Hypospadias is the result of an abnormal development of the urethra causing an ectopic and more proximal placement of the meatus in the ventral side of the penis. Recent molecular studies, and a better understanding of the normal development of the genital tubercle, have demonstrated a complex interaction between genes, hormones and the environment with the development of hypospadias. It is possible that hypospadias is the phenotypic expression of different diseases. Recently a new candidate gene for hypospadias has been discovered, called MAMLD1, which is involved in the normal production of testosterone during fetal development.
Las hipospadias resultan de un arresto en el desarrollo normal de la uretra, creando un meato ectópico proximal en la cara ventral del pene. Estudios moleculares y un mejor conocimiento de la embriogénesis del tubérculo genital demuestran una compleja interacción de genes, hormonas y el ambiente con el desarrollo de hipospadias. Es posible que esta entidad sea la expresión fenotípica común de varias enfermedades diferentes. Recientemente se describió un nuevo gen candidato en el desarrollo de hipospadias llamado MAMLD1, involucrado en la producción normal de testosterona durante la embriogénesis.
Hypospadias is a condition characterized by the presence of an ectopic urethral orifice in along the ventral side of the penis. Most of the cases are sporadic and isolated. It has been described an increasing trend in prevalence in different regions around the world. The exact cause is unknown but an environmental effect thereon is proposed. In Colombia, our group has described a prevalence for isolated hypospadias of 1.4–1.7 per 1000 live births.1,2 Recently, great efforts have been devoted to the study of molecular pathways and the embryology of genital tubercle. Different genes involved in its development have been described and to date there are around 26 genes described. Around 64% of proximal hypospadias cases have a positive molecular diagnosis. Clearly, the role played by testosterone is very important. Mutations in genes such as the androgen receptor (AR) and the 5-a-reducatase have been described in a minority of hypospadias cases. Thus there may be a multiple different genes involved in the development of hypospadias. Recently a new candidate gene, the MAMLD1 has been identified. The purpose of our review is to describe this new gene and its effects on the pathophysiology of hypospadias and the normal developement of the genital tubercule.
EmbriologyThe development of the external genitalia is a complex process in which a cascade of molecular events interactions occurs. These include gene expression, enzyme activity, cell differentiation and hormone signaling pathways.3,4 Genital development is divided in two phases. One that is hormonal independent and a later phase that is hormonal dependent.5 In the initial independent phase which takes place between the fifth and eighth week of gestation, a growth of the genital tubercle (GT) occurs and is dependent on the expression of Shh, HOX, FGF, BMP Wnt/beta catenin genes. The hormone-dependent phase between weeks 8 to 20, the androgens are very important. Important genes in this phase are the AR the 5aR, 17aHSDH, FKBP52, MAMLD1, ATF3, Dhh, HOX. Early studies and descriptions of the urethral embryology described by Glenister in 1954 where the first to mention the concept of urethral plate.6,7 It is important to note that most processes occur simultaneously and interaction between each process is very common. For example during week 5 of gestation, cloacal membrane formation and the initial steps in the gonadal development start.8 The first critical step is to determine the sex of the fetus, which occurs at the moment of fertilization. When sex is genetically determined, the next step is the expression of SRY gene which will stimulate differentiation of primitive cells into Sertoli cells located in the primitive sex cords. If there is no normal expression of the SRY then these cells will continue their process and become follicular cells of the ovary. When Sertoli cells begin their activity and gene expression occurs, Müllerian inhibiting substance production becomes crucial. This occurs around week 7–10.
Between weeks 9 and 10 Leydig cells evolve. Their origin and molecular differentiention process is unclear. Once mature, Leydig cell begins testosterone production around weeks 8–12. This is key to continue the process of masculinization. Usually androgen stimulus in the development of the external genitalia occurs in late stages of embryogenesis.9
Initially, the caudal portion of the fetus, the cloacal membrane composed only by ecto- and endoderm running from the navel to what in the future will be the perineum is formed. In these first steps, mesoderm migration toward the cloacal membrane occurs. These mesodermal cells will give rise to the muscles of the perineum, the symphysis pubis and the anterior wall of the bladder. By the end of fourth week migration is complete.
Cloacal folds are formed during the fifth week of gestation; these are the same in both sexes and are developed on the sides of the cloacal membrane. At the end of 5th week, they merge into at the anterior and medial region to form the genital tubercle (GT) located ahead of the urogenital sinus. This process is completed by the end of week 6. This GT consists of a lateral plate composed by mesoderm and ectoderm. The urethral plate originates from endoderm. As in limb formation, guidance and proximal to distal growth is very important in the future development of the penis. The distal portion is known as distal urethral epithelium (EUD). At this level the expression of genes such as Shh, FGF and BMP is very important.
Hormone independent phaseIn the initial independent hormonal phase, the growht orientation is given mainly by the expression of Fgf8 gene that is expressed at the EUD. This is a regulatory center for longitudinal growth of the future penis.10 However, it is not completely clear the specific mechanism of action of Fgf8 since the inactivation of it does not always inhibits the GT development. Other genes involved in this phase are the Fgfr2 Fgf10.11 Among the many functions described, the Fgf8 stimulates the expression of other genes such as Bmp4 Bmp7 Wnt7 and Msx1.12,13 The expression of these genes is very important in mesenchyme-epithelial interactions. In this initial phase of development, independent of the androgen stimulus, Sonic hedgehog (Shh) expression at the GT has been described as one of the early genes involved in the development and growth of the GT. It has beenIt described that Shh stimulates the expression of other genes such as: Fgf8, Bmp7, Ptch1, Fgf10, Bmp2, Bmp4, HOXA13, Wnt5a and Msx1. Studies of Shh knockout mice, present penile agenesis. The same has been described for the lack of Wnt5a expression.14,15 Similar results have been seen in knockout mice for the Gli2 gene, however, some of the mice did develop a phallus but with a lack of normal urethral formation.16
By suppressing the expression of Shh a reduction in genital development occurs and cells arrest in G1 phase.17 The expression of Shh has been suggested in two main phases. An initial development of GT and cloacal end and a second one later during urethral closure phase.17,18 In later stages when a distal development of GT occurs, the expression of Hoxha-13 and Bmp7 become important for the formation of future corpora cavernosa.19 The Bmp7 described in multiple organs such as limbs, eyes, kidneys, also participates in GT development and apparently its expression enables the development and proper interaction with epithelial mesenchyme.20 This interaction is partly regulated by the expression of the FGF family and BMP genes. For the first time during the formation of GT, the expression of HOX genes (HOXA13 and Hoxd13) occurs and regulates the expression of other genes such as the Bmp7 and Fgf8. Another regulatory gene is Fgf8 and Wnt5a, whose additional function during mesenquimo-epithelial interaction is homeostasis and proper urethral formation.21 Although uncommon, some cases have been described were mutations are present and patients develop severe proximal hypospadias. The HOX genes, are also important in hormone dependent-phase. Knock-out mice for the HOX genes present an abnormal genital developement with cloacal persistance.19 Vasculogenesis at the distal end of the GT also express high levels of EphA6 and EphA7 genes.22
Simultaneously with the above process during the fifth week, urogenital folds merge into the medial portion generating the urogenital groove. The edges of the grooves become the urethral folds that will be formed at the end of the sixth week. During this second month of pregnancy, Leydig cells develop after fetal testicular differentiation on the eighth week.23 This whole process ends at about weeks 15–16. Previously it was believed that navicular fossa was formed by invagination from distal to proximal at the Guerin lagoon. It has now been found that the endodermal urothelium has the ability to differentiate into the squamous epithelium and therefore originates the whole urethra including the distal part. The foreskin is formed by a ventral closure of the urethral folds (ectoderm) covering the endoderm (urethra). Fgfr2IIIb and Fgf1 genes are involved in the foreskin development. I was recently identified that rats exposed to flutamide develope proximal hypospadias and low expression in preputial epidermis the protein induced by prolactin (PIP).24
Hormone dependent phaseMasculinization of the genitals begins around week 10–14 of gestation and hormone androgen stimulation is very important. However earlier stages can be influenced by androgens before noticing masculinazing changes.25 Initially, hormonal stimulation is produced by the placenta with human chorionic gonadotropin (hCG) production starting on the 9th week of gestation with a peak between the 11th and 18th weeks. This production is the main stimulator for GT masculinization. Then once they have differentiated and Leydig cells are formed, the latter will start producing testosterone. Later, testosteron will be transformed into Dehydrotestosterone (DHT), its most potent form thanks to the 5-α-reductase. DHT will be more important than testosterone in the development of the external genitalia. The place where the first androgen receptors are expressed is prostatic ventral urogenital sinus mesenchyme.26 It has been found that androgens influence the expression of other genes such as Cyp1b1, FKBP51, Fgf10 receptor, MafB and Fgfr2. All of them involved in the development of the GT.27
Within the first signs of genital virilization an increase in the distance between the anus and the male genitalia is seen. Subsequently elongation of the phallus occurs with the formation of the penile urethra and foreskin. The latter starts on the tenth week. For the week 15–16, the process of the formation of the external genitalia is complete in most fetuses.
MAMLD1 geneInitially known as CXorf6 (open reading frame X Chromosome 6) or F18.28MAMLD1 (Mastermind like domain protein 1) is a gene initially described by Laporte in 1997 in patients with ambiguous genitalia during the study of X-linked miotubilar miotrofia.29 Later Fukami, et al. focused their research patients with microdeletions on the MTM1 that extended and included the MAMLD1 did also had hypospadias.28,30 This opened the path to future research on the MAMLD1 gene in the field of hypospadias.
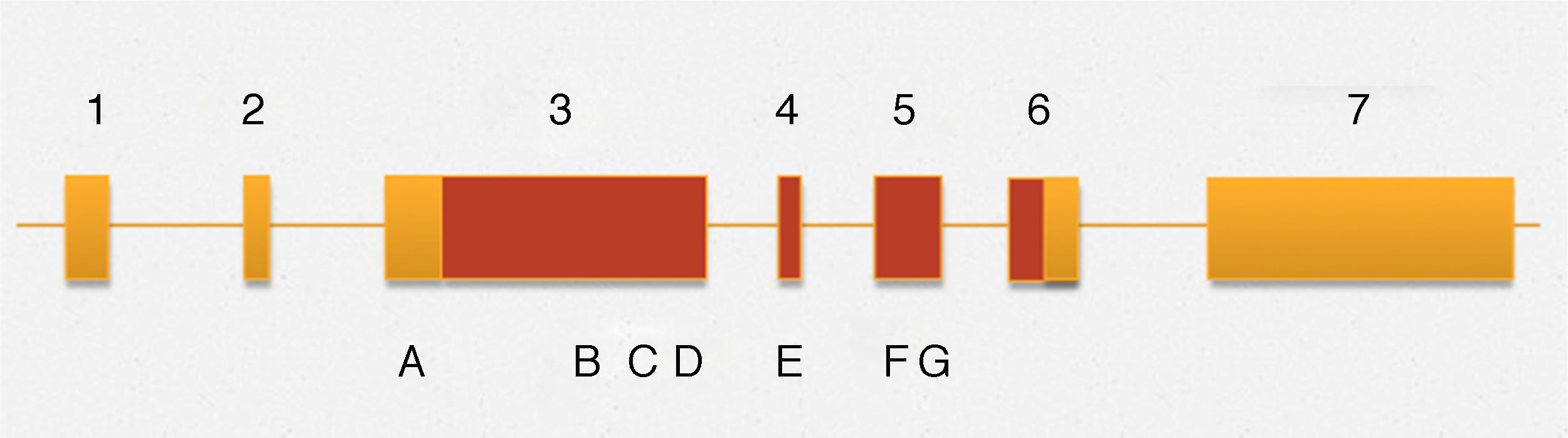
The gene is located on the long arm of the X chromosome in band 28 (Xq28). It consists of 100kb with a total of 7 exons of which two are non-coding. It presents an open reading frame 3–6. It encodes two proteins of 701 and 660 amino acids due to splicing with and without exon 4. The highest expressing variant is one that is positive for the splicing of exon 4 that is negative.31 Tsai et al., identified that the exon 3 codifies 80% of the information for protein production.32,33 So far most mutations are present in exon 3. An important feature is to codify a proline-rich domonio making it a characteristic DNA-binding protein. Structurally, the protein encoded by the MAMLD1 is very similar to the one produced by the MAML2. The MAML2 is characterized by exerting an important role in cellular differentiation in many tissues by induction or inhibition of different genes and by formation of an intracellular protein complex. It has been described that MAMLD1‘s function is to transactivate the HES3 promoter (hairy/enhancer) by additional interaction with STAT3 and increase of the expression of CYP17A1 gene which is achieved have adequate testosterone production in Leydig cells during embryogenesis.32In vitro studies have shown that the decreased expression of the gene to generate 25–30% testosterone levels are reduced to 50–60% of normal production, but never become undetectable.34 The hCG stimulation test is not altered before the deletion MAMLD1.
It is striking that patients with mutations in this gene have normal testosterone levels when being evaluated. Animal studies showed expression in Leydig and Sertoli cells during major periods of genital organogenesis (E12,5 to E14). Expression levels decreased significantly afterwards. During embryogenesis it has also been found a co-expression with the steroidogenic factor (SF1) described previously in gonadal development.35 This SF1 has an upstream binding site on intron 2 in the coding region that exerts MAMLD1 activation.36
Among the variants described associated with the development of hypospadias, in this gene are the following (Fig. 1): 370G-T (E124X), p.589C-T (Q197X), 1957C-T(R653X), 1295T>C (V432A), 325deG, p.Q529K, p.D686D,c.2065+8a>t and p.531ins3Q (CAG10>CAG13),36,37 and the insertion of 9 nucleotides, which generates the presence of 3 additional glutamines. Previously a similar type of insertions was described in the androgen receptor, however, it remains unclear whether this phenomenon is directly associated with the development of hypospadias.36 This is rare in malformations of the genitourinary tract, although in multiple degrees of hypospadias it has been observed that an exon 3 mutation with a stop codon is associated with proximal hypospadias.
Outline MAMLD1 gene. The numbers correspond to different exons that comprise the gene. Areas in yellow and terracotta are non-coding and coding regions respectively. The letters stand for: A c.865C > T (p.P286S) B c.1295T B > C (p.V432A) C c.1585C C > A (p.Q529K), D c.1591ins (CAG) 3 (p.531ins3Q), E c.1766A > G (p.N589S), F c.2058C > T (p.D686D) and G c.2065 + > t.
A variant recently described in a case of hypospadias, is p.P286. Additionally the p.P589S variant also recently described in a case of hypospadias, was identified at a low rate in healthy patients.28,36 Therefore, this haplotype described as SS has a high association with hypospadias unable to be statistically conclusive relationship.
DiscussionRecently the large amount of molecular information has allowed new insight into the pathophysiology of hypospadias. Although the exact mechanisms are still elusive, it is clear the interaction between genotype and environment. Bearing in mind the two phases in the development of GT and multiple genes involved in this process, it is possible to propose that hypospadias are the phenotypic expression of different diseases. Although there are still details to know, it could explain the cascade of molecular events with the action of different genes in a chronological order and correlate them with the severity of hypospadias. So that alterations in early stages correlate with more severe cases. The MAMLD1 gene is an example of the genotype–phenotype expression at a specific time during embryogenesis and the importance of the expression amount expressed in amount of testosterone production as a mechanism of abnormal development of the urethra correlation. Perhaps this quantitative dependence of expression explain why individuals with the mutation have different degrees of hypospadias. The utility of future studies will allow us to know more about this group of diseases expressed as hypospadias.
Level of evidenceLevel III.
Conflict of interestThe authors declare no conflict of interest.