A 47-year-old female patient with a medical history of epilepsy, psychotic disorder, and recurrent episodes of bilateral mastitis presented with cyclic mastalgia and pain persisting for ten years. The patient denied any prior breast prosthesis implantation or free silicone infiltration.
On physical examination, both breasts exhibited induration in the upper outer quadrants, with no evident cutaneous alterations.
Mammography revealed extensive regional asymmetries in both upper outer quadrants. Markedly hyperdense external and superior interquadrant lines were observed, displaying an internal multinodular pattern with calcified walls. Bilateral skin thickening was more pronounced in the lower quadrants, with no axillary lymphadenopathy detected (Fig. 1).
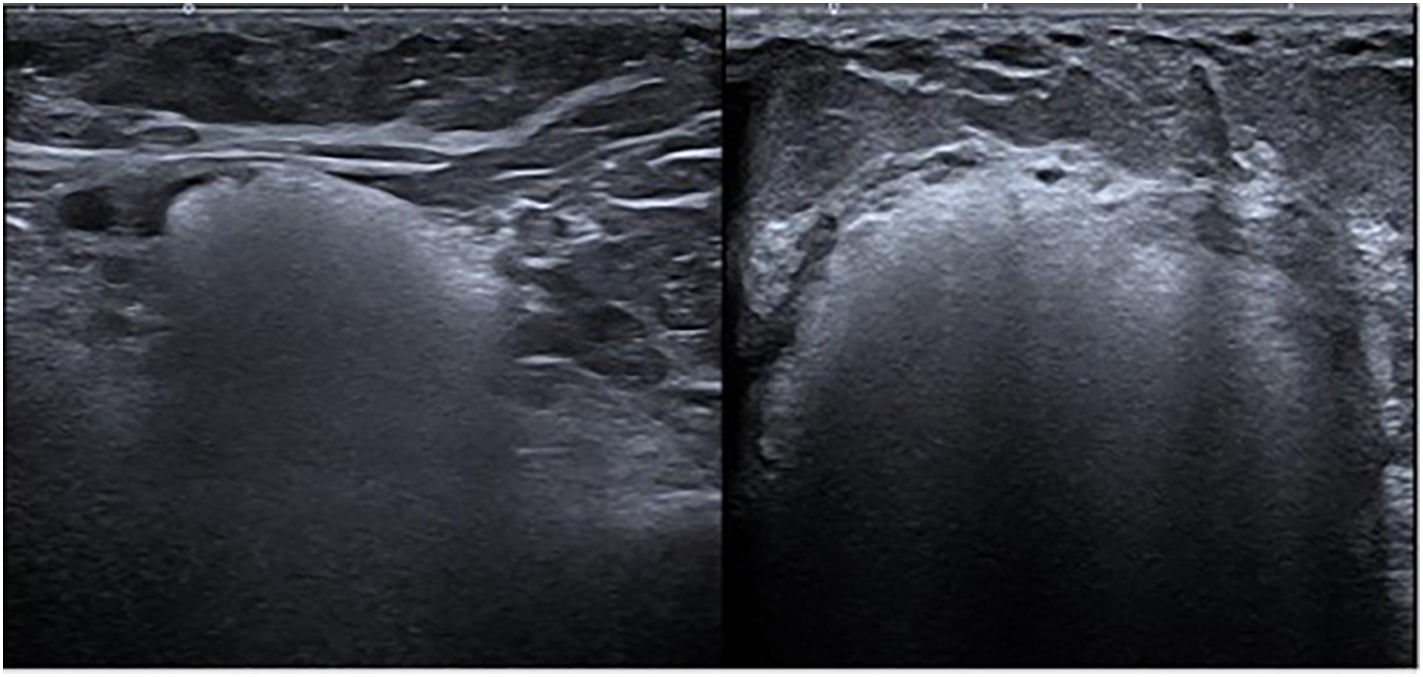
Ultrasound imaging demonstrated extensive hyperechoic areas with a “snowstorm” artefact in both breasts, indicative of intraparenchymal silicone and multiple bilateral oil cysts, along with axillary siliconomas (Fig. 2).
Based on these findings, the patient was diagnosed with breast siliconomas and subsequently underwent a mastectomy with immediate reconstruction.
Breast siliconomas are chronic inflammatory reactions caused by the migration or leakage of silicone into adjacent tissue, typically resulting from implant rupture or degradation. Clinically, they present as palpable nodules, pain, and inflammation. In some cases, silicone may migrate to axillary lymph nodes, leading to inflammatory lymphadenopathy[1,2].
Diagnosis is primarily based on imaging studies, with magnetic resonance imaging (MRI) being the most sensitive technique for detecting extracapsular silicone and assessing granuloma extension. Ultrasound also plays a crucial role, revealing characteristic patterns such as the “snowstorm” artefact associated with free silicone [2].
Treatment varies from observation in mild cases to surgical excision of granulomas and implant removal in severe cases, often followed by the placement of a new prosthesis [3]. These complications highlight the importance of regular follow-up in patients with breast implants.
Patient consentWe confirm that written informed consent was obtained from all participants prior to the study.
Ethical considerationsThe authors of this scientific work declare that we have fulfilled all the ethical responsibilities required for the preparation, writing and publication of the manuscript. This statement reflects our commitment to international ethical standards and the editorial policy of the journal to which the article is being submitted.
AuthorshipAll authors of the manuscript contributed substantially to the development of the study and met the established criteria for authorship.
FundingNo specific support from public sector agencies, commercial sector, or not-for-profit organizations was received for this research study. The authors conducted this research independently and with their own resources.
The authors have no conflict of interest to declare.