of cerebral venous thrombosis post ChAdOx1 vaccination, an online questionnaire was sent to neurology departments in Germany. They reported a total of 62 cases of cerebrovascular outcomes within 1 month after ChAdOx1 or BNT162b2 vaccination. In these cases, 75.8% were female with a mean age of 46.7 years old, and 45 cases were from cerebral venous thrombosis (CVT). Thirty-seven after ChAdOx1 and 8 after BNT162b2. In general, the estimated incidence of CVT after first dose vaccination is 6.51% per 100 000 person-years, 17.91% after vaccination with ChAdOx1 per 100 000 person-years, and 1.32% after BNT162b2 per 100 000 person-years.88
A single-blind, randomised, controlled phase II/III trial in the UK determined that AZD1222 has comparable immunogenicity across all age groups. Of the 560 participants enrolled, 50% were female, and 160 were 56–69 years old. Local and systemic reactions were more common in younger adults than older individuals (aged ≥56 years).89
Convidencia (AD5-nCOV) by CanSino Biologics IncCanSino's and Beijing Institute of Biotechnology vaccine or Convidencia ™ is a non-replicating viral vector vaccine. That uses Ad5-nCov as a vector to induce an immune response that will prepare the body for future COVID-19 infections. Adverse effects were reported by 81% of volunteers in 7 days after vaccination. The most common adverse effects were pain in the injection site (54% of all participants), fever (46%), fatigue (44%), headache (39%), and muscle pain (17%).90
Rapid binding antibodies (RBA) are responsible for binding on COVID-19 and alerting the immune system to destroy it. These antibodies were observed in all groups at day 14 post-first dose. On day 28, 100% of participants in the high-dose group had RBA levels quadruplicated, while 97% and 94% of middle- and low-dose groups, respectively, had this increase.90
Neutralising antibodies against SARS-CoV-2 increased on day 14 post-vaccination and peaked on day 28. A 4-fold increase was observed in 50% of participants in the low- and middle-dose groups and 75% in the high-dose group.90 In a double-blind, randomised, international, placebo-controlled phase III clinical trial, the calculated Convidencia™ vaccine efficacy was 57.5% against symptomatic infection and 91.7% against severe disease. Convidencia™ can produce high levels of anti-RBA, and its efficacy peaks 2 to 4 weeks after vaccination, gradually diminishing afterwards.91 In addition, a study with healthcare workers showed a 48% effectiveness against PCR confirmed illness.92
Johnson & Johnson/JanssenJohnson e Johnson company acquired Janssen Pharmaceutical that developed the Ad26.COV2. S vaccine. This vaccine is a recombinant replication-incompetent adenovirus, which means it lacks essential genes for viral reproduction.93
Ad26.COV2. S vaccine immunogenicity was tested unblinded, measuring the binding-antibody geometric mean concentration (GMC), the average concentration of a specific antibody. After 29 days post the first dose, all the 5 groups in cohort 1 had a 95% increase in GMC values. Surprisingly, all groups had 100% seroconversion (development of specific antibodies) before the second dose except high dose/high-dose participants with a 97% immunisation.94
In the phase III trial, 19 630 SARS-CoV-2–negative participants received Ad26.COV2.S and 19 691 received placebo. Twenty-eight days after a single dose, the efficacy was 66.9% against moderate to severe critical COVID-19 and 85.4% against critical COVID-19. The main systemic symptoms were fatigue, headache, myalgia, nausea, and pyrexia (fever) and the main local symptoms were erythema , pain, and swelling. In general, systemic symptoms were felt by 65% of low dose, 84% high dose, and 26% of placebo participants.95
The real-world effectiveness of Ad26.COV2. S is shown by a study that enrolled participants between 27 February and 14 April 2021. Only 3 of 1779 vaccinated participants tested positive for COVID-19 and 128 of 17 744 in the unvaccinated individuals representing a 73.6% effective in preventing COVID-19 at least 2-week after a single dose.96
After more than 7 million doses of Ad26.COV2. S in April 2021, there were 6 cases in vaccinated individuals of cerebral venous sinus thrombosis with thrombocytopaenia in the USA. Females only reported the cases between 18 and 48 years and 13 days or more after vaccination. Furthermore, in the UK, there were 23 patients with the same case 61% was female, their ages were between 21 and 77 years, and thrombosis and thrombocytopaenia happened 6–24 days after vaccination.97
The Janssen vaccine was able to induce median pseudovirus neutralising antibody titers that were 5.0- and 3.3-fold lower against the B.1351 and P.1 variants, respectively, compared to the original Wuhan strain.98
Sputnik VGam-COVID-Vac, also known as Sputnik V, is a vaccine with recombinant adenoviral with a non-replicating vector such as rAd26-S and rAd5-S. In brief, viral vector vaccines use a harmless vector synthesising spike proteins specific to COVID-19. After that, the body learns how to defend itself from future COVID-19 infections.99
In phase I, specific COVID-19 IgGs were detected 14 days after the first dose in 88,9% of volunteers who received rAd26-S and 84,5% in those who had rAd5-S. Subsequently, after 21 days, 100% of participants in phase I had IgGs in their blood, including frozen and lyophilised vaccines. Similarly, in Phase II, after 14 days of the first dose, 85% of participants had IgGs in their blood, and after 21 days, 100% of them this data also combines frozen and lyophilised vaccines. The most common local reactions from both phases were: pain at the injection site (58%), hyperthermia (50%), asthenia (28%), and muscle and joint pain (24%).100
Therefore, the cellular immune response was detected in 100% of participants after 28 days. The median cell proliferation (increase in cell growth and division) in frozen formulations were 2,5% CD4+ and 1,3% CD8+. However, in lyophilised formulas 1,3% CD4+ and 1,1% CD8. In conclusion, the vaccine-induced humoral and cellular responses occurred in 100% of healthy adult volunteers and seroconversion in all participants after 42 days.100
In a randomised, double-blind, placebo-controlled, phase III clinical trial, the vaccine's efficacy was tested with interim results. Of the 21 977 participants, 61.5% were male, and the majority were Caucasian (98.5%). Data collected on day 21 after the first dose (the day the participants received the second dose) analysed that Gam-COVID-Vac is 91.6% efficacious against COVID-19.101
This vaccine is currently being used in a single dose regimen in some countries. However, a study investigated the immunogenicity of the vaccine in 327 naïve individuals, of which 88.7% were seroconverted. While this is highly immunogenic, using a 2-dose regimen is likely to be more beneficial.102
The effectiveness of the Sputnik V vaccine was analysed against SARS-CoV-2 variants. The B.1.351 variant reveal a potential of B.1.351 to escape the neutralising antibody responses.103 An Argentinean cohort was able to evaluate the presence of neutralising response in 285 participants who received the Gam-COVID-Vac. At day 42 after the second dose, 99.65% of the participants had detectable antibodies, but only 23.15% of them were negative for neutralising antibodies detection. Regarding the P.1 variant, there was a 0.94- and 1.68-fold decrease of the neutralisation response for this variant compared to the Wuhan strain. However, the effectiveness of the vaccine against the variant was not able to be calculated since the study did not have a control group.104
Updated performance of COVID-19 vaccine against SARS-CoV-2 variantsFig. 2 summarises the efficacy and effectiveness of the vaccines addressed in this review. It is observed that the efficacy and effectiveness of the 11 vaccines against SARS-CoV-2 are satisfactory. However, the performance of vaccines against SARS-CoV-2 variants is concerning. This issue has been discussed throughout the text, but this section aims to address vaccine performance against SARS-CoV-2 variants, as shown in Table 2. The effectiveness of Pfizer/BioNTech and Oxford/AstraZeneca vaccines remained preserved against the B.1.1.7 variant, indicating protection of 87.0–93.4% and 66.1–70.4%,105 respectively, while the Moderna vaccine showed a 1.2-fold reduction.106 The effectiveness against the P.1 variant was 68.1% for the J&J/Janssen vaccine and 41.6% for the CoronaVac/Sinovac vaccine,46 while Pfizer/BioNTech, Moderna, and Oxford/AstraZeneca vaccines showed preserved or little reduced neutralisation.107
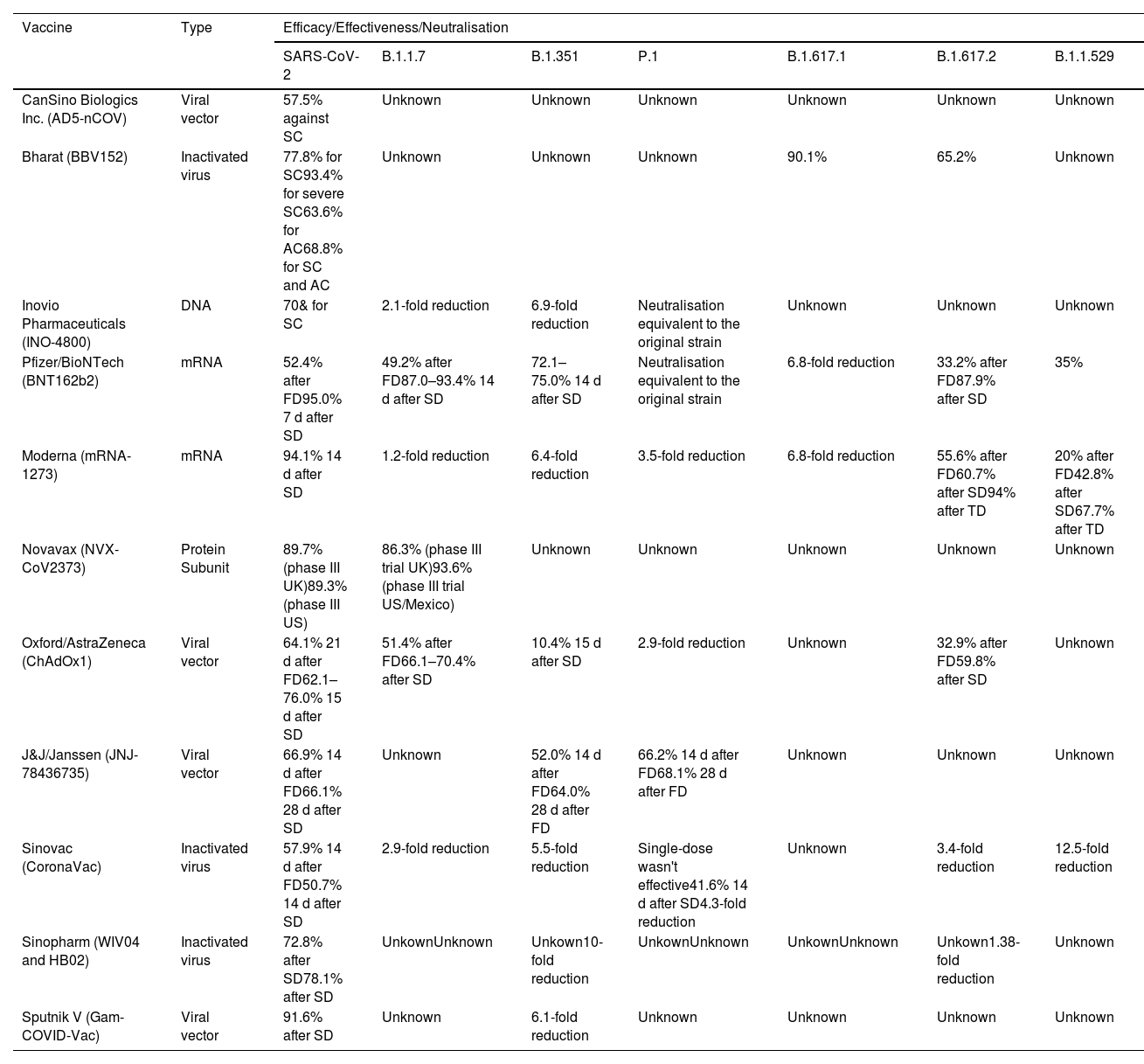
Efficacy and effectiveness of the vaccines against SARS-CoV2 and its variants.
| Vaccine | Type | Efficacy/Effectiveness/Neutralisation | ||||||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | B.1.1.7 | B.1.351 | P.1 | B.1.617.1 | B.1.617.2 | B.1.1.529 | ||
| CanSino Biologics Inc. (AD5-nCOV) | Viral vector | 57.5% against SC | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Bharat (BBV152) | Inactivated virus | 77.8% for SC93.4% for severe SC63.6% for AC68.8% for SC and AC | Unknown | Unknown | Unknown | 90.1% | 65.2% | Unknown |
| Inovio Pharmaceuticals (INO-4800) | DNA | 70& for SC | 2.1-fold reduction | 6.9-fold reduction | Neutralisation equivalent to the original strain | Unknown | Unknown | Unknown |
| Pfizer/BioNTech (BNT162b2) | mRNA | 52.4% after FD95.0% 7 d after SD | 49.2% after FD87.0–93.4% 14 d after SD | 72.1–75.0% 14 d after SD | Neutralisation equivalent to the original strain | 6.8-fold reduction | 33.2% after FD87.9% after SD | 35% |
| Moderna (mRNA-1273) | mRNA | 94.1% 14 d after SD | 1.2-fold reduction | 6.4-fold reduction | 3.5-fold reduction | 6.8-fold reduction | 55.6% after FD60.7% after SD94% after TD | 20% after FD42.8% after SD67.7% after TD |
| Novavax (NVX-CoV2373) | Protein Subunit | 89.7% (phase III UK)89.3% (phase III US) | 86.3% (phase III trial UK)93.6% (phase III trial US/Mexico) | Unknown | Unknown | Unknown | Unknown | Unknown |
| Oxford/AstraZeneca (ChAdOx1) | Viral vector | 64.1% 21 d after FD62.1–76.0% 15 d after SD | 51.4% after FD66.1–70.4% after SD | 10.4% 15 d after SD | 2.9-fold reduction | Unknown | 32.9% after FD59.8% after SD | Unknown |
| J&J/Janssen (JNJ-78436735) | Viral vector | 66.9% 14 d after FD66.1% 28 d after SD | Unknown | 52.0% 14 d after FD64.0% 28 d after FD | 66.2% 14 d after FD68.1% 28 d after FD | Unknown | Unknown | Unknown |
| Sinovac (CoronaVac) | Inactivated virus | 57.9% 14 d after FD50.7% 14 d after SD | 2.9-fold reduction | 5.5-fold reduction | Single-dose wasn't effective41.6% 14 d after SD4.3-fold reduction | Unknown | 3.4-fold reduction | 12.5-fold reduction |
| Sinopharm (WIV04 and HB02) | Inactivated virus | 72.8% after SD78.1% after SD | UnkownUnknown | Unkown10-fold reduction | UnkownUnknown | UnkownUnknown | Unkown1.38-fold reduction | Unknown |
| Sputnik V (Gam-COVID-Vac) | Viral vector | 91.6% after SD | Unknown | 6.1-fold reduction | Unknown | Unknown | Unknown | Unknown |
D: days; FD: first dose; SD: second dose; TD: third dose; SC symptomatic case; AC asymptomatic case; *Reduction in neutralisation by sera from vaccinated individuals.
Regarding the B.1.351 variant, Pfizer/BioNTech and J&J/Janssen vaccines were 72.1–75.0% and 64.0% effective, respectively.105 Oxford/AstraZeneca vaccine was ineffective, and the Moderna vaccine had a 6.4-fold reduction.108 Pfizer/BioNTech and Moderna vaccines showed a 6.8-fold efficacy reduction against the B.1.617.1 variant. A similar situation occurs against the B.1.617.2 variant, where there are only data on 87.9% and 59.8% of vaccine effectiveness from Pfizer/BioNTech and Oxford/AstraZeneca, respectively.109
Oxford/AstraZeneca efficacy against B.1.351 (South Africa variant) was obtained in a trial with 20 026 participants enrolled between 24 June and 9 November their median age was 31 years old and happened in South Africa during the B.1.351 variant epidemic. In general, 23/717 participants (3.2%) of the placebo group and 19/750 participants (2.5%) who received 2 doses of ChAdOx1 developed COVID-19. All 42 COVID-19 cases were mild (15 vaccine group and 17 placebo) or moderated (4 vaccine group and 6 placebo). 95.1% of COVID-19 cases were B.1.351 variant and 4.9% B.1.1.1 and B.1.144 strains. Overall vaccine efficacy was 21.9%. Efficacy against B.1.351 (South Africa strain) was 10.4%.108
Challenges with COVID-19 vaccinesThe emergence and spread of mutated strains will challenge the vaccine's effectiveness, and more global vaccination cycles may be needed. Countries such as the USA and UK, which had reduced cases since the start of vaccination in December 2020, returned to an increase in COVID-19 cases in July 2021,4 attributed to Delta variant quick spread.110
The recent advance of Omicron leads to another wave of increased cases and uncertainty. This new variant has mutational changes more than 3 times denser than previous variants, which allows a significant decrease in neutralisation titers for vaccine and naturally immune sera, with many demonstrating complete neutralisation failure. An antigenic map was build comparing the neutralisation of SARS-CoV-2 strains. While early pandemic viruses and Alpha are close to the centre, Beta/Gamma diverge in one direction and Delta goes to the opposite side. Omicron has the most antigenically distant point in the map, meaning that Omicron serum would poorly neutralise other variants.111
So far, the disease has been considered mild, and the vaccines could partially protect against severe disease and hospitalisation. It is, however, highly transmissible, and studies reported doubling times of 3.38 days. Monoclonal antibody cocktails do not work against this variant, and reinfections are common, but the risk of hospitalisation is significantly lower in these cases.38
New vaccines specifically targeting Omicron and other variants must be created. Moderna's mRNA-Omicron, for instance, has been recently tested in macaques as a booster after a 2-dose regimen of mRNA-1273. The study's findings demonstrate that both vaccines elicit a similar neutralising response against the variant; no additional benefit was provided by the omicron spike-specific vaccine.112
This raises the contentious issue of booster vaccination, both homologous and heterologous. In response to diminishing antibody titres 3–6 months after vaccination, numerous countries began to offer boosters to their population, even after WHO's appeal to ensure vaccination to all adults with 2 doses—particularly in underdeveloped countries—before offering boosters.38
A phase IV, masked participant study was made in Brazil with participants who had received a 2-dose regimen of CoronaVac 6 months previously. After receiving the third dose, the participants significantly increased binding and neutralising antibodies. All heterologous regimens (Ad26.COV2-S, BNT162b2, and AZD12222) had superior anti-spike IgG responses if compared to the homologous regimen.113 Further studies are, however, necessary to better understand the optimal combinations, intervals, and doses.
Another challenge is the hesitation of part of the population to get vaccinated. The WHO itself recognises this as a threat to international public health security. Social, cultural, and political differences, and doubts about the effectiveness and safety of COVID-19 vaccines are some factors that influence people's willingness to be vaccinated. Community education is the main weapon that needs to be improved to accept COVID-19 vaccines better.114
In addition, immune senescence is a well-described phenomenon whereby responses to pathogens and vaccines are impaired/dysregulated with age.115 For example, effective seasonal influenza vaccination of the elderly is a significant public health challenge due to greater morbidity and mortality in this group. Lower neutralising antibody titres using standard-dose influenza vaccines in elderly individuals have been addressed by using higher dose vaccines, highlighting that understanding age-related heterogeneity in vaccine responses can lead to health policy change.116
ConclusionThis review addresses the different platforms of SARS-CoV vaccines—DNA, RNA, recombinant, inactivated—and some characteristics of 11 vaccines currently in use or under development. The clinically tested vaccines provide satisfactory efficacy and high immunogenicity, with only mild to moderate adverse effects. However, the loss of effectiveness against COVID-19 variants is concerning, especially regarding Omicron with high transmissibility. Therefore, the production of new vaccines against the current strains of concern is urgent. More studies on booster and heterologous vaccination against current variants should be done to analyse the most effective prophylactic measures against this disease.
Conflict of interest/Competing interestsThe authors report there are no competing interests to declare.