Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosVaccine safety is a major barrier to the uptake of the COVID-19 vaccine by pregnant women. To bring confidence among pregnant women towards vaccine intake, there is a need to synthesize evidence on safety profile of vaccination.
ObjectiveTo assess adverse events (AEs) following COVID-19 vaccination among pregnant women.
Materials and methodsA vaccine safety surveillance was conducted at 2 rural primary health centers (PHC) located in Anantapur District, India. A total of 420 pregnant women were monitored for AEs following COVID-19 vaccination for a period of 30 min and followed for 1 month for late reactions through telephonic interviews. All AEs were subjected to causality and severity assessment. Descriptive statistics were used to represent adverse events.
ResultsThe COVID-19 vaccine acceptance rate among pregnant women was 64.4%. A total of 420 pregnant women received 670 vaccine doses (Covishield = 372, Covaxin = 298) against COVID-19. Majority of vaccine intake was observed during the second trimester. The incidence rate of AEs following the COVID-19 vaccine among pregnant women was 93.8%, and the majority include injection site pain (28.4%, 29.6%), fever (25.5%, 19.0%), myalgia (8.21%, 12.3%), and malaise (13.6%, 8.4%). Most AEs notified are probable and mild in nature.
ConclusionThe COVID-19 vaccine acceptance rate among pregnant women was 64.4%. A 30 days incidence rate of AEs following COVID-19 vaccination among pregnant women was 93.8%, with the most common mild events like injection site pain, and fever. A further follow-up cohort study by taking an adequate sample size was recommended to capture fetal–maternal outcomes.
La seguridad de la vacuna es una barrera importante para la adopción de la vacuna COVID-19 por parte de las mujeres embarazadas. Para llevar confianza entre las mujeres embarazadas hacia la ingesta de vacunas, es necesario sintetizar la evidencia sobre el perfil de seguridad de la vacunación.
ObjetivoEvaluar los eventos adversos (EA) después de la vacunación contra la COVID-19 en mujeres embarazadas.
Materiales y métodosSe llevó a cabo una vigilancia de la seguridad de las vacunas en dos centros rurales de atención primaria de salud (PHC) ubicados en el distrito de Anantapur, India. Un total de 420 mujeres embarazadas fueron monitoreadas para detectar EA después de la vacunación COVID-19 durante un período de 30 minutos y seguidas durante un mes para detectar reacciones tardías a través de entrevistas telefónicas. Todos los EA se sometieron a una evaluación de causalidad y gravedad. Se utilizaron estadísticas descriptivas para representar los eventos adversos.
ResultadosLa tasa de aceptación de la vacuna COVID-19 entre las mujeres embarazadas fue del 64,4%. Un total de 420 mujeres embarazadas recibieron 670 dosis de vacunas (Covishield = 372, Covaxin = 298) contra COVID-19. La mayoría de la ingesta de vacunas se observó durante el segundo trimestre. La tasa de incidencia de EA después de la vacuna COVID-19 entre las mujeres embarazadas fue del 93,8%, y la mayoría incluye dolor en el lugar de la inyección (28,4%, 29,6%), fiebre (25,5%, 19,0%), mialgia (8,21%, 12,3%) y malestar general (13,6%, 8,4%). La mayoría de los EA notificados son de naturaleza probable y leve.
ConclusiónLa tasa de aceptación de la vacuna COVID-19 entre las mujeres embarazadas fue del 64,4%. Una tasa de incidencia de EA a 30 días después de la vacunación contra COVID-19 entre las mujeres embarazadas fue del 93,8%, con los eventos leves más comunes como dolor en el lugar de la inyección y fiebre. Se recomendó un estudio de cohorte de seguimiento adicional mediante la toma de un tamaño de muestra adecuado para capturar los resultados maternos fetales.
The Coronavirus disease 2019 (COVID-19) has shown serious and lethal effects among the elderly and co-morbid people.1 Evidence shows that pregnant women are at increased risk to develop severe symptoms, hospitalizations, intensive care unit admissions, invasive ventilation, extracorporeal membrane oxygenation, and mortality due to COVID-19.2 Safe and effective COVID-19 vaccines are a game-changing tool in the battle against COVID-19 infection. Globally, multiple vaccines were launched and shown a wide range of safety and efficacy profiles. A scientific report on mRNA vaccines from the USA revealed that COVID-19 vaccination is safe to administer among pregnant women.3 Post-authorization safety studies like active surveillance or registry-based monitoring are vital to re-establishing the safety profile of the COVID-19 vaccine in pregnant women.4
There was a scarcity of evidence about the safety profile of the COVID-19 vaccine for pregnant women from a real-world evidence perspective, specifically from low- and middle-income countries (LMICs), such as India.5 Majority of the published evidence on the safety of the COVID-19 vaccine reflects towards high-income countries.6 The WHO Strategic Advisory Group of Experts on Immunization (SAGE) and National Technical Advisory Group on Immunization (NTAGI) recommend that pregnant women can receive the COVID-19 vaccine.7 Considering the safety profile of the COVID-19 vaccine in pregnant women generated by other countries, India started vaccinating pregnant women based on emergency use license from the first week of July 2021.8
In India, at present 2 vaccines have received approval. One of them is an inactivated viral vaccine (Covaxin) and the other one is based on a non-replicating viral vector vaccine (Covishield). The vaccination can be recommended in all trimesters of pregnancy.9 The guidelines of India emphasize the importance of collection of the safety profile of the COVID-19 vaccine among pregnant women. Close screening and monitoring of adverse events following COVID-19 vaccine in pregnant women can generate the evidence on potential risks attributed with vaccine and promote the confidence in intake of the vaccine. Surveillance of adverse events following vaccination also helps in identification of time for vaccination in pregnancy and interval between 2 doses.
Evidence shows that vaccine safety is a major barrier in acceptance of COVID-19 vaccine among pregnant women, same has reported by the majority of pregnant women in various studies.10–12 Therefore, a prospective observation study was conducted which aimed to actively monitor the adverse events following COVID-19 vaccination among pregnant women.
Materials and methodsStudy design and settingsThis is a prospective active vaccine safety surveillance study conducted at 2 primary health centers (PHC) located in Bathalapalli, and Sarada Nagar, Anantapur District, Andhra Pradesh, India.
Study participants and samplingAll pregnant women irrespective of their trimester came for COVID-19 vaccination were recruited from September 2021 to February 2022 with a follow-up period of 1 month per individual. Non-pregnant and lactating women were excluded from the study. A non-probable convenient sampling technique was used to select the subjects into the study.
Ethical considerationsThe study was performed after getting protocol clearance (RIPER/IRB/PP/2021/001) from the Institutional Review Board (IRB) of RIPER. The confidentiality of participants' identifiers was maintained throughout the study period. The pregnant women who are willing to participate were recruited after getting oral and written informed consent. Before consent, all pregnant women have explained regarding objectives, procedure, and outcomes of the study in a local language.
Data collection tools and techniquesData collection formThe data collection form comprises information regarding: (1) Demographics, (2) vaccination information, (3) adverse events, and (4) causality and severity.
DemographicsDemographic characteristics like age, location, contact details, and allergic, obstetric, and medical history were included in this section.
Vaccination informationName of the vaccine, manufacturer details, type of vaccine, date of administration, route of administration, batch number, and the number of doses (first or second) details were added to the vaccination information.
Adverse eventsThe adverse events reported in the previous COVID-19 vaccine safety studies like injection site pain, fatigue, headache, myalgia, chills, fever, nausea, joint pains, injection site swelling, injection site reactions, vomiting, abdominal pain, diarrhea, thrombocytopenia, and anaphylaxis were added in the form to monitor suspected events followed by vaccination. This section also consists to record new events upon COVID-19 vaccination.
Causality and severityThe cause-and-effect relationship between the COVID-19 vaccine and the event was evaluated by using a WHO-AEFI Causality Assessment Brighton Criteria for COVID-19 vaccines. According to the WHO-AEFI scale, causality can be graded as certain, probable, possible, unlikely, unrelated, and unclassifiable.13
Naranjo scale was also added to this section to assess the causality of developing adverse events following COVID-19 vaccination. With the score obtained from the Naranjo scale, the causality can be graded as doubtful (≤0), possible (1–4), probable (5–8), and definite (≥9).14
The severity of the adverse event following COVID-19 vaccination was assessed by using a modified Hartwig and Siegel scale. This scale categorizes the severity into 3 levels mild (Level 1–2), moderate (Level 3–4), and severe (Level 5–7).15
Data collection techniqueAfter getting an oral and written informed consent, a pre-designed data collection form was used to collect data from the eligible participants. All subjects were monitored for 30 min in the waiting area of the PHC for the development of adverse event following COVID-19 vaccination. Each pregnant women under the study were followed for 1 month for the development of late reactions following vaccination. The follow-up was assisted with the telephonic interview. The telephonic follow-up was conducted every week till the end of the month after vaccination.
Participants who developed adverse events following COVID-19 vaccination were subjected to drug therapy to resolve the event. Outcomes of interventions recommended for AEFI among pregnant women were collected. All pregnant women were instructed to report to the healthcare professionals if any untoward reaction was encountered in home settings. Pregnant women are advised to take drugs only after consultation with a physician. If any untoward event (immediate or late) was observed after COVID 19 vaccination, it was recorded and subjected for causality and severity assessment by using Naranjo ADR probability scale and Hartwig's and Siegel scale.
Data analysisThe collected data was entered into the spreadsheet (Microsoft Office – 2013) and performed data analysis by using Epi-Info 7 (Center for Disease Control (CDC), USA). Descriptive statistics like frequency and percentage were used to represent demographics, adverse events observed, causality and severity of adverse events following COVID-19 vaccine.
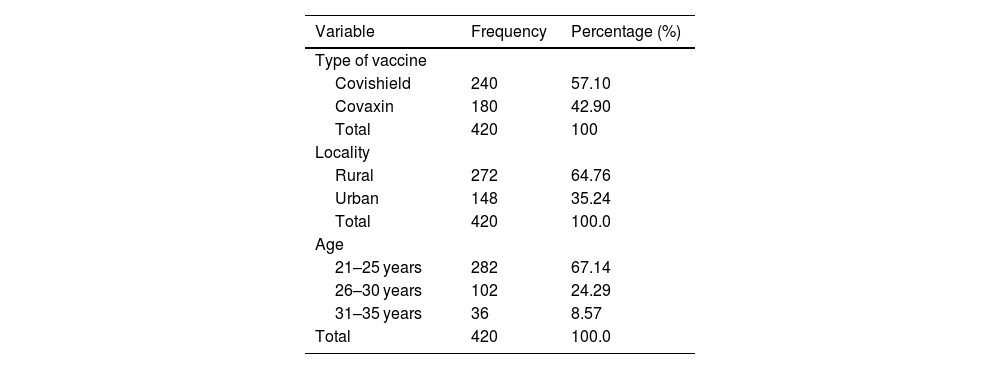
ResultsA total 652 pregnant women visited for antenatal care during study period at 2 primary health centers. Among 652 pregnant women, 420 accepted to take COVID-19 vaccine. The rate of COVID-19 vaccine acceptance rate among pregnant women was found to be 64.4%. Majority of women were recommended Covishield vaccine (240; 57.10%), aged between 21 and 25 years (282; 67.14%), and from rural residence (272; 64.76%). The distribution of participants’ demographics and type of vaccine recommended were shown in Table 1.
Demographics and COVID-19 vaccination profile of pregnant women.
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Type of vaccine | ||
| Covishield | 240 | 57.10 |
| Covaxin | 180 | 42.90 |
| Total | 420 | 100 |
| Locality | ||
| Rural | 272 | 64.76 |
| Urban | 148 | 35.24 |
| Total | 420 | 100.0 |
| Age | ||
| 21–25 years | 282 | 67.14 |
| 26–30 years | 102 | 24.29 |
| 31–35 years | 36 | 8.57 |
| Total | 420 | 100.0 |
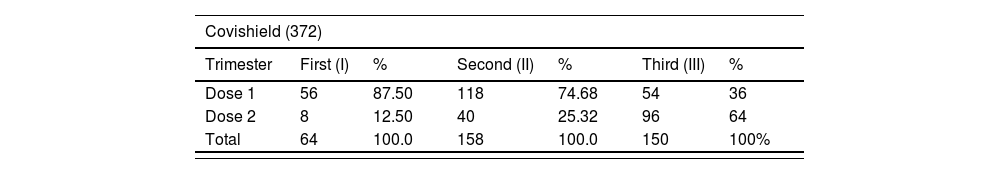
A total of 420 pregnant women received 670 doses (Covishield = 372, Covaxin = 298) of vaccine against COVID-19 infection. Majority of COVID-19 vaccine intake was observed during second trimester than first and third trimester. The distribution of the doses of Covishield and Covaxin according to the trimester were shown in Table 2.
Trimester wise distribution of COVID-19 vaccine administration.
| Covishield (372) | ||||||
|---|---|---|---|---|---|---|
| Trimester | First (I) | % | Second (II) | % | Third (III) | % |
| Dose 1 | 56 | 87.50 | 118 | 74.68 | 54 | 36 |
| Dose 2 | 8 | 12.50 | 40 | 25.32 | 96 | 64 |
| Total | 64 | 100.0 | 158 | 100.0 | 150 | 100% |
| Covaxin (298) | ||||||
|---|---|---|---|---|---|---|
| Trimester | First (I) | % | Second (II) | % | Third (III) | % |
| Dose 1 | 38 | 73.08 | 82 | 67.21 | 56 | 45.17 |
| Dose 2 | 14 | 26.92 | 40 | 32.79 | 68 | 54.83 |
| Total | 52 | 100 | 122 | 100% | 124 | 100 |
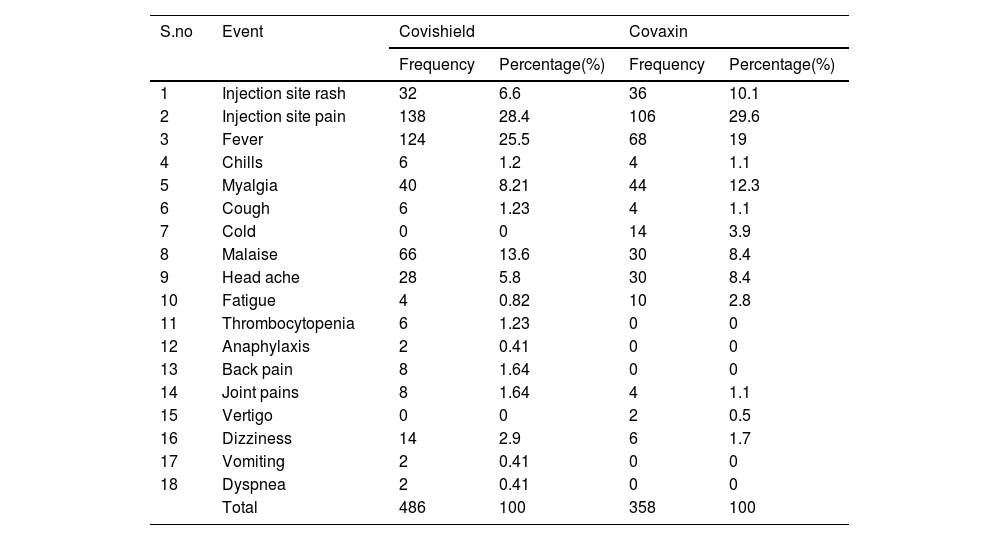
A total of 844 adverse events were notified in 394 women upon 670 COVID-19 vaccine doses given to 420 pregnant women. The incidence rate of adverse event following COVID-19 vaccine among pregnant women was 93.8%. The most common adverse events following COVID-19 vaccine (Covishield and Covaxin) include injection site pain (28.4%, 29.6%), fever (25.5%, 19.0%), myalgia (8.21%, 12.3%), and malaise (13.6%, 8.4%). The distribution of the adverse events following Covishield and Covaxin vaccine administration were represented in Table 3.
Adverse events following COVID-19 vaccination among Pregnant Women.
| S.no | Event | Covishield | Covaxin | ||
|---|---|---|---|---|---|
| Frequency | Percentage(%) | Frequency | Percentage(%) | ||
| 1 | Injection site rash | 32 | 6.6 | 36 | 10.1 |
| 2 | Injection site pain | 138 | 28.4 | 106 | 29.6 |
| 3 | Fever | 124 | 25.5 | 68 | 19 |
| 4 | Chills | 6 | 1.2 | 4 | 1.1 |
| 5 | Myalgia | 40 | 8.21 | 44 | 12.3 |
| 6 | Cough | 6 | 1.23 | 4 | 1.1 |
| 7 | Cold | 0 | 0 | 14 | 3.9 |
| 8 | Malaise | 66 | 13.6 | 30 | 8.4 |
| 9 | Head ache | 28 | 5.8 | 30 | 8.4 |
| 10 | Fatigue | 4 | 0.82 | 10 | 2.8 |
| 11 | Thrombocytopenia | 6 | 1.23 | 0 | 0 |
| 12 | Anaphylaxis | 2 | 0.41 | 0 | 0 |
| 13 | Back pain | 8 | 1.64 | 0 | 0 |
| 14 | Joint pains | 8 | 1.64 | 4 | 1.1 |
| 15 | Vertigo | 0 | 0 | 2 | 0.5 |
| 16 | Dizziness | 14 | 2.9 | 6 | 1.7 |
| 17 | Vomiting | 2 | 0.41 | 0 | 0 |
| 18 | Dyspnea | 2 | 0.41 | 0 | 0 |
| Total | 486 | 100 | 358 | 100 | |
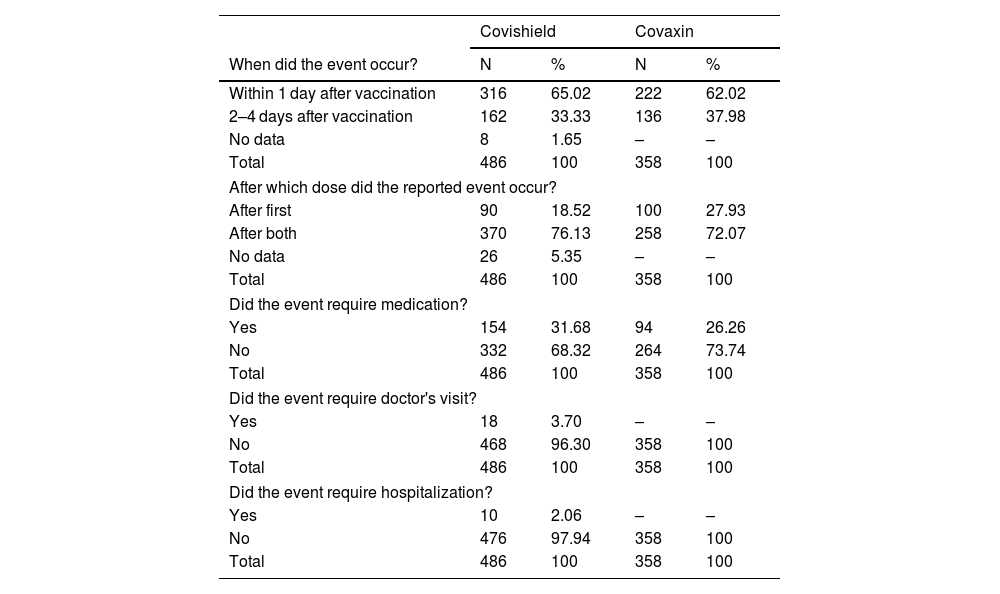
Majority of the adverse events were observed within 1 day (65.02%, 62.02%), and after 2 doses (76.13%, 72.07%) of COVID-19 vaccine (Covishield and Covaxin) administration. For majority of adverse events, no medication (68.32%, 73.74%), no physician consultation (96.30%, 100.0%), and no hospitalization (97.94%, 100.0%) are required after COVID-19 vaccination (Covishield and Covaxin) among pregnant women. The distribution of the adverse events characteristics after COVID-19 vaccine were represented in Table 4.
Characteristics of adverse events upon COVID-19 vaccination among pregnant women.
| Covishield | Covaxin | |||
|---|---|---|---|---|
| When did the event occur? | N | % | N | % |
| Within 1 day after vaccination | 316 | 65.02 | 222 | 62.02 |
| 2–4 days after vaccination | 162 | 33.33 | 136 | 37.98 |
| No data | 8 | 1.65 | – | – |
| Total | 486 | 100 | 358 | 100 |
| After which dose did the reported event occur? | ||||
| After first | 90 | 18.52 | 100 | 27.93 |
| After both | 370 | 76.13 | 258 | 72.07 |
| No data | 26 | 5.35 | – | – |
| Total | 486 | 100 | 358 | 100 |
| Did the event require medication? | ||||
| Yes | 154 | 31.68 | 94 | 26.26 |
| No | 332 | 68.32 | 264 | 73.74 |
| Total | 486 | 100 | 358 | 100 |
| Did the event require doctor's visit? | ||||
| Yes | 18 | 3.70 | – | – |
| No | 468 | 96.30 | 358 | 100 |
| Total | 486 | 100 | 358 | 100 |
| Did the event require hospitalization? | ||||
| Yes | 10 | 2.06 | – | – |
| No | 476 | 97.94 | 358 | 100 |
| Total | 486 | 100 | 358 | 100 |
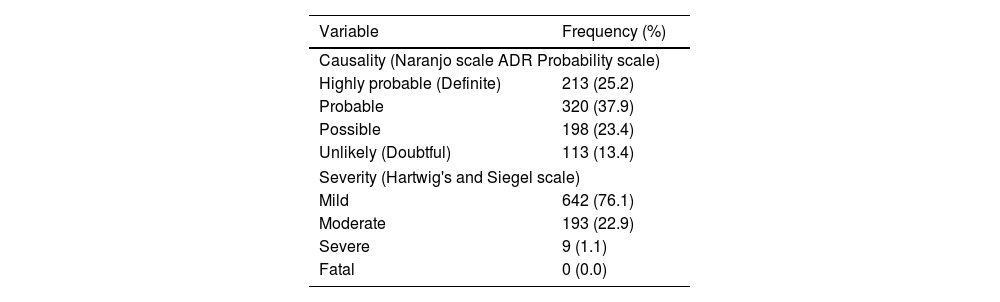
The causality and severity assessment findings of adverse events following COVID-19 vaccination towards pregnant women revealed that majority of events were probable and mild as per Naranjo causality scale and Thornton and Hartwig severity scale. Distribution of the causality and severity levels of adverse events following COVID-19 vaccination were represented in Table 5.
Distribution of causality and severity levels of adverse events following COVID-19 vaccination among pregnant women (n = 844).
| Variable | Frequency (%) |
|---|---|
| Causality (Naranjo scale ADR Probability scale) | |
| Highly probable (Definite) | 213 (25.2) |
| Probable | 320 (37.9) |
| Possible | 198 (23.4) |
| Unlikely (Doubtful) | 113 (13.4) |
| Severity (Hartwig's and Siegel scale) | |
| Mild | 642 (76.1) |
| Moderate | 193 (22.9) |
| Severe | 9 (1.1) |
| Fatal | 0 (0.0) |
ADR: Adverse Drug Reaction.
Pregnant women are excluded from clinical trials. So, the current study is attempting to generate evidence on safety profile of COVID-19 vaccine in pregnant women. This will promote the confidence among pregnant women to accept vaccine intake and promotes the vaccine coverage.
The COVID-19 vaccine acceptance rate among pregnant attending rural primary health centers located in South India was 64.4%. Studies conducted by Lipkind et al. and Theiler et al. in USA has shown less acceptance of 21.8% and 7.0%, respectively.16,17 The wide variation in the acceptance rate between studies was majorly due to change in the time period of vaccine recommendation. The studies conducted in USA had enrolled the cohort between December 2020 and July 2021. Whereas, the current study was conducted in between September 2021 and February 2022. The primary reason for low acceptance in elsewhere studies is due to concern regarding safety of COVID-19 vaccine in pregnant women. Other factors for low acceptance may include lack of information, and attitude of pregnant women regarding COVID-19 vaccine.11 Findings of the studies conducted in Czech Republic, and Ethiopia reported an acceptance rate of 66.7%, and 62.04%, respectively, for COVID-19 vaccine acceptance among pregnant women which were nearly similar to our study.18,19 A 6-country survey in the European region also reported similar rate of vaccine acceptance rate (61.0%) as that of our study.20 The high rate of COVID-19 vaccine acceptance among pregnant women in the current study may also due to wide acceptance among adults and pediatrics population that was reported in the India.21,22
Findings of our study revealed that majority of the pregnant women undergone COVID-19 vaccination during second trimester. Similar findings are also observed in the study conducted at Brigham and Women's Hospital, Boston, MA.23 In contrast to our study, Lipkind et al. reported that majority of the women received vaccine during third trimester.16
A 30 days incidence rate of adverse event following COVID-19 vaccination was 93.8%. The most common adverse events following COVID-19 vaccine (Covishield and Covaxin) include injection site pain, fever, myalgia, and malaise. Injection site pain/soreness was the most common adverse event reported following COVID-19 vaccine in the USA after first dose.24 Only 1.1% of the pregnant women experienced severe adverse events following COVID-19 vaccination like severe vomiting and giddiness. Similar severe adverse events are also reported among 234 pregnant women vaccinated with Covaxin in Mumbai, India.8
In our study, majority of the adverse events following COIVD-19 vaccination were reported within 1 day. Similar findings are also observed in the studies conducted by Goldshtein et al. and Gandhi et al.8,25 It indicates that majority of the adverse events are either fever or injection site pain that are nearly similar with the routinely used pediatric or adult vaccines. The current study actively captured all events within 30 min after COVDI-19 vaccination among pregnant women, thereafter pregnant women passively report the adverse events experienced in the 30 days after vaccination. So, the study provides both acute and delayed events of COVID-19 vaccination among pregnant women.
Strengths and limitationsThe primary strength of the current study is prospective active surveillance design, so it will limit the recall bias. Also, the design helps to estimate true incidence rate of adverse events, which is not possible in passive surveillance (spontaneous reporting) of adverse event monitoring system. This is the first study that explores adverse events following COVID-19 vaccination among pregnant women in rural primary healthcare settings of South India.
Due to time limitation, the study was not focused on the impact of COVID-19 vaccination on perinatal outcomes in pregnancy. Also, the study findings were captured from single region of the rural India.
ConclusionThe study concludes that the COVID-19 vaccine acceptance rate among pregnant women was 64.4% in rural South India. A 30 days incidence rate of adverse event following COVID-19 vaccination among pregnant women was 93.8%, with a most common events like injection site pain, and fever. The study findings help in bringing confidence among pregnant women to vaccinate against COVID-19 infection and prevent the severe or critically ill COVID-19. The study also suggests that there should be a nation-wide adverse event monitoring system for COVD-19 vaccination among pregnant women.
FundingNil.
Authors contributionAll authors contributed in drafting and revising the manuscript. NG, BK, NN, BB, HRG, KVV, JOCE and VB were involved in the design of the study, data collection, and data analysis. NG, BK, NN, BB, and HRG were involved in theoretical formalism, data collection, data analysis, interpretation, and revision of the manuscript. All authors have read and approved the final manuscript.
We acknowledge Dr. Praveen Kumar, and Dr. Jyothi Suchitra, Rural Development Trust (RDT) Hospital for providing platform to enroll the subjects from study sites.