Coronavirus disease 2019 (COVID-19) is a pandemic caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The spread of the virus was rapid and currently COVID-19 cases are present worldwide in 213 countries, area or territories. Researchers worldwide are working and sharing their contribution regarding epidemiology, prevention, treatment, clinical and diagnostic patterns of the COVID-19. Current review is another contribution to the current knowledge, presenting the data in organized and systematic format about the current pandemic of COVID-19. The epidemiological information presented in the paper is subject to change as new cases are diagnosed and status of active cases is updated on daily basis.
La enfermedad por coronavirus de 2019 (COVID-19) es una pandemia causada por un nuevo coronavirus, el coronavirus causante del síndrome respiratorio agudo severo 2 (SARS-CoV-2). La difusión del virus fue rápida y, actualmente, existen casos de COVID-19 a nivel mundial en 213 países, áreas o territorios. Los investigadores internacionales trabajan y comparten sus contribuciones en cuanto a epidemiología, prevención, tratamiento, patrones clínicos y diagnósticos de COVID-19. La presente revisión es otra contribución al conocimiento actual, que presenta los datos sobre la pandemia de COVID-19 en formato organizado y sistemático. La información epidemiológica presentada en el documento está sujeta a cambios, a medida que se diagnostiquen nuevos datos y se actualice el estatus de los casos activos, de manera diaria.
Late in December 2019, in Wuhan, the capital city of Hubei Province, China, local health authorities reported unknown viral pneumonia cases. Soon after, the cases rapidly spread to the other parts of China. By January 7, 2020, with the use of real-time reverse transcription polymerase chain reaction (RT-PCR), scientists in China isolated a novel coronavirus from these patients with viral pneumonia. The virus was accordingly named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The diseases was later designated as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO).
On January 30, 2020, the WHO declared COVID-19 pandemic, a Public Health Emergency of International Concern (PHEIC). On 7 February 2020, 43103 COVID-19 cases were confirmed in twenty-five (25) countries. Similarly, during February 2020, 77780 cases were confirmed in China alone and 2459 cases in 33 other countries of the world. The total count was 80239 with 2700 fatalities worldwide. With gradual recognition of coronavirus; professional consensus, criteria and guidelines for diagnosis, treatment and preventing transmission has been established.1,2
Pneumonia linked with SARS-CoV-2, is the incessant disease worldwide. Coronavirus have high recombination and mutation rate due to unique replication mechanism, which facilitates them to acclimatize to new host and ecological niches.3 Until 2003, about these fatal viruses, a limited research data was available and only ten coronaviruses known. However, in 2003, severe acute respiratory syndrome (SARS), a viral induced respiratory infection came out and spread in more than twelve states of America, Asia and Europe, 800 individuals died in SARS outbreak.4
Coronavirus structureSARS-CoV-2, have a single stranded, enveloped positive sense RNA (ssRNA), belongs to coronaviruses (CoVs) family, known since 1960s. The virus can infect humans and animals, causing respiratory, hepatic, gastrointestinal and neurologic diseases.5 The name coronavirus is due to spikes like projections on its surface under electron microscope that gives crown like appearance. After emergence of novel coronavirus (CoVs), different novel CoVs were discovered. International Committee on Taxonomy of Viruses (ICTV) has classified these CoVs groups into various genera such as Alpha, Beta, Gamma and Delta coronaviruses.6 Several human coronaviruses (alpha-CoVs HCoVs-NL63, beta-CoVs HCoVs-OC43, HCoVs-229E, HCoVs-HKU1, Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV) and acute respiratory distress syndrome (ARDS) have been identified. Periodically new coronaviruses appear due to their large genetic diversity, rapid mutation rate, high prevalence and wide distribution.7
For emergence of CoVs, animals and birds serve as reservoir due to their ability to fly and habit of walking in groups. According to literature, birds have potential to transmit viruses to humans and other animals. It is assumed that birds may bring viruses including SARS-CoV-2 to china due to their diverse routes of migration.8,9
Source and transmission of SARS-CoV-2SARS-CoV-2 has less define pathophysiological characteristics, and there is uncertainty regarding the spread and transmission of virus.10 According to literature, there are three condition for wide spread and transmission of viruses including source of infection, route of transmission and susceptibility.
Source of infectionIt is considered that the outbreak is most probably started from a zoonotic transmission (most likely bats) in a Huanan seafood wholesale market mainly trading in live wild animals. According to Institute Pasteur of Shanghai, bats might be the natural host of SARS-CoV-2 while Peking University studies suggest that snakes could be the natural host. However, later research studies indicate that there is no evidence about snakes.11 According to Wuhan institute of virology studies, there is 96.2% gene sequence similarity between bat coronavirus and SARS-CoV-2, while other study based on pangolins report 99% similarity.12 It is assumed that bats and pangolins are possible source of SARS-CoV-2. Although it is yet to be fully elucidated about the potential source of SARS-CoV-2. However, at present, it is clear that person to person is the main source of transmission of SARS-CoV-2 infection.7,13
Route of transmissionThe spread of SARS-CoV-2 occurs from close human-to-human contact and droplets. The viral RNA can be detected on surfaces and materials including plastic and steel. According to literature, currently SARS-CoV-2 patients are the main source of virus transmission. From SARS-CoV-2 patients, respiratory droplets are the main route of transmission including also airborne transfer. While during initial viral incubation period, the host body play the main role in transmission of CoVs.14 However, it can be transmitted through virus-contaminated food and by touching surfaces where virus is present. It is reported that newborn baby born to mother diagnosed with SARS-CoV-2 was also infected by SARS-CoV-2, suggesting that SARS-CoV-2 can transmit vertically from mother to the newborn babies.7,15
SusceptibilityAccording to epidemiological investigation, elderly citizens, median age of 75 years, with comorbidities and history of surgery are the most susceptible group for SARS-CoV-2. The clinical features of SARS-CoV-2 patients show that viral incubation period range from 0 to 24 days while first symptom to death is 14 days. However, SARS-CoV-2 have long incubation period than other coronaviruses that may increase the risk of virus transmission.7,16
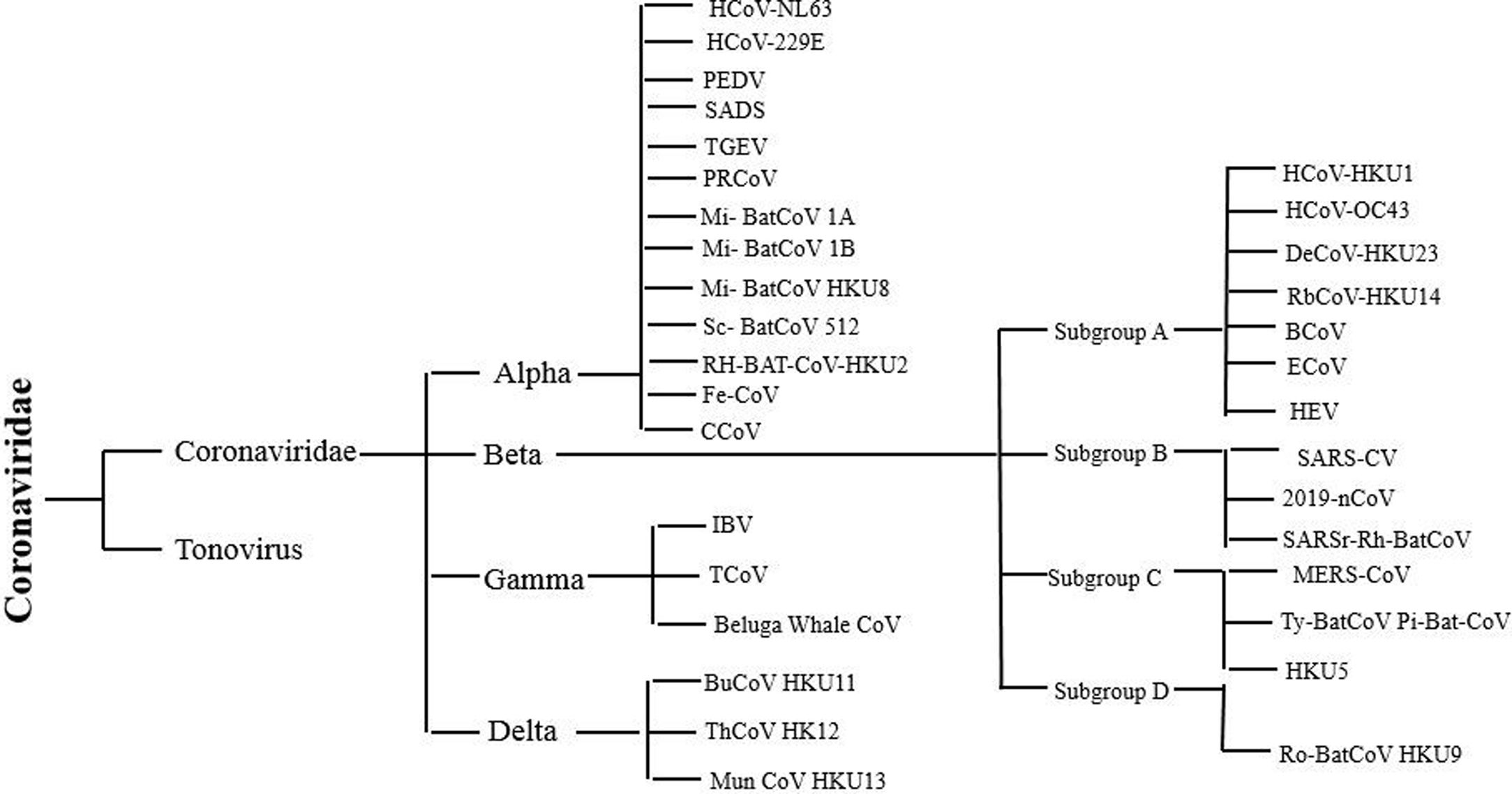
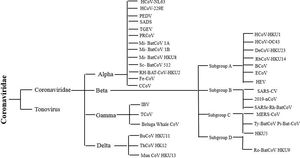
Classification of SARS-CoV-2SARS-CoV-2 belongs to Nidovirales order. This order is further divided into three families Coronaviridae, Arteriviridae, and Roniviridae. The classification chart of the taxonomy of the order Coronaviridae is shown in Fig. 1. Coronaviridae family is categorized into two subfamilies: Coronavirinae and Torovirinae. Coronavirinae is then further classified into alpha, beta, gamma and delta coronavirus.17 The current SARS-CoV-2 or COVID-19 belongs to Beta genera of coronavirus.18 Just like SARS coronavirus, this virus also uses ACE2 (angiotensin converting enzyme 2) receptors of host for entering host cell.19 Among all the four genera of coronaviruses, alpha and beta coronaviruses infect mammals, gamma coronaviruses infect avian species, and delta coronaviruses infect both mammalian and avian species.20
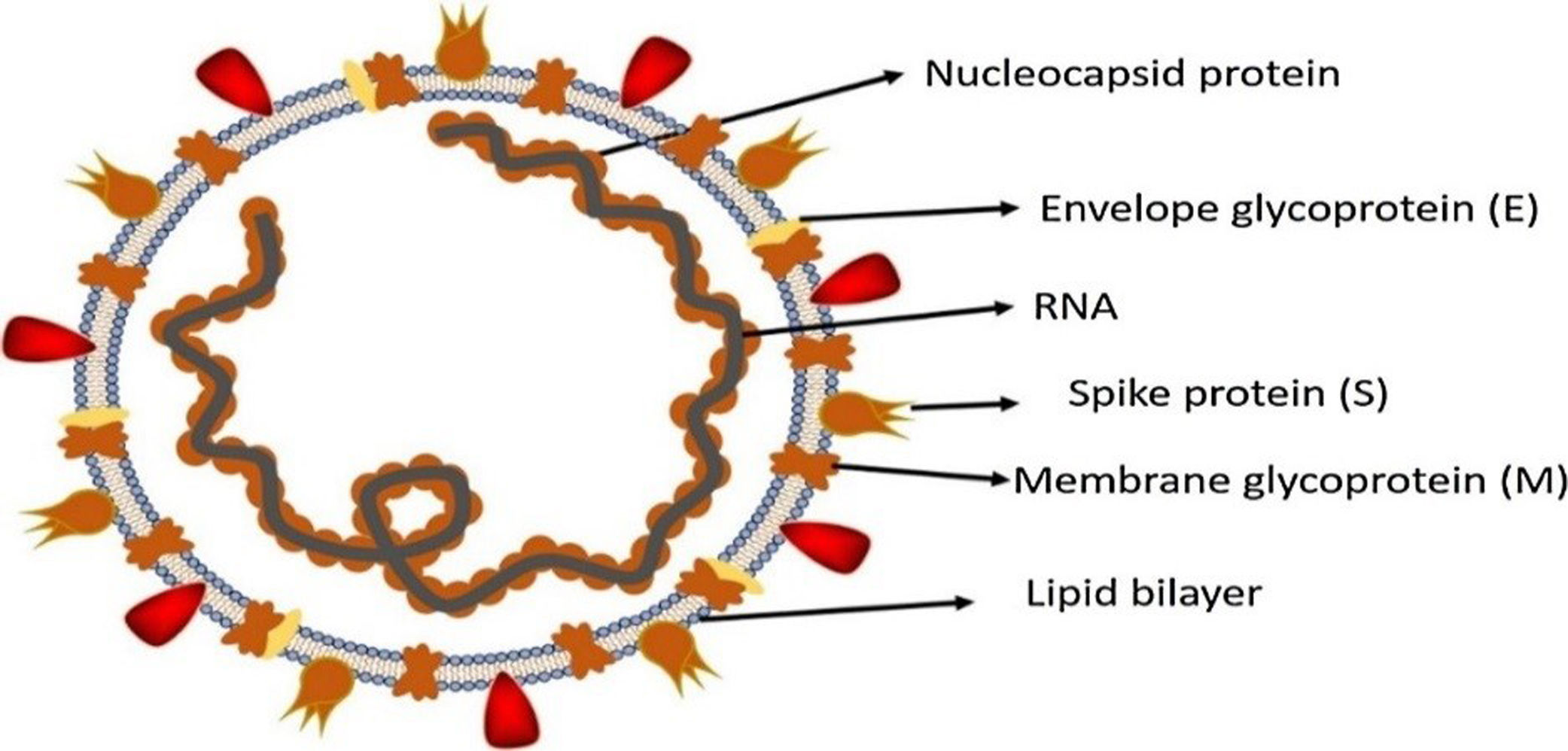
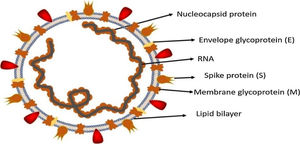
Morphology and genomic organization of SARS-CoV-2SARS-CoV-2 is enveloped positive sense single stranded RNA and has a crown shaped structure. It has spike like proteins called S proteins that is required for attachment with host cell receptor, membrane protein or M protein, nucleocapsid protein and E protein also called enveloped protein as shown in Fig. 2. Beside these SARS-CoV-2 also contain some additional structural proteins like Haemagglutinin esterase or HE protein.21 SARS-CoV-2 contain the largest genome size among all RNA viruses ranging from 26.4 to 31.7kb. All coronaviruses except for gamma coronaviruses contain six ORFs.18 The genomic organization of beta coronavirus consists of 5′-UTR and 3′-UTR. Between these two untranslated regions is ORF1a and ORF1b, spike protein, envelop protein, membrane protein, nucleocapsid and additional proteins.22 The two third part of coronavirus genome is possessed by two large ORFs, ORF1a, ORF1b which translate to form nsp (non-structural proteins) pp1a and pp1ab together these two proteins form an enzyme called RdRp/replicase.23
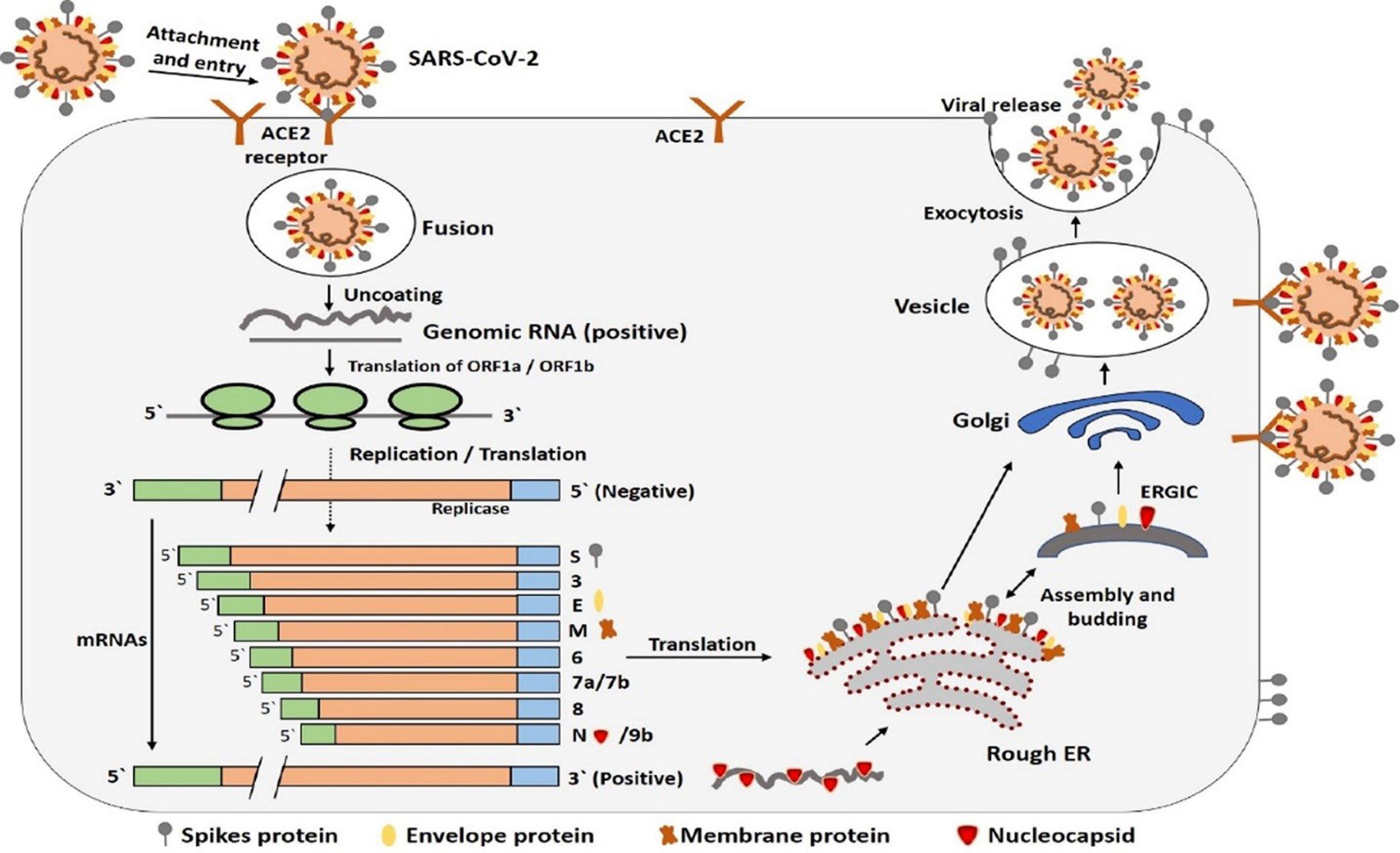
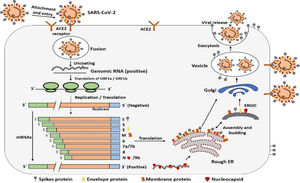
Infectious cycle of SARS-CoV-2SARS-CoV-2 bind with the host cell receptor ACE2 (angiotensin converting enzyme 2) through its spike protein or S protein. The S protein of SARS-CoV-2 consists of two subunits S1 and S2. S1 is the receptor binding subunit while S2 is the membrane fusing subunit. S1 facilitate binding of virus with the host cell receptor while S2 fuses membrane of both host cell and virus. After binding of membrane of both host and virus the virus genome then enters the host cell.20 However, before entering cell through its spike proteins the spike proteins must be primed by an enzyme called protease TMPRSS2. Entering of virus requires attachment with ACE2 and activation by TMPRSS2 enzyme.18 Once the genome of coronavirus enters the host cell then its genome is translated. Initially a small part of viral genome ORF1a, ORF1b translate to form nsp (non-structural proteins) pp1a and pp1ab. These two proteins together form an enzyme called RdRp (RNA dependent RNA polymerase). This enzyme then induces the ER (endoplasmic reticulum) to make double membrane vesicles (DMV). DMV together with RdRp makes RTC (replication and transcription complex). The RTC functions either continuous to make minus sense strand RNA to make a copy of genomic RNA or dis-continuous to make sg ms RNA (sub-genomic minus strand RNA) that serve as a template for making a sg mRNA23 and then these sub-genomic nested mRNA then translated into relevant viral proteins like S, E, M and N and finally all viral proteins along with genomic RNA are assembled together in the endoplasmic reticulum and Golgi apparatus to make new virus particles. These new virus particles are then released outside the cell in the form of vesicles.24 The entire mechanism of replication of SARS-CoV-2 in host cell is shown in Fig. 3.21
Epidemiology and clinical features of SARS-CoV-2It is difficult to control human to human transmission due to viral asymptomatic incubation period that is why cases exponentially increased, after 8 months and 27 days, of disease outbreak, the disease is pandemic, the number of infected individuals with confirmed cases of SARS-CoV-2 has reached up to 32730204 and deaths up to 991211 worldwide.25
The highest number of cases (6960152) are reported in United States of America (USA). The regional distribution of confirmed cases and deaths are presented in Table 1 with Americas contributing 49.6% of the total cases and 55.2% of total deaths. Males have comparatively more number of cases than females. Similar to earlier outbreaks of SARS and MERS, SARS-CoV-2 is observed less frequently in children than adults. Most cases are present in individuals above the age of 18 years and only 8.4% of the cases are reported below the age of 18 years. Similarly, large number of deaths (94.8%) are reported in individuals aged 50 years and above (Table 2).
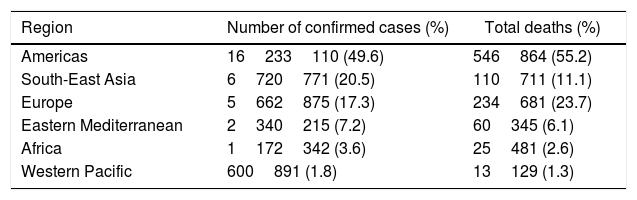
COVID-19 confirmed cases and deaths presentation numbers by region according to the WHO as of September 28, 2020.
| Region | Number of confirmed cases (%) | Total deaths (%) |
|---|---|---|
| Americas | 16233110 (49.6) | 546864 (55.2) |
| South-East Asia | 6720771 (20.5) | 110711 (11.1) |
| Europe | 5662875 (17.3) | 234681 (23.7) |
| Eastern Mediterranean | 2340215 (7.2) | 60345 (6.1) |
| Africa | 1172342 (3.6) | 25481 (2.6) |
| Western Pacific | 600891 (1.8) | 13129 (1.3) |
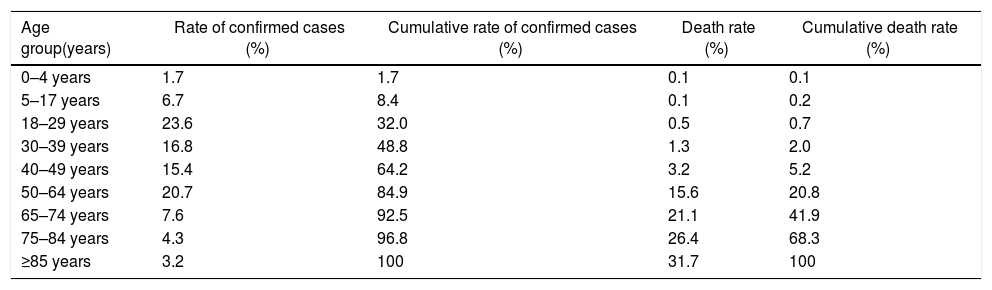
COVID-19 cases and deaths presentation rate by age groups according to the CDC as of September 27, 2020.
| Age group(years) | Rate of confirmed cases (%) | Cumulative rate of confirmed cases (%) | Death rate (%) | Cumulative death rate (%) |
|---|---|---|---|---|
| 0–4 years | 1.7 | 1.7 | 0.1 | 0.1 |
| 5–17 years | 6.7 | 8.4 | 0.1 | 0.2 |
| 18–29 years | 23.6 | 32.0 | 0.5 | 0.7 |
| 30–39 years | 16.8 | 48.8 | 1.3 | 2.0 |
| 40–49 years | 15.4 | 64.2 | 3.2 | 5.2 |
| 50–64 years | 20.7 | 84.9 | 15.6 | 20.8 |
| 65–74 years | 7.6 | 92.5 | 21.1 | 41.9 |
| 75–84 years | 4.3 | 96.8 | 26.4 | 68.3 |
| ≥85 years | 3.2 | 100 | 31.7 | 100 |
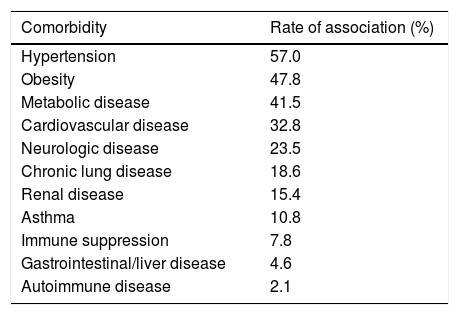
SARS-CoV-2 clinical features vary from asymptomatic to symptomatic. The mean incubation period is about 3–9 days with the range values between 0 and 24 days. Asymptomatic period lasts up to 14 days. During symptomatic period, the clinical features include fever, cough, fatigue, dyspnea, anorexia, productive sputum, myalgia and conjunctivitis. The most common symptoms associated with SARS-CoV-2 positive cases are fever and cough. After first week of SARS-CoV-2, disease turn into pneumonia, respiratory failure and death.26 This progression is associated with increase in release of inflammatory cytokines such as IL2, IL7, IL10, MIP1A, MCP1, IP10, GCSF and TNFα. The median time is 5 days from onset of symptoms to dyspnea, at 7th day hospitalization and at 8th day acute respiratory distress syndrome (ARDS). Complication include ARDS, acute lung injury, kidney injury and shock. The most common comorbidity associated with COVID-19 cases is hypertension followed by obesity (Table 3).
Rate of comorbidities associated with laboratory-confirmed COVID-19 hospitalized cases according to the CDC as of September 27, 2020.
| Comorbidity | Rate of association (%) |
|---|---|
| Hypertension | 57.0 |
| Obesity | 47.8 |
| Metabolic disease | 41.5 |
| Cardiovascular disease | 32.8 |
| Neurologic disease | 23.5 |
| Chronic lung disease | 18.6 |
| Renal disease | 15.4 |
| Asthma | 10.8 |
| Immune suppression | 7.8 |
| Gastrointestinal/liver disease | 4.6 |
| Autoimmune disease | 2.1 |
Adapted from 40.
According to published data, 25–30% affected patients need intensive care. Recovery of patients start in 2nd or 3rd week. The case-fatality rate (CFR) is different for different regions, highest for elderly citizen age group of ≥75 (Table 2). SARS-CoV-2 also infects children, however the CFR for infants and neonates is the lowest and is significantly milder than their adult counterparts.5,12
Clinical diagnosis of SARS-CoV-2Clinical diagnosis includes physical examination, nucleic acid detection, CT imaging and other techniques based on blood culture and immune identification such as enzyme-linked immunosorbent assay (ELISA) and Point-of-care Testing (POCT) of IgM/IgG.
Physical examinationSARS-CoV-2 infected patient's clinical signs and symptoms are relatively similar to that of seasonal influenza i.e. fever, cough, fatigue, dyspnea, anorexia, productive sputum, myalgia, conjunctivitis and other respiratory symptoms (moist rales in lungs, shortness of breath, weakened breath sounds).
Nucleic acid detection technologyFor lab diagnosing purpose various specimens such as nasal swabs, trachea or nasopharynx extract, sputum, lung tissue, blood and faeces should be retained from patients. Most preferable specimen are lower respiratory tract specimens. Diagnosis of SARS-CoV-2 are real time quantitative polymerase chain reaction (RT-qPCR) and high throughput sequencing method. The most accurate method is high throughput sequencing and virus blood culture, while the use of high throughput sequencing is limited due to high cost and equipment dependency. However, RT-qPCR is simple and effective method and most commonly used for viral nucleic acid detection from respiratory specimen and blood. In China after SARS-CoV-2 outbreak, Chinese companies launched RT-qPCR test kits, approved by Chinese Center for Disease Control and Prevention (China CDC).12,27 Diagnosis kits are also available for rapid COVID-19 diagnosis that target antibodies or antigens such as ELISA and POCTof IgM/IgG kits have been developed and have higher detection rate.12 However, leukocytes number decrease or keeps normal, with increase or normal monocytes or decrease lymphocytes can also indicates the diagnosis of SARS-CoV-2.7
CT imaging examinationCT Imaging is important diagnostic method, although RT-qPCR is specific method for diagnosis of SARS-CoV-2 but its false negative results should not be ignored. In that case clinicians proposed CT scans.12 The image results vary with patient's age, immunization status, drug interventions, underlying disease and disease at stage of scanning. At early stage of pneumonia cases, chest X-ray images show small patchy shadows however in severe cases, infiltrating shadow and pulmonary consolidation (with infrequent pleural effusion).2,28 Pulmonary lesions are more clearly shown in chest CT scan than X-ray examination as well as segmental consolidation and ground glass opacity in bilateral lungs while in children's (severe infection) in both lungs multiple lobar lesions may be present. According to research studies, COVID-19 image finds are similar to those reported with MARS and SARS.7,29,30
Treatment of SARS-CoV-2Currently there is no available treatment for SARS-CoV-2 but there are some supportive treatments such as the use of broad-spectrum antibiotics to protect from secondary bacterial infections, oxygen therapy and conservation of fluid management.31 Different potential drugs have also been proposed to assess their efficacy.32 Around 351 clinical trials are underway to check the effect of drugs for coronavirus in which 291 trials are specifically designed for SARS-CoV-2. Out of these 291 trials, 109 clinical trials are pharmacological based trials in adult patients. In which 82 are interventional with 29 placebo-controlled trials. The potential drugs which are underway for the clinical trials are chloroquine, hydroxychloroquine, Lopinavir/ritonavir,32 remdesivir, nucleoside analogues, umifenovir (arbitol), tenofovir disoproxil and lamivudine which are DNA synthesis inhibitors.32,33 Preliminary clinical studies have shown that chloroquine and hydroxychloroquine both causes alterations in post translational modification of viral proteins specially glycosylation in many viral proteins.33,34 Very less amount of data is available for the optimum dosage of chloroquine and hydroxychloroquine. However, 500mg of chloroquine is being used orally once or twice a day35 and for hydroxychloroquine the loading dosage is 400mg twice for 1 day then followed by 200mg twice daily.29
Remdesivir was another drug that was discovered during the screening of antimicrobials against RNA viruses. Remdesivir known as GS-5734, is a monophosphate inactive form and when it metabolizes then it converts to C-adenosine nucleoside triphosphate that is the active form of this drug. A recent study was conducted in Vitro which showed that remdesivir and as well as chloroquine both are effective towards SARS-CoV-2 due to their broad-spectrum.29,36
Umifenovir (arbitol) is another antiviral drug which is understudy for COVID-19 because this drug was found to be effective against SARS-CoV-2.37 This drug targets the S protein/ACE2 interaction and prevents the interaction of host and virus membranes.38 Additional therapies that can be used for SARS-CoV-2 is use of corticosteroid, immunomodulatory agents and immunoglobulins. The purpose of using corticosteroid is to decrease the inflammation of lungs due to lung infection, which may cause lung injury, but use of corticosteroid may increase the risk of secondary infection and delay the cleansing of virus. Another adjunctive therapy is use of monoclonal antibodies or immunomodulatory agents. These modulatory agents act as antagonists against certain cytokines/interleukins that increases the immune response of the body in the form of inflammation, which may damage organs. The key cytokine that enhance the inflammation of lungs is IL-6 and the monoclonal antibody against this cytokine is Tocilizumab. This monoclonal antibody is FDA approved to act against IL-6. There is another treatment therapy for SARS-CoV-2 though in very early phases, the use of immunoglobulins from the recovered patients that is the blood plasma of the recovered patients that already has antibodies against the virus.33
Prevention of SARS-CoV-2As there is no exact treatment for SARS-CoV-2, therefore the best way to prevent from wide spread of coronavirus is to avoid being exposed to the virus.7 WHO has recommended some control measures such as, maintaining social distancing, use of masks, regular hand washing and incase water is not available then hand sanitizer can also be used containing 60% alcohol as a disinfectant. People are also recommended to refrain from touching their eyes, nose and mouth without washing their hands.39 People should also avoid contact with any wild animals, if a person is suffering from any respiratory infection so, they should use disposable tissues and clothing and follow the cough etiquette. People should refrain from close contact with the person having respiratory infections.32
WHO has also recommended guidelines for the use of facial masks in home, in community and in the health care centers. The health care workers are recommended to use N95 masks when dealing with suspected or confirmed SARS-CoV-2 patients. All those people who are suffering from this disease should use medical masks both in home and health care centers and properly dispose the masks to avoid any transmission of virus.41
Conclusion and future prospectsDue to pandemic outbreak and lack of research studies of SARS-CoV-2, many facts regarding SARS-CoV-2 particularly actual source, virulence, mode of transmission, treatment and vaccine production are under investigation. Current available data related to SARS-CoV-2 is limited which still need to be explored. Particularly, the source of viral infection is still unknown that is why authorities face hurdles in control of virus. However, the first case reported was confirmed from China (Seafood wholesale market). Moreover, transmission through human to human has been confirmed and healthcare workers were also infected and face risk of viral infection. The researchers are working on priority bases on SARS-CoV-2 medication but still un-succeed in production of specific medicine for treatment of SARS-CoV-2 disease while vaccine is under trialed.9 Moreover, research studies are required to understand the virulence of SARS-CoV-2 that will help in production of SARS-CoV-2 antiviral medicines.9,26
After declaration of SARS-CoV-2 pandemic by WHO, all countries and their responsible authorities performing their best to control the transmission of disease directing their citizens to strictly follow the preventive measures by social distancing, prohibited public gathering and lock down the cities and countries. This is the current order of the day until the researchers succeed in finding the specific drug and more particularly the vaccine.
FundingThe authors declare that the current study was not funded.
Conflict of interestThe authors declare that they have no conflict of interest.