“One-size-fits-all” is a conventional medical approach to treating patients with similar symptoms with similar types of drugs. The effectiveness of various targeted therapies in such diverse tumors has entered an era in which treatment is determined based on the tumor's molecular abnormality profile, or “characteristics,” rather than on the type of tumor tissue or the site of anatomical origin. It improves the prognosis of patients and determines their quality of life. The evolving field of personalized medicinal drugs has significant potential in cancer prevention, diagnosis, and therapeutics. Identifying cancer susceptibility genes, such as the BRCA gene in breast cancer, helps screening programs identify patients at risk for developing cancer and make decisions about individual risk-modifying behaviors. Precision medicine plays a vital role in breast cancer management, e.g., using intracellular agents (Vemurafenib and Olaparib) and monoclonal antibodies (Cetuximab and Trastuzumab) in HER2-positive breast cancer. This review mainly focuses on the role of precision medicine in preventing and treating breast cancer.
El enfoque de “solución única” es un enfoque médico convencional para tratar a los pacientes con síntomas similares, con tipos de fármacos similares. La efectividad de las diversas terapias dirigidas para los diversos tumores ha iniciado una era en la que el tratamiento viene determinado sobre la base del perfil de anomalía molecular tumoral, o las “características”, en lugar del tipo de tejido tumoral o el sitio de origen anatómico. Mejora el pronóstico de los pacientes y determina su calidad de vida. El campo en evolución de los fármacos medicinales personalizados tiene un potencial significativo en la prevención, diagnóstico y terapéutica del cáncer. Identificar los genes de la susceptibilidad del cáncer, tales como el gen BRCA en el cáncer de mama, ayuda a los programas de cribado que identifican a los pacientes con riesgo de desarrollar cáncer, y a tomar decisiones sobre los comportamientos modificadores del riesgo del individuo. La Medicina de precisión juega un rol esencial en el manejo del cáncer de mama, como por ejemplo utilizar agentes intracelulares (Vemurafenib y Olaparib) y anticuerpos monoclonales (Cetuximab y Trastuzumab) en el cáncer de mama HER2 positivo. Esta revisión se centra principalmente en el papel de la Medicina de precisión a la hora de prevenir y tratar el cáncer de mama.
We have been approaching the ‘One-Size-Fits-All’ method for the last two decades. All individuals are not the same, i.e., everyone has different genomic characteristics, environmental exposure, diet, and lifestyles.1,2 The ‘One-Size-Fits-All’ is a form of medical approach that uses information about a person's genomic data and protein information, variability in environment, and lifestyle to create a personalized medical plan to prevent, diagnose, and treat disease.3
In today's era, precision medicines have shown rapid growth in cancer treatment, especially after the discovery of different genetic aspects of the disease.2,4 Research shows that precision medicine has shown more effective outcomes than traditional medicine.1 In the case of cancer, precision medicine uses specific information about a patient's tumor to make a diagnosis, plan treatment, determine how effective the treatment is, or make a prognosis.5 Identification of both individual and constituted carcinogenesis is essential for the development of cancer therapy.6 This understanding paves the way for precision medicine, which can inhibit the expression of different oncogenes by targeting cancer-related pathways, such as RAS, PI3K, and AKT.7
Breast cancer is a major global public health issue and the second leading cause of cancer deaths.8 Although various genes involved in DNA repair, such as ATM, PALB2, RAD51C, CHEK2, and BRIP1, are associated with breast cancer, BRCA1, BRCA2, TP53, and PTEN genes are primarily responsible for inherited breast cancers.9–12
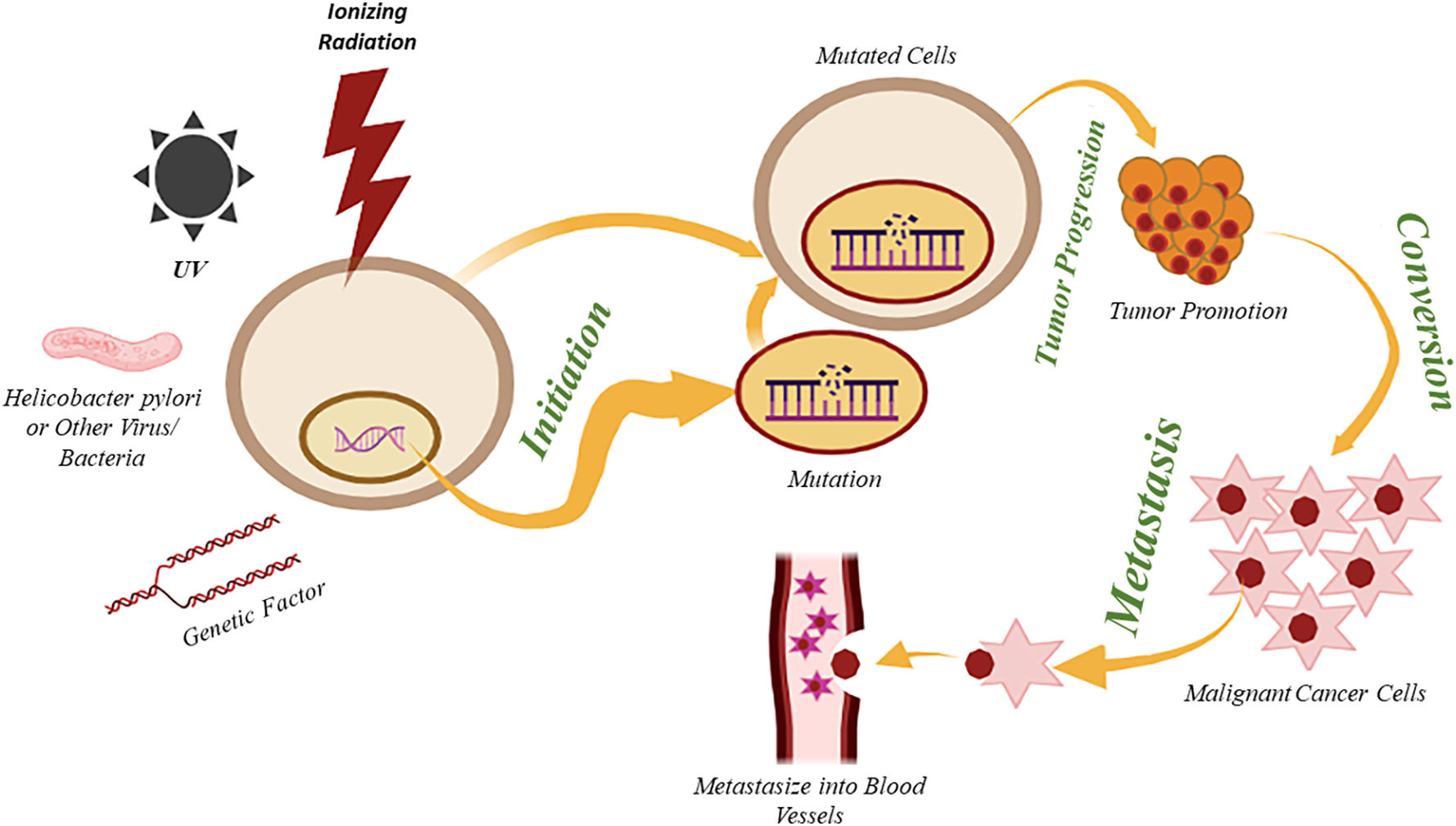
The pictorial representation below (Fig. 1) illustrates how the individual and family history (Genetic Factors) may impact the risk of developing breast cancer. It also highlights other contributing factors, including exposure to ionizing radiation, hormonal imbalances, reproductive factors, toxic chemicals, and UV radiation, which lead to irreversible cellular mutations that disrupt the regulation of oncogenes, the cell cycle, and DNA transcription. As a result, uncontrolled cell growth occurs, progressing to tumor formation, local tissue invasion, and eventually metastasis.5 BRCA 1 and BRCA 2 are significant genes associated with breast and ovarian cancer risks.13 BRCA1 and BRCA2 are located on chromosomes 17 and 13, respectively, and are tumor suppressor genes that control cell growth. A mutation in any or both of the genes leads to uncontrolled cell growth and tumor formation.14
Depending on their subtypes, breast cancer may be broadly classified into two categories, i.e., histological and molecular subtypes.15 Histologically, it is further divided into two main categories, i.e., Invasive Ductal Carcinoma and Invasive Lobular Carcinoma.15
As the name suggests, invasive ductal carcinoma occurs when cancer develops in the breast ducts (mammary ducts and lactiferous ducts) and metastasizes into the surrounding breast tissues. In contrast, invasive lobular carcinoma appears in the lobules (milk-producing glands) and metastasizes to lymph nodes and other parts of the body. According to the source of the tumor, they can be divided into several other types, as shown in Fig. 2A.16 Among all types of breast cancer, the most common type of carcinoma is invasive ductal carcinoma, accounting for around 50–80% of cases, whereas only 5–15% of breast cancers can be categorized as invasive lobular carcinoma.15 Among all types of invasive ductal carcinoma, apocrine carcinoma is the rarest form, i.e., it comprises only 4% of all breast malignancies.17
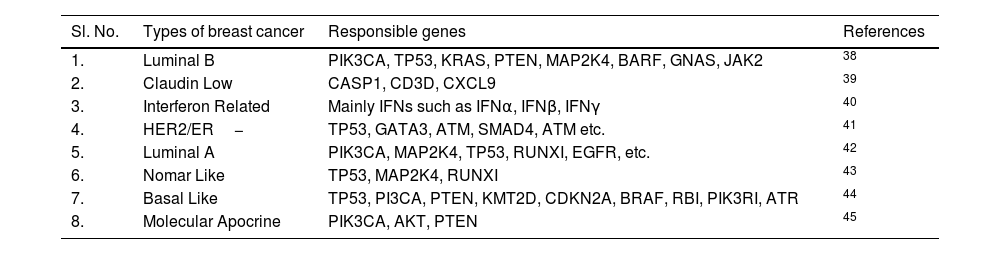
The breast cancer types depend on the kind of breast cells that lead to cancer. Breast cancer is a highly diverse disease with various genetic subtypes and associated complications. The terminology “inter-tumor heterogeneity” reflects the diversity in tumor molecules across persons.18 Variations within a single tumor mass are classified as intratumor (spatial) heterogeneity, whereas variations that occur due to the period of tumor growth and the pressure of treatment therapy are described as temporal heterogeneity. Precision medicine seeks to understand and target the heterogeneity of tumors, enabling physicians to design personalized treatments based on specific molecular markers.19,20 Breast cancer restricted to localized breast lesions is referred to as localized or early restricted to a localized breast lesion is referred to as localized, early, or curable. Unfortunately, it is often spread through lymph channels or blood to a distant site (micro-metastases), even in its early stages, which cannot be detected through current diagnostic methods.21,22–25 When the cancer is detected at a distance from the breast, it is referred to as advanced or metastatic breast cancer.22,26 As shown in (Fig. 2B), there is a total of seven molecular subtypes of breast cancer available.15 BLBC or Basal-like breast cancer is considered a triple negative (HER2−, ER−, PR−) breast cancer, i.e., it does not adequately express only HER2 but also ER and PR genes.27 Luminal Breast cancer can be divided into two categories, i.e., Luminal A (LumA) and Luminal B (LumB). LumA (HER2-, ER+, and PR+) accounts for 40–60% of cases, whereas LumB (HER2+/−, ER+, and PR−) comprises 15% of the cases. HER2/ER− lacks the expression of ER (ER−) and PR (PR−); however, it expresses HER2 (HER2+), which has an overall survival rate (95% CI) of 66–88.7%. Like BLBC, Claudin-Low breast cancer lacks the expression of both HER2, ER, and PR.15 Claudin-low tumors show a lower proliferation rate compared to BLBC, but higher than LumA and LumB. Ki-67, encoded by the MKI67 gene, is most commonly used as a proliferation marker in the case of the Claudin-Low subtype. In contrast, cytokeratins such as CK5 and CK14 are used as proliferation markers in the case of BLBC.27,28–30 In the case of molecular apocrine carcinoma, ER and PR expressions are negative, while HER2 can occasionally be positive. Although CK16/17 can be used as a marker for Molecular apocrine tumors, the expression of the Androgen Receptor (AR) is primarily used as a prognostic marker for such tumors.16,31–33 In the case of Interferon (IFN)-related Breast tumors, the expression of PR is still unknown. In contrast, the expression of ER is negative, and the expression of HER2 is rarely positive. Tumors with high IFN expression are 1.7 times more prone to metastasize than those with low IFN expression. The expression of the STAT1 and STAT2 genes can be used as a prognostic marker for interferon-related breast cancer.15,16,34–36 For normal breast-like or Normal-like breast cancer, it is closely related to LumA. The expression of HER2 is negative, the expression of ER is rarely positive, and the expression of PR is still unknown in typical breast cancer.5,15,16,37 The classification of breast cancer based on molecular profiling and its responsible genes is shown below (Table 1).
Classification of breast cancer based on molecular profiling.
| Sl. No. | Types of breast cancer | Responsible genes | References |
|---|---|---|---|
| 1. | Luminal B | PIK3CA, TP53, KRAS, PTEN, MAP2K4, BARF, GNAS, JAK2 | 38 |
| 2. | Claudin Low | CASP1, CD3D, CXCL9 | 39 |
| 3. | Interferon Related | Mainly IFNs such as IFNα, IFNβ, IFNγ | 40 |
| 4. | HER2/ER− | TP53, GATA3, ATM, SMAD4, ATM etc. | 41 |
| 5. | Luminal A | PIK3CA, MAP2K4, TP53, RUNXI, EGFR, etc. | 42 |
| 6. | Nomar Like | TP53, MAP2K4, RUNXI | 43 |
| 7. | Basal Like | TP53, PI3CA, PTEN, KMT2D, CDKN2A, BRAF, RBI, PIK3RI, ATR | 44 |
| 8. | Molecular Apocrine | PIK3CA, AKT, PTEN | 45 |
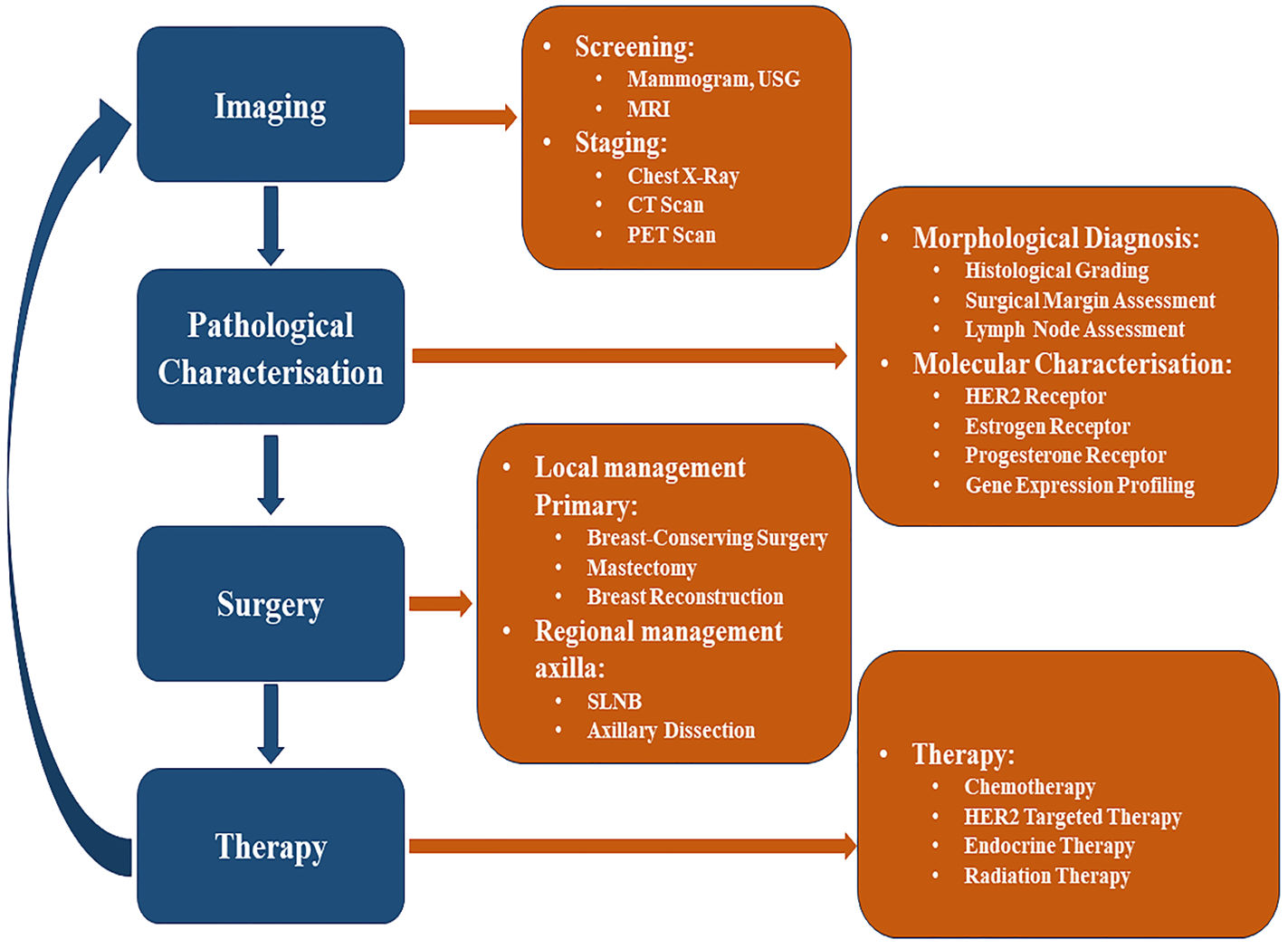
The various approaches used for the management (i.e., Diagnosis and Treatment) of breast cancer are illustrated below (Fig. 3).
ScreeningMost breast cancers are usually recognized via screening or a symptom (e.g., pain or a palpable mass) that results in the diagnosis. Screening a more significant number of people has the potential to uncover tiny lesions, has a more negligible risk of spreading, are much more amenable to breast-protecting and limited axillary surgery, and is less likely to require chemotherapy.
Breast mammography, MRI, and ultrasounds are used to diagnose cancer, though they have a sensitivity for malignancy. MRI has a higher sensitivity for malignancy (84.6%) compared to ultrasound (39.6%) or mammography (38.6%).46 Furthermore, employing MRI as an adjunct to mammography has a sensitivity to malignancy of around 92.7%, whereas using ultrasound as an adjunct has a sensitivity for malignancy of around 52%.47 As a result, women with a higher risk of breast cancer are recommended to have breast MRI as an accompaniment to mammography.
Ultrasound screening is applicable for a woman with dense breasts or intermediate risk, who cannot have an MRI. The main drawback of ultrasound screening is the high rate of false positive reports.48
Researchers are working to enhance breast cancer detection methods by utilizing Computer-Aided Design, AI, and deep learning models to predict or identify early-stage breast cancer accurately. In this regard, the automated breast ultrasound system was trained and evaluated with breast ultrasound images, achieving an accuracy rate exceeding 95%.49–53
Pathological evaluationAfter diagnosis through various screening methods, some pathological evaluations are necessary, for which diseased tissue is obtained by fine-needle aspiration, surgical excision, or core biopsy. Ancillary immune-histochemical (ICH) and molecular tests are done to characterize morphology. The size of the tumor is determined carefully with screening and pathological examination.54–56
Prevention of breast cancer“Prevention is better than cure”, so if we can prevent breast cancer rather than cure it, the person at high risk can get relief from the regular long-term treatment cycle consisting of surgery, radiation therapy, and chemotherapy. So, researchers are now analyzing different risk factors of breast cancer and possible preventive measures. Early menstruation, entering menopause at a late stage, being older at the birth of a first child, or having never given birth, etc., are some significant risk factors for breast cancer. Those are the risks caused by long-term exposure to estrogen. Some estrogen modulators (SERMs), such as tamoxifen, have been shown to reduce breast cancer incidence by 16–49%. However, the long-term benefits and the side effects are still unknown. Raloxifene is another SERM with fewer side effects and is a promising alternative to tamoxifen.57 A woman with a familial history of the BRCA gene can test for the expression of the BRCA1 and BRCA2 genes. These two genes are mainly 45–65% responsible for developing breast cancer. Prophylactic options, such as the removal of breast tissues, significantly reduce the risk of breast cancer, although prophylactic mastectomy involves significant surgical and psychological risks.5
Breast cancer treatmentThe traditional treatment of breast cancer is done by surgery, followed by either chemotherapy or radiation. Surgery is the primary solution for the treatment of breast cancer. In the early 20th century, women diagnosed with breast cancer were most commonly treated with radical mastectomy. With the advancement in breast cancer screening, the recognition of nonpalpable cancers led to the need for more effective localization techniques to implement surgical treatments. Such surgery can be categorized into Breast-Conserving, Non-breast-conserving, and Axilla Staging. However, surgeries are selected based on the characteristics of the tumor. Neoadjuvant chemotherapy may be used to optimize the surgical procedure by reducing tumor size to an operable range. Following surgery, if desirable, chemotherapy is recommended for the patient. The indications for chemotherapy include ER-negative, PR-negative, and HER2-negative tumors, as well as HER2-positive tumors, larger tumor sizes, and positive lymph nodes. Radiation therapy is also suggested, subsequently, following surgery or chemotherapy. BCS (Breast-conserving surgery) and radiation show a promising local control rate of 90–95% in the preserved breast within 10 years of treatment.58
Advancement of precision medicine in treating breast cancerTrastuzumab is a well-known example of precision medicine in treating breast cancer. It is a humanized IgG1 monoclonal antibody used to treat HER2-positive breast cancer.59 It is used as an additive to the standard chemotherapy drugs in patients who overexpress the HER2 growth factor. Research demonstrated that the rate of recurrent breast cancer can be reduced by 40% through the use of trastuzumab.5
Neratinib, a commonly used TKI drug, and pilaralisib or alpelisib, used as a PI3K inhibitor, have shown potential in targeting the HER2 growth factor in breast cancer. Antibody drugs, such as patritumab, T-DM1, and enhertu have been considered for metastatic tumor targets and cannot be controlled with drugs targeting their metabolic action.60
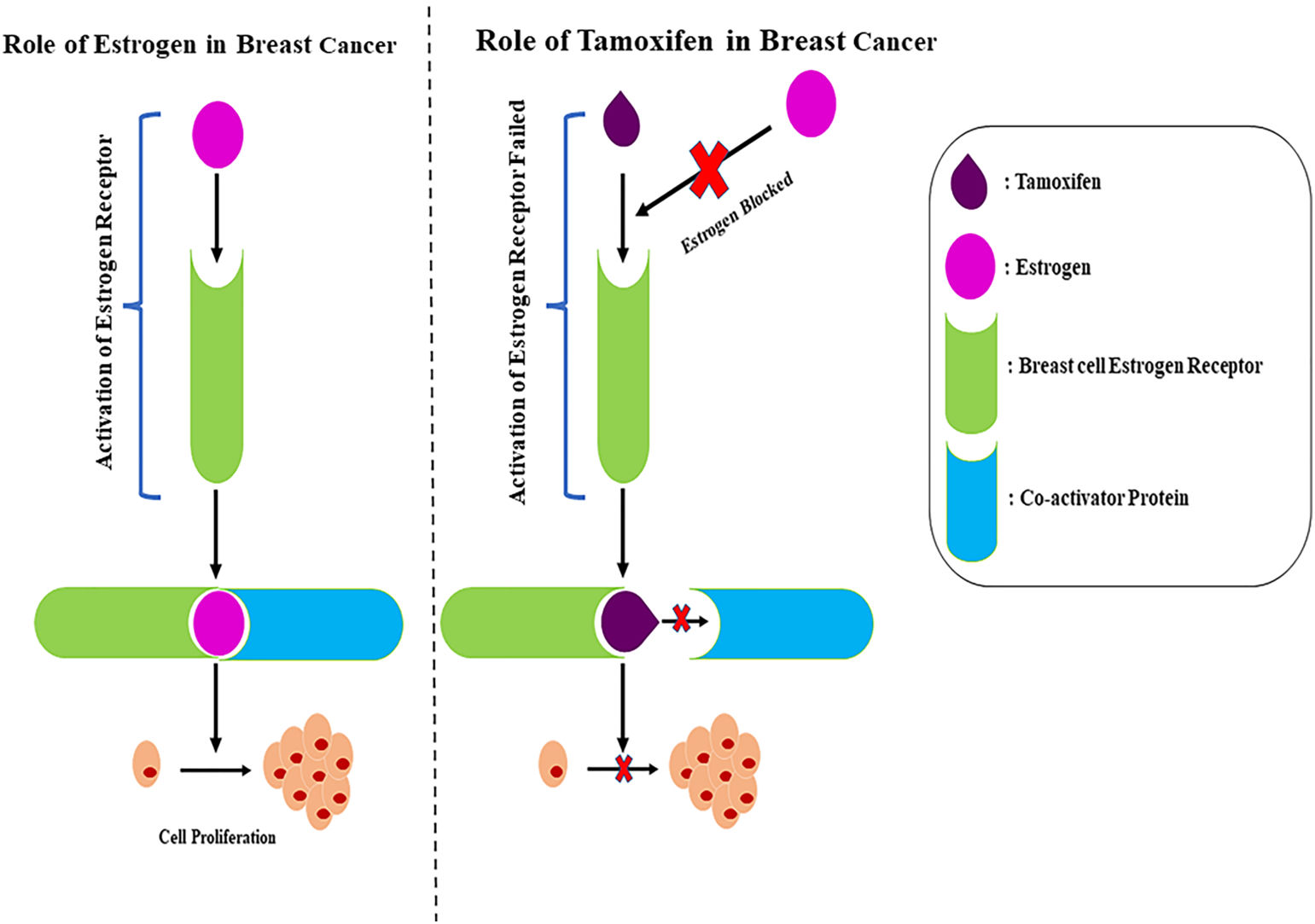
Tamoxifen, as shown in Table 2, has demonstrated an effective role in blocking estrogen uptake by receptors (Fig. 4). The drug has enhanced potential in targeting ER+ breast cancer and is also being used in cancer recurrence cases with ER+ profiles. This therapy for breast cancer patients with an ER+ profile can provide a better prognosis in both initial tumor and recurrent cases. Another standard profile of breast cancer patients includes estrogen production in the post-menopausal stage. The enzyme-mediated estrogen synthesis can be inhibited using aromatase inhibitors, which have been shown to improve disease-free survival and lower local recurrence rates in individuals. The identification of newer generation aromatase inhibitors such as letrozole, exemestane, and anastrozole has shown better prognosis and increased survival rates of patients diagnosed with HR+ metastatic breast cancer.61 Several cancers including breast cancer such as HR+, HER2−, TNBC, etc., small cell lung cancer and non-small cell lung cancer are also regulated by uncontrolled cell division caused by the dysregulation of cyclin-dependent kinases such as CDK4/6. Selective inhibitors such as ribociclib, palbociclib, and abemaciclib have shown potential in targeting cancers by inhibiting the regulation of CDK4/6.62 The regulation of CDK4/6, downstream of the mutagenic signaling, shows that the combination of these inhibitors is required for effective targeting in specific breast cancer profiles.
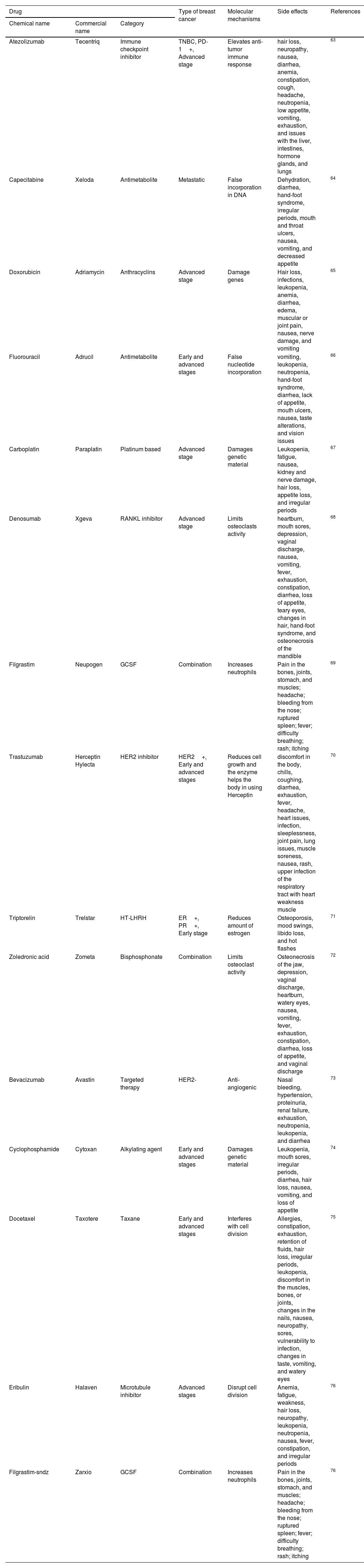
Overview of therapeutic medications that are used for the treatment of various kinds of breast cancer, as well as their present state.
| Drug | Type of breast cancer | Molecular mechanisms | Side effects | References | ||
|---|---|---|---|---|---|---|
| Chemical name | Commercial name | Category | ||||
| Atezolizumab | Tecentriq | Immune checkpoint inhibitor | TNBC, PD-1+, Advanced stage | Elevates anti-tumor immune response | hair loss, neuropathy, nausea, diarrhea, anemia, constipation, cough, headache, neutropenia, low appetite, vomiting, exhaustion, and issues with the liver, intestines, hormone glands, and lungs | 63 |
| Capecitabine | Xeloda | Antimetabolite | Metastatic | False incorporation in DNA | Dehydration, diarrhea, hand-foot syndrome, irregular periods, mouth and throat ulcers, nausea, vomiting, and decreased appetite | 64 |
| Doxorubicin | Adriamycin | Anthracyclins | Advanced stage | Damage genes | Hair loss, infections, leukopenia, anemia, diarrhea, edema, muscular or joint pain, nausea, nerve damage, and vomiting | 65 |
| Fluorouracil | Adrucil | Antimetabolite | Early and advanced stages | False nucleotide incorporation | vomiting, leukopenia, neutropenia, hand-foot syndrome, diarrhea, lack of appetite, mouth ulcers, nausea, taste alterations, and vision issues | 66 |
| Carboplatin | Paraplatin | Platinum based | Advanced stage | Damages genetic material | Leukopenia, fatigue, nausea, kidney and nerve damage, hair loss, appetite loss, and irregular periods | 67 |
| Denosumab | Xgeva | RANKL inhibitor | Advanced stage | Limits osteoclasts activity | heartburn, mouth sores, depression, vaginal discharge, nausea, vomiting, fever, exhaustion, constipation, diarrhea, loss of appetite, teary eyes, changes in hair, hand-foot syndrome, and osteonecrosis of the mandible | 68 |
| Filgrastim | Neupogen | GCSF | Combination | Increases neutrophils | Pain in the bones, joints, stomach, and muscles; headache; bleeding from the nose; ruptured spleen; fever; difficulty breathing; rash; itching | 69 |
| Trastuzumab | Herceptin Hylecta | HER2 inhibitor | HER2+, Early and advanced stages | Reduces cell growth and the enzyme helps the body in using Herceptin | discomfort in the body, chills, coughing, diarrhea, exhaustion, fever, headache, heart issues, infection, sleeplessness, joint pain, lung issues, muscle soreness, nausea, rash, upper infection of the respiratory tract with heart weakness muscle | 70 |
| Triptorelin | Trelstar | HT-LHRH | ER+, PR+, Early stage | Reduces amount of estrogen | Osteoporosis, mood swings, libido loss, and hot flashes | 71 |
| Zoledronic acid | Zometa | Bisphosphonate | Combination | Limits osteoclast activity | Osteonecrosis of the jaw, depression, vaginal discharge, heartburn, watery eyes, nausea, vomiting, fever, exhaustion, constipation, diarrhea, loss of appetite, and vaginal discharge | 72 |
| Bevacizumab | Avastin | Targeted therapy | HER2- | Anti-angiogenic | Nasal bleeding, hypertension, proteinuria, renal failure, exhaustion, neutropenia, leukopenia, and diarrhea | 73 |
| Cyclophosphamide | Cytoxan | Alkylating agent | Early and advanced stages | Damages genetic material | Leukopenia, mouth sores, irregular periods, diarrhea, hair loss, nausea, vomiting, and loss of appetite | 74 |
| Docetaxel | Taxotere | Taxane | Early and advanced stages | Interferes with cell division | Allergies, constipation, exhaustion, retention of fluids, hair loss, irregular periods, leukopenia, discomfort in the muscles, bones, or joints, changes in the nails, nausea, neuropathy, sores, vulnerability to infection, changes in taste, vomiting, and watery eyes | 75 |
| Eribulin | Halaven | Microtubule inhibitor | Advanced stages | Disrupt cell division | Anemia, fatigue, weakness, hair loss, neuropathy, leukopenia, neutropenia, nausea, fever, constipation, and irregular periods | 76 |
| Filgrastim-sndz | Zarxio | GCSF | Combination | Increases neutrophils | Pain in the bones, joints, stomach, and muscles; headache; bleeding from the nose; ruptured spleen; fever; difficulty breathing; rash; itching | 76 |
Mechanism of action of tamoxifen. On the left panel, the role of estrogen in breast cancer is shown. Estrogen binds to the estrogen receptor in the breast cell and activates the breast cell receptor, and then, the estrogen receptor along with estrogen binds to the Co-activator protein inducing cell proliferation. On the right panel, the role of tamoxifen in breast cancer treatment is shown. Tamoxifen binds to the estrogen receptor blocking the binding of estrogen failing activation of the estrogen receptor. Furthermore, the estrogen receptor and tamoxifen block the Co-activator protein's binding, thus preventing cell proliferation.
The continuous evaluation of different methods of breast management has helped bring about a paradigm shift from the traditional one-size-fits-all approach to a more precise and patient-centric approach, namely precision medicine or Personalized medicine, which targets the unique genetic structure of patients and tumors. Precision medicine seems to have a better approach in managing breast cancer in the future. However, no precision medicine available will eliminate the standard approach. Currently, precision medicine can only be used to support standard treatment methods. Furthermore, the precision medicine regimen suggests that all prognoses may eventually depend on precision medicine. In this report, we observe that medications such as tamoxifen and raloxifene can help prevent breast cancer by reducing estrogen exposure to breast tissues. Nevertheless, specific drugs may be administered to treat breast cancers by targeting particular genes, tissues, hormones, and cell signaling pathways. Though conventional medicines help traditional treatments, the futures of precision medicines seem to have a greater scope in treating breast cancer.
FundingThis investigation did not receive specific help from agencies of the public sector, commercial sector, or profit-making entities.
Ethical disclosuresI confirm that the photos and data included in the referenced article do not contain personal details, images of patients, research subjects, or other individuals who require consent under applicable law.
Therefore, the requirement to obtain and submit consent for publication does not apply to this review manuscript.
CRediT authorship contribution statementSuman Bhadra: Writing – original draft, Writing – review & editing. Sohini Kulavi: Conceptualization, Writing – review & editing. Jaya Bandyopadhyay: Supervision.
The authors declare that they have no conflict of interest.
This work was supported by the M.Tech Biotechnology PG Teaching Program funded by the Department of Biotechnology (Government of India), All India Council for Technical Education (AICTE) for the Doctoral Fellowship to SK, and the Department of Biotechnology (MAKAUT, WB).