For patients with a first psychotic episode (FPE), the international clinical guides recommend antipsychotic treatment, clozapine in case of pharmacoresistance, electroconvulsive therapy (ECT) in certain situations and metabolic syndrome monitoring.1 In spite of this, clinical practice is heterogeneous and sometimes differs from these recommendations.2–4 To the knowledge of the authors of this work, no studies have been published on the attitudes of Spanish psychiatrists to the treatment of patients with a FPE. We present here the results obtained in a sample of psychiatrists who practice in Catalonia.
We undertook a descriptive cross-sectional survey-type study of specialist doctors and psychiatric interns practicing in Catalonia. We prepared a questionnaire containing 18 items covering demographic and professional data and the treatment of patients with a FPE (Appendix A). This questionnaire was self-administered and anonymous, and it was distributed in 3 regional scientific meetings selected by convenience in 2018 and 2019. We describe the sample and results in terms of absolute and relative frequencies, as well as by using centralization and dispersion parameters. Bivariate comparisons were carried out using the corresponding statistical techniques (chi squared, Fisher's exact test, the Student t-test or the Mann-Whitney U). Version 23 of the SPSS statistical programme was used. This study was approved by the Clinical Research Ethics Committee of the Hospital Clínic, Barcelona (HCB/2017/0952).
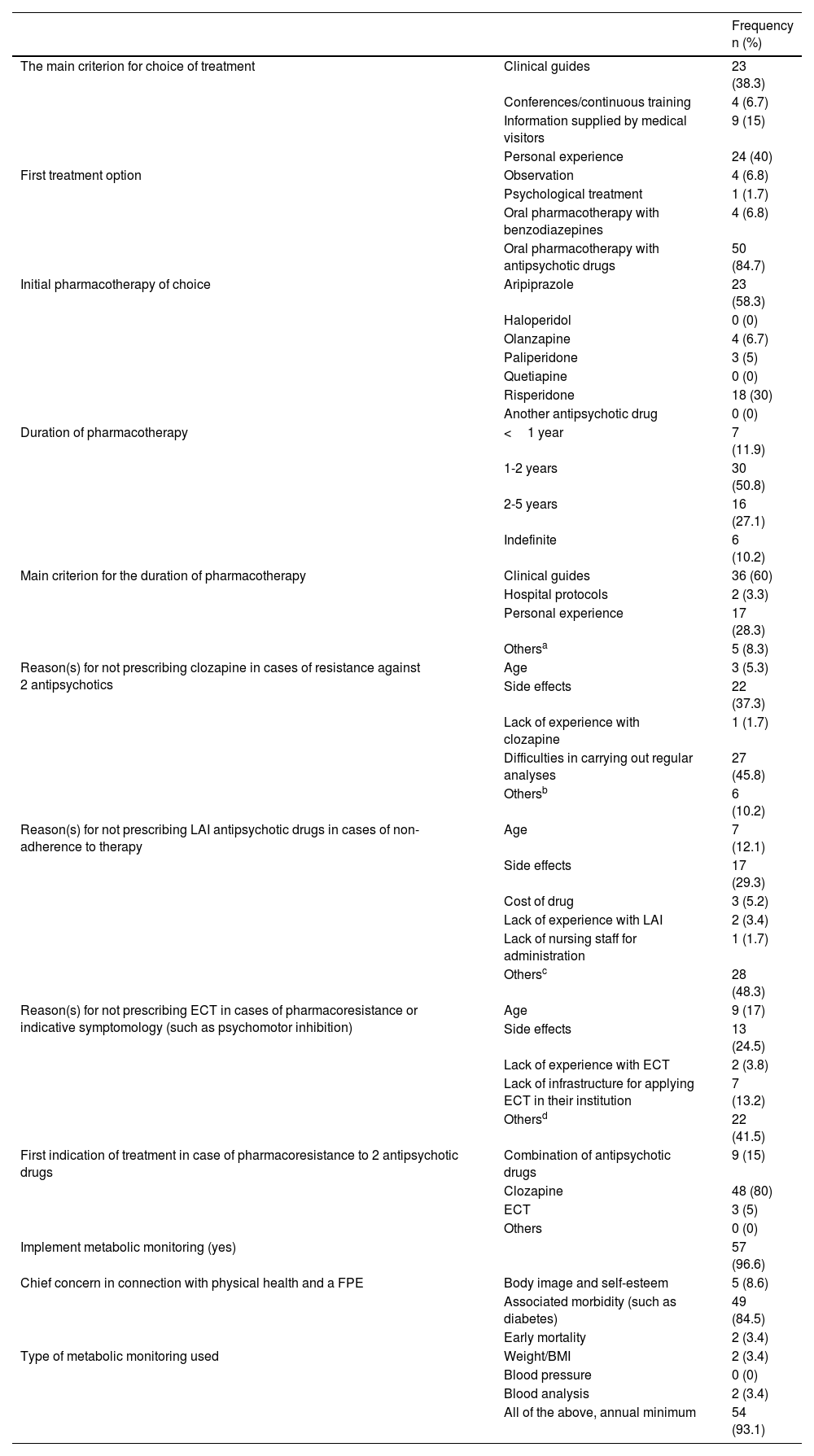
60 professionals answered the questionnaire (Table S1). Regarding the choice of treatment for a FPE, the most important influences were personal experience (40%) and the clinical guides (38.3%). The majority selected an antipsychotic drug as the first choice (84.7%), with treatment lasting for 1-2 years according to 50.8% of the sample. Side effects stood out as barriers against the use of clozapine, long-acting injectable antipsychotic drugs and electroconvulsive therapy (36.7%; 28.3% and 21.7%, respectively). In the case of pharmacoresistance, 80% of the sample prescribed clozapine, followed by a combination of antipsychotic drugs (15%). 96.6% of the sample mentioned the need for metabolic monitoring (Table 1).
Attitudes of the psychiatrists surveyed (n=60) to the treatment of individuals with a first psychotic episode.
| Frequency n (%) | ||
|---|---|---|
| The main criterion for choice of treatment | Clinical guides | 23 (38.3) |
| Conferences/continuous training | 4 (6.7) | |
| Information supplied by medical visitors | 9 (15) | |
| Personal experience | 24 (40) | |
| First treatment option | Observation | 4 (6.8) |
| Psychological treatment | 1 (1.7) | |
| Oral pharmacotherapy with benzodiazepines | 4 (6.8) | |
| Oral pharmacotherapy with antipsychotic drugs | 50 (84.7) | |
| Initial pharmacotherapy of choice | Aripiprazole | 23 (58.3) |
| Haloperidol | 0 (0) | |
| Olanzapine | 4 (6.7) | |
| Paliperidone | 3 (5) | |
| Quetiapine | 0 (0) | |
| Risperidone | 18 (30) | |
| Another antipsychotic drug | 0 (0) | |
| Duration of pharmacotherapy | <1 year | 7 (11.9) |
| 1-2 years | 30 (50.8) | |
| 2-5 years | 16 (27.1) | |
| Indefinite | 6 (10.2) | |
| Main criterion for the duration of pharmacotherapy | Clinical guides | 36 (60) |
| Hospital protocols | 2 (3.3) | |
| Personal experience | 17 (28.3) | |
| Othersa | 5 (8.3) | |
| Reason(s) for not prescribing clozapine in cases of resistance against 2 antipsychotics | Age | 3 (5.3) |
| Side effects | 22 (37.3) | |
| Lack of experience with clozapine | 1 (1.7) | |
| Difficulties in carrying out regular analyses | 27 (45.8) | |
| Othersb | 6 (10.2) | |
| Reason(s) for not prescribing LAI antipsychotic drugs in cases of non-adherence to therapy | Age | 7 (12.1) |
| Side effects | 17 (29.3) | |
| Cost of drug | 3 (5.2) | |
| Lack of experience with LAI | 2 (3.4) | |
| Lack of nursing staff for administration | 1 (1.7) | |
| Othersc | 28 (48.3) | |
| Reason(s) for not prescribing ECT in cases of pharmacoresistance or indicative symptomology (such as psychomotor inhibition) | Age | 9 (17) |
| Side effects | 13 (24.5) | |
| Lack of experience with ECT | 2 (3.8) | |
| Lack of infrastructure for applying ECT in their institution | 7 (13.2) | |
| Othersd | 22 (41.5) | |
| First indication of treatment in case of pharmacoresistance to 2 antipsychotic drugs | Combination of antipsychotic drugs | 9 (15) |
| Clozapine | 48 (80) | |
| ECT | 3 (5) | |
| Others | 0 (0) | |
| Implement metabolic monitoring (yes) | 57 (96.6) | |
| Chief concern in connection with physical health and a FPE | Body image and self-esteem | 5 (8.6) |
| Associated morbidity (such as diabetes) | 49 (84.5) | |
| Early mortality | 2 (3.4) | |
| Type of metabolic monitoring used | Weight/BMI | 2 (3.4) |
| Blood pressure | 0 (0) | |
| Blood analysis | 2 (3.4) | |
| All of the above, annual minimum | 54 (93.1) |
LAI: long acting injectable; BMI: body mass index; FPE: first psychotic episode; ECT: electroconvulsive therapy.
Other criteria (specified by the respondents): “Scientific study” (n=2; 3.3%), “evolution, comorbidities” (n=1; 1.7%), “Agreement with the patient” (n=1; 1.7%), not specified (n=1; 1.7%).
Regarding the first treatment option for a FPE, a smaller proportion of the professionals in the Programme of Specialized Care for Incipient Psychotic Disorder (PAE-TPI) tended to prescribe antipsychotic drugs (70% vs. 91.9%; P=.054), while fewer of the respondents who exclusively treated the paediatric and young population selected clozapine as the first option in case of pharmacoresistance (53.3% vs. 90.5%; P=.003).
Personal experience stands out in clinical decision-making, and almost half of the respondents selected this option to describe their approach to a FPE. It should be pointed out that evidence-based medicine requires clinical experience, although it has to be appropriately combined with scientific knowledge and patients’ values and circumstances. Assigning too much weight to experience may partially explain the proportion of respondents whose attitudes differ from the recommendations. On the one hand, and in line with studies in other similar socioeconomic regions,5–7 the psychiatrists surveyed consider the side effects of clozapine, long-lasting injectable antipsychotic drugs or ECT to be relevant barriers against their use. It should therefore not be forgotten that the clinical guides include calculations of the risks and benefits of treatments, and that these 3 types of treatment have unequivocally been shown to have a positive balance when they are indicated. The lower usage of clozapine in cases of pharmacoresistance by the professionals who treated paediatric and young patients may be because they are less familiar with this drug. This would have important implications: delay in establishing an effective treatment for patients with schizophrenia gives rise to a poorer long-term prognosis, which is already worse in the case of early onset psychosis.8 The finding that members of the PAE-TPI tend to prescribe less antipsychotic therapy as the first option in cases of a FPE may be explained by the programme itself, which offers the possibility of more intensive monitoring (as this favours a more conservative initial approach), or by their clinical guide (which permits observation and the use of benzodiazepines if a FPE may have been associated with substance abuse).9
The limitations of this study include the possible compromise of the extrapolation of its results, distortion due to complacency or a conflict of interests with the pharmaceutical industry, or the use of a non-validated questionnaire.
To conclude, our work indicates that in general the psychiatrists practicing in Catalonia following the recommendations of clinical guides when treating individuals with a FPE, while it also underlines the need to continue working to bring clinical practice closer to evidence-based medicine, above all in certain areas such as the paediatric-young population.
Conflicts of interestsMSV has received fees or support for travel to conferences from Lundbeck, Janssen-Cilag and Casen Recordati.
IB has received fees or support for travel to conferences from Angelini, Janssen and Lundbeck.
DB has received fees for attending conferences or support to attend congresses from Otsuka Pharmaceuticals and Casen Recordati.
MB has received fees or support for travel to conferences from Adamed, Angelini, Ferrer, Janssen-Cilag, Lundbeck, Neuraxpharm, Otsuka, Pfizer and Sanofi, as well as support for research from the Instituto de Salud Carlos III.
CGR has received fees or support for research or to attend conferences from Adamed, Angelini, Casen Recordati, Janssen-Cilag and Ludbeck.
MC has received support to attend conferences from Angellini and Lundbeck.
XL has received fees or support for travel to conferences from Angelini, Janssen, Lundbeck and Otsuka.
AM has received fees or support for travel to conferences from Janssen-Cilag, Angelini and Otsuka.
IM has received fees or support to attend conferences from Angellini, Janssen Cilag, Otsuka Pharmaceutical and Lundbeck.
VSG has received fees or support for travel to conferences from Janssen-Cilag, Lundbeck and Otsuka.
MC and JU have no conflicts of interest to declare.
We would like to thank the Societat Catalana de Psiquiatria i Salut Mental, the Societat Catalana de Psiquiatria Infanto-juvenil and the Comisión Pedagógica del Programa de Atención Específico al Trastorno Psicótico Incipiente.
IB would like to thank the Instituto de Salud Carlos III for its support for (INT19/00021) “Una manera de hacer Europa”.