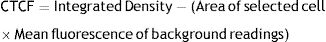Fertilin β is a sperm surface protein that can mediate sperm-egg membrane interaction. This study was conducted to determine whether the expression of fertilin β after intrauterine insemination (IUI) in donors with normal parameters after standard semen analysis is related to low success rate or failure of fertilization.
MethodsWe examined the sperm of 30 male donors who have normal as controls, oligozoospermia, and unexplained infertility as the clinically indication for IUI. Fertilin β has been labeled with the ADAM2 antibody by indirect immunofluorescence (IF) assay. To evaluate the reproducibility of the test, we selected four sperm samples scale of 0 to +++ according to the distribution of fluorescence label.
ResultsThe results were highly correlated with the corrected total cell fluorescence (CTCF) (Rp=0.9972, P<0.05). We suggest that the relationship between infertility and fertilin β may be due to the distribution of this protein on the sperm surface. Male partners of couples with unexplained infertility showed a low distribution of fertilin β by a decrease of the fluorescence signal in the IF labeling (scale of +++ by 7.4±10.32%, P<0.0001, ±SD).
DiscussionAbnormal fertilin β function may be a potential mechanism that could lead to fertilization failure.
La fertilinβ es una proteína de la superficie del esperma que puede mediar entre la interacción del espermatozoide y la membrana del óvulo. Este estudio se realizó para determinar si la expresión de fertilinβ después de la inseminación intrauterina (IIU) en donantes con parámetros normales después del análisis estándar de semen está relacionada con una baja tasa de éxito o fracaso de la fertilización.
MétodosExaminamos los espermatozoides de 30 donantes masculinos que tenían controles normales, oligozoospermia e infertilidad inexplicable como indicación clínica de IIU. La fertilinβ ha sido etiquetada con el anticuerpo ADAM2 mediante un ensayo de inmunofluorescencia indirecta (IF). Para evaluar la reproducibilidad de la prueba, seleccionamos cuatro muestras de esperma en una escala de 0 a +++ según la distribución de la etiqueta de fluorescencia.
ResultadosLos resultados estuvieron altamente correlacionados con la fluorescencia celular total corregida (Rp=0,9972, p<0,05). Sugerimos que la relación entre la infertilidad y la fertilinβ puede deberse a la distribución de esta proteína en la superficie del esperma. Los compañeros masculinos de parejas con infertilidad inexplicable mostraron una baja distribución de fertilinβ por una disminución de la señal de fluorescencia en el etiquetado IF (escala de +++ en 7,4 ±10,32%, p<0,0001, ±DE).
ConclusiónLa función anormal de la fertilinβ puede ser un mecanismo potencial que podría conducir al fracaso de la fertilización.
ADAMs are multi-part proteins, ∼750 amino acids long, and have multiple domains (active sites). These regions consist of pro-domain and metalloprotease region, disintegrin region, a cysteine-rich region, epidermal growth factors (EGF)-like region, a transmembrane part, and cytoplasmic tail part. Each of these parts (except the transmembrane domain) has proven to have not only a structural role but also a functional role.1,2 Active metalloprotease moieties of 29 of 41 known ADAM proteins are known to be functional proteases. In the other 12 ADAM proteins, the proteolytic activity of the metalloprotease is thought to be inhibited.3 ADAM proteins are closely related to cell migration, cell interaction, cell adhesion and the regulation of growth factors, cytokines and their receptor shedding. These are involved in biological processes such as fertilization, neurogenesis, myogenesis, cancer and inflammation.4 Recently the localizations, complex formations, and processings of reproductive ADAM proteins were detailed in a review4 with their functional interactions between ADAM proteins. Among the ADAM proteins, a few of them have been identified as active in adhesion. In the ADAM protein family, such as ADAM2 and many members play important roles in the fertilization process.5 ADAM2 is a heterodimer protein containing two subunits known as fertilin α and β.3,4 The tripeptide Arginine-Glycine-Aspartate (RGD) is found in the disintegrin regions of the proteins as in the somatic cells of the rat fertilin protein, while the Threonine-Aspartate-Glutamate (TDE) tripeptide is present in the guinea pig fertilin protein.6 It has been stated that the fertilin protein in humans is expressed from the 8th chromosome's band p11.2. Researchers have revealed amino acid sequence, tissue specificity, and chromosome mapping by cDNA cloning of human fertilin. The amino acid sequence of human fertilin; they reported that it consists of metalloprotease, disintegrin-, cysteine-rich, epidermal growth factor-like (EGF) repeat, transmembrane and cytoplasmic tail regions.7 It has been noted that the amino acid sequence of human fertilin shows 90% homology to the monkey, 56% to guinea, and 55% to the mouse, respectively. A Phenylalanine-Glutamate-Glutamate (FEE) binding tripeptide is present in the disintegrin region of human fertilin instead of RGD or TDE. It is thought that it can recognize and bind integrins with FEE.7
During the maturation of spermatozoon in the testis and epididymis, it has been reported that fertilin undergoes several different proteolytic processes.8 The spermatozoa are formed during their development in the seminiferous tubule before the α subunit of the fertilin appears on the cell surface.8,9 It was stated that the subunit of the fertilin occurred after the fertilin α, during the passage of spermatozoon through the epididymis. It is known that spermatozoa gain some of its motility and fertilization ability between the distal corpus and proximal cauda of the epididymis. Due to the proteolytic processing of guinea pig fertilin β in these parts, it was thought that fertilin plays a role in spermatozoon motility and fertilization.8 It has been reported that the fertilinα has a catalytic sequence (HEXXH) on the metalloprotease site. However, once these sperm pass through the epididymis during maturation, the protein migrates to the back area of the sperm head on the plasma membrane.10
Male infertility has been proven to be a complex pathology, and its basic physiological and biochemical characteristics need to be further understood. Many men confirmed to have infertility received this diagnosis, but there was no explanation for the accompanying cause. Many research projects focus on exploring the genetic basis of male infertility, but so far, they have been able to explain no more than 15% of infertility cases.11–13
For the reproduction process of mammals to proceed smoothly, there must be a complex molecular mechanism. The key factors play an important role in sperm by acquiring the ability to fertilize in the male testis, such as spermatogenesis, sperm maturation during epididymal transport, sperm migration, and sperm-egg fusion in the female reproductive tract. Myles14 proposed that during spermatogenesis and epididymal transport, the proteins on the sperm surface are located in specific areas. The surface of the sperm is a dynamic structure, and it will change even after the sperm leaves the male. Ding et al.15 revealed that fertilin β localization on the human sperm changes due to capacitation and acrosome reaction and showed by immunofluorescence and immunoelectron microscopy techniques. The study's results were as below. Firstly, during capacitation, fertilin β was restricted to the anterior head. Then, during the acrosome reaction, the expression and localization of fertilin β changed dramatically on the anterior head, and finally restricted to the lateral of the posterior head. Fertilin β is restricted to the front part of human spermatozoa, indicating that fertilin β may be involved in the binding of sperm to fallopian tube epithelial cells. The restriction of fertilin β to the occipital domain of acrosome reaction in spermatozoa indicates its function in sperm-egg binding and fusion.15
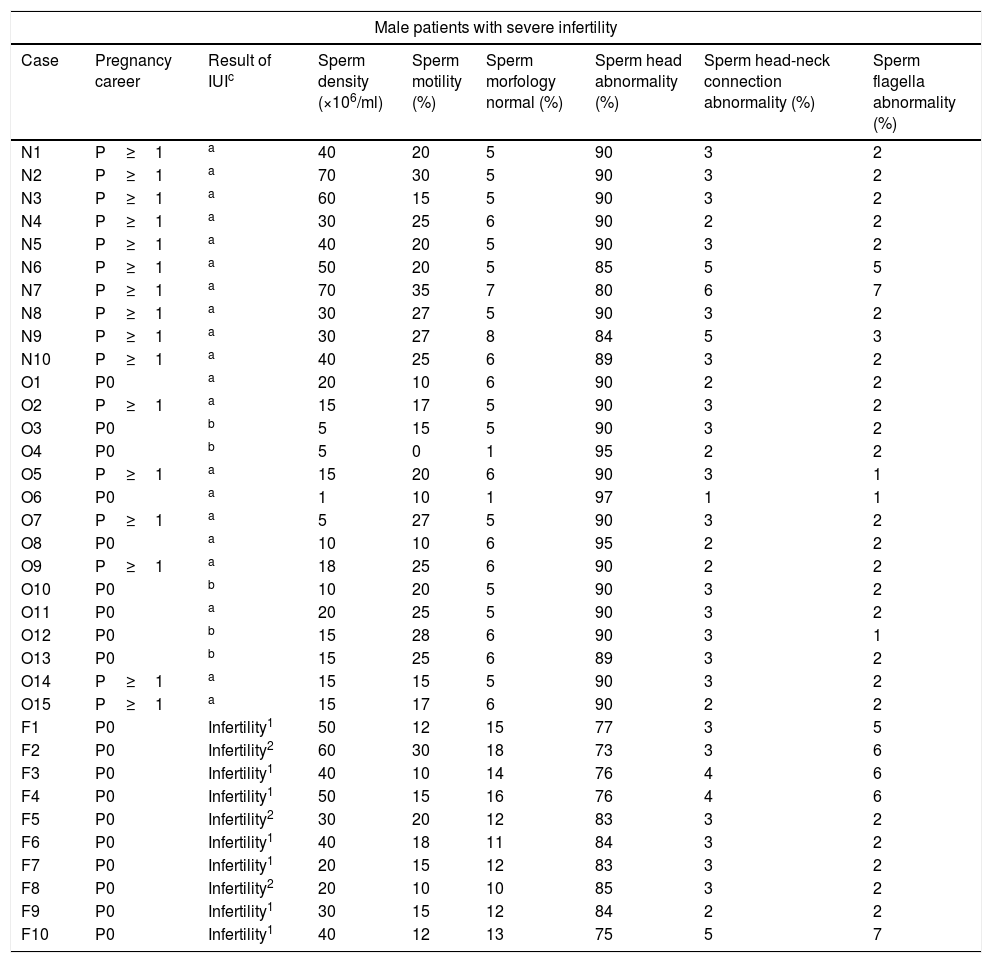
In this case, we used human sperm to form our research to determine the presence and location of fertilin β. We examined the sperm of 30 male donors who have normal as controls, oligozoospermia, and unexplained infertility as the clinically indication for IUI. Their pregnancy career, sperm density, sperm motility, and the result of IUI (Intra Uterine Insemination) are shown in Table 1. The expression of fertilin β was revealed by comparing each group.
Normal, oligozoospermia and unexplained infertility diagnosed donors’ pregnancy career, sperm density, sperm motility, and the result of IUI.
| Male patients with severe infertility | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case | Pregnancy career | Result of IUIc | Sperm density (×106/ml) | Sperm motility (%) | Sperm morfology normal (%) | Sperm head abnormality (%) | Sperm head-neck connection abnormality (%) | Sperm flagella abnormality (%) |
| N1 | P≥1 | a | 40 | 20 | 5 | 90 | 3 | 2 |
| N2 | P≥1 | a | 70 | 30 | 5 | 90 | 3 | 2 |
| N3 | P≥1 | a | 60 | 15 | 5 | 90 | 3 | 2 |
| N4 | P≥1 | a | 30 | 25 | 6 | 90 | 2 | 2 |
| N5 | P≥1 | a | 40 | 20 | 5 | 90 | 3 | 2 |
| N6 | P≥1 | a | 50 | 20 | 5 | 85 | 5 | 5 |
| N7 | P≥1 | a | 70 | 35 | 7 | 80 | 6 | 7 |
| N8 | P≥1 | a | 30 | 27 | 5 | 90 | 3 | 2 |
| N9 | P≥1 | a | 30 | 27 | 8 | 84 | 5 | 3 |
| N10 | P≥1 | a | 40 | 25 | 6 | 89 | 3 | 2 |
| O1 | P0 | a | 20 | 10 | 6 | 90 | 2 | 2 |
| O2 | P≥1 | a | 15 | 17 | 5 | 90 | 3 | 2 |
| O3 | P0 | b | 5 | 15 | 5 | 90 | 3 | 2 |
| O4 | P0 | b | 5 | 0 | 1 | 95 | 2 | 2 |
| O5 | P≥1 | a | 15 | 20 | 6 | 90 | 3 | 1 |
| O6 | P0 | a | 1 | 10 | 1 | 97 | 1 | 1 |
| O7 | P≥1 | a | 5 | 27 | 5 | 90 | 3 | 2 |
| O8 | P0 | a | 10 | 10 | 6 | 95 | 2 | 2 |
| O9 | P≥1 | a | 18 | 25 | 6 | 90 | 2 | 2 |
| O10 | P0 | b | 10 | 20 | 5 | 90 | 3 | 2 |
| O11 | P0 | a | 20 | 25 | 5 | 90 | 3 | 2 |
| O12 | P0 | b | 15 | 28 | 6 | 90 | 3 | 1 |
| O13 | P0 | b | 15 | 25 | 6 | 89 | 3 | 2 |
| O14 | P≥1 | a | 15 | 15 | 5 | 90 | 3 | 2 |
| O15 | P≥1 | a | 15 | 17 | 6 | 90 | 2 | 2 |
| F1 | P0 | Infertility1 | 50 | 12 | 15 | 77 | 3 | 5 |
| F2 | P0 | Infertility2 | 60 | 30 | 18 | 73 | 3 | 6 |
| F3 | P0 | Infertility1 | 40 | 10 | 14 | 76 | 4 | 6 |
| F4 | P0 | Infertility1 | 50 | 15 | 16 | 76 | 4 | 6 |
| F5 | P0 | Infertility2 | 30 | 20 | 12 | 83 | 3 | 2 |
| F6 | P0 | Infertility1 | 40 | 18 | 11 | 84 | 3 | 2 |
| F7 | P0 | Infertility1 | 20 | 15 | 12 | 83 | 3 | 2 |
| F8 | P0 | Infertility2 | 20 | 10 | 10 | 85 | 3 | 2 |
| F9 | P0 | Infertility1 | 30 | 15 | 12 | 84 | 2 | 2 |
| F10 | P0 | Infertility1 | 40 | 12 | 13 | 75 | 5 | 7 |
The number of insemination is indicated in superscript for unknown infertility patients.
IUI: intra uterine insemination.
This study was performed by the guidelines of the Ethics Committee of Istanbul University Cerrahpaşa Faculty of Medicine Clinical Research with the approval number of B.30.2. İST.0.30.90.00/4580.
Collection of human sperm and formation of study groupsThe study was conducted with three groups. Semen samples were taken from donors who applied to Istanbul University Cerrahpaşa Faculty of Medicine, Department of Urology-Infertility were used. General information about the donors has been collected. Insemination was only done after previous examination found no indications of tubular damage. All patients were ovulating, either as part of their natural cycle, or following low-dose FSH stimulation or stimulation with clomifene. Ovulation was triggered by the administration of human chorionic gonadotropin (hCG). Inclusion criteria for this prospective analysis were a maximum patient age of 38 years, limited sperm motility of the patient's partner and stimulation with FSH. A total sperm count of at least 10million/ml and a motility of at least 35% were required for insemination. To examine the relationship between spermatozoon fertility and spermatozoon surface proteins, paid attention that the duration of sexual abstinence of the donors whose semen will be taken is 3–5 days. After the spermiogram and morphological tests of the donors who gave samples to the andrology laboratory, the rest of the sperm suitable for our experimental group from the samples with determined characteristics were used as the experimental materials for this study. For semen analysis, freshly ejaculated semen was collected and analyzed for motility, concentration, viability, and morphology as previously described by Kaleli et al.16; morphology was evaluated according to strict Kruger criteria. The Diff–Quik method was used to determine the morphology of the sperm.
Preparation of spermatozoaThe sperm was mixed with 1:1 SupraSperm (Origio-SupraSperm-100 100% solution) wash medium and centrifuged at 1800×g for 15min. Discarded the supernatant and added to 80% percoll as Sutovsky17 to the pellet. Mixed and centrifuged at 1800×g for 10min. The supernatant was discarded, and the 1:1 washing medium SupraSperm was added to the pellet and mixed. Then centrifuge at 1500×g for 10min. Discarded the supernatant and centrifuged the pellet 3 times in 1:1 PBS (1500×g, 10min, 25°C). Then the sperm was suspended in 1:2 PBS, took on slides, and let it dry. Then fixed in cold methanol (−20°C).
Indirect immunofluorescence stainingFor fertilin β, immunofluorescence staining was performed as explained by Nagdas S.K. et al.18 Slides were blocked with 10% goat serum blocking buffer (Santa Cruz Biotechnology) and incubated with Fertilin β (sc25131) antibody (1:100 dilutions with PBS supplemented 1% blocking buffer). Donkey anti-goat IgG-FITC antibody (Santa Cruz Biotechnology, sc-2024) was used as the secondary antibody at 1:200 dilutions with PBS. Applied mounting agent and coverslip. Stored the slides in a dark and humid room. The negative control was the same except that the primary antibody is missing. Finally, LSCM (Carl Zeiss LSM-510, Jena, Germany) was used to evaluate fluorescently stained sperm samples at a magnification of 100×. The emission wavelength was 536/40nm and the excitation wavelength was 482/35nm (wavelength/width). Used the attached GraphPad Prism 5 software to process the graphics. The indirect immunofluorescence analysis was repeated at least three times.
Scales according to the distribution of fluorescence labelWe selected four sperm samples scale of 0 to +++ according to the distribution of the fluorescence label by visual scoring. 0, negative; +, weak; ++ moderator, +++ strong reaction by fluorescence for sperm surface antibody. As seen in Fig. 1, 0 denoted no staining, while + indicated fluorescence in up to approximately one-quarter; ++ indicated fluorescence in up to approximately half; +++ indicated fluorescence in up to approximately three-quarter of the spermatozoa heads from the top to the equatorial plane of the cells. The score of +++ indicated staining was present on almost all surfaces of the acrosomal membrane and equatorial plane of the cells. Visual scoring was done by using Zen 2.3 Blue Edition software, fluorescence microscope. In a blinded manner, we tested each sample 15 times (5 tests each on three separate days).
Representative microscope images from the localization of fertilin β on human spermatozoa by indirect immunofluorescence analysis. A. arrow 0, negative; arrowhead +, weak, B. ++ moderator, C. +++ strong reaction, D. Negative control (performed with primary antibody deficiency). Magnification 100×.
Sperm showed fluorescence from the primary and secondary antibody complexes and was considered positive for the fertilin β antibody. Image J software was used to quantify the level of fluorescence. This was performed by circling the head of the cells for each group. The fluorescence intensity obtained from the control and +, ++, +++ samples are plotted as a graph to measure the mean fluorescence intensity (MFI). We performed 6 tests on each sample in a blinded manner (3 tests were performed in two days). Fluorescence images were acquired with a single 300ms exposure. Using Zen 2.3 Blue Edition software, measured the fluorescence intensity of each cell in a 60×60 pixel box, and took the average of at least 100cells per cover glass (n=6).19
ReproducibilityTo evaluate the reproducibility of the test, the scales according to the distribution of fluorescence label results were correlated with the measurement (CTCF) based on fluorescence intensity. Used the following formula to calculate the corrected total cell fluorescence (CTCF).20
Statistical analysesStatistical analysis was performed using GraphPad (Prism 5) software. MFI values for fertilin β in control and samples were evaluated by one-way analysis of variance (ANOVA) Multiple comparisons were made using Tukey's procedure. P<0.05 was considered statistically significant. Associations between individual sperm scale score and fertilin β were assessed using the Pearson correlation test (Rp) because CTCF data were normally distributed. Fluorescence intensity assessment of fertilin β and sperm scale scores are presented as the mean±SD. Differences were considered significant at two-tailed P<0.05.
ResultsIndirect immunofluorescence stainingWe compared the changes in the locations of fertilin β on sperm of 30 male donors who have normal, oligozoospermia, and unexplained infertility as the clinically indication for IUI by using the “indirect immunofluorescence technique” staining protocol. Indirect immunofluorescence technique staining is a reliable and cheap technique that can be used to detect proteins in cell experiments or cell experiments.21 The comparison is based on the distribution of the fluorescence label by the fertilin β antibody in each group and the fluorescence intensity (described in material and method).
Our findings demonstrated that the Fertilin β had different markings in density and distribution according to the groups. Representatively the sample of fluorescence-labeled normal spermatogenic parameter diagnosed group is shown in Fig. 2. Although the distribution was different, the samples taken from all groups were very bright and dense marking was observed of Fertilin β. The most intensive marking was seen in the samples taken from the normal and oligospermia diagnosed group.
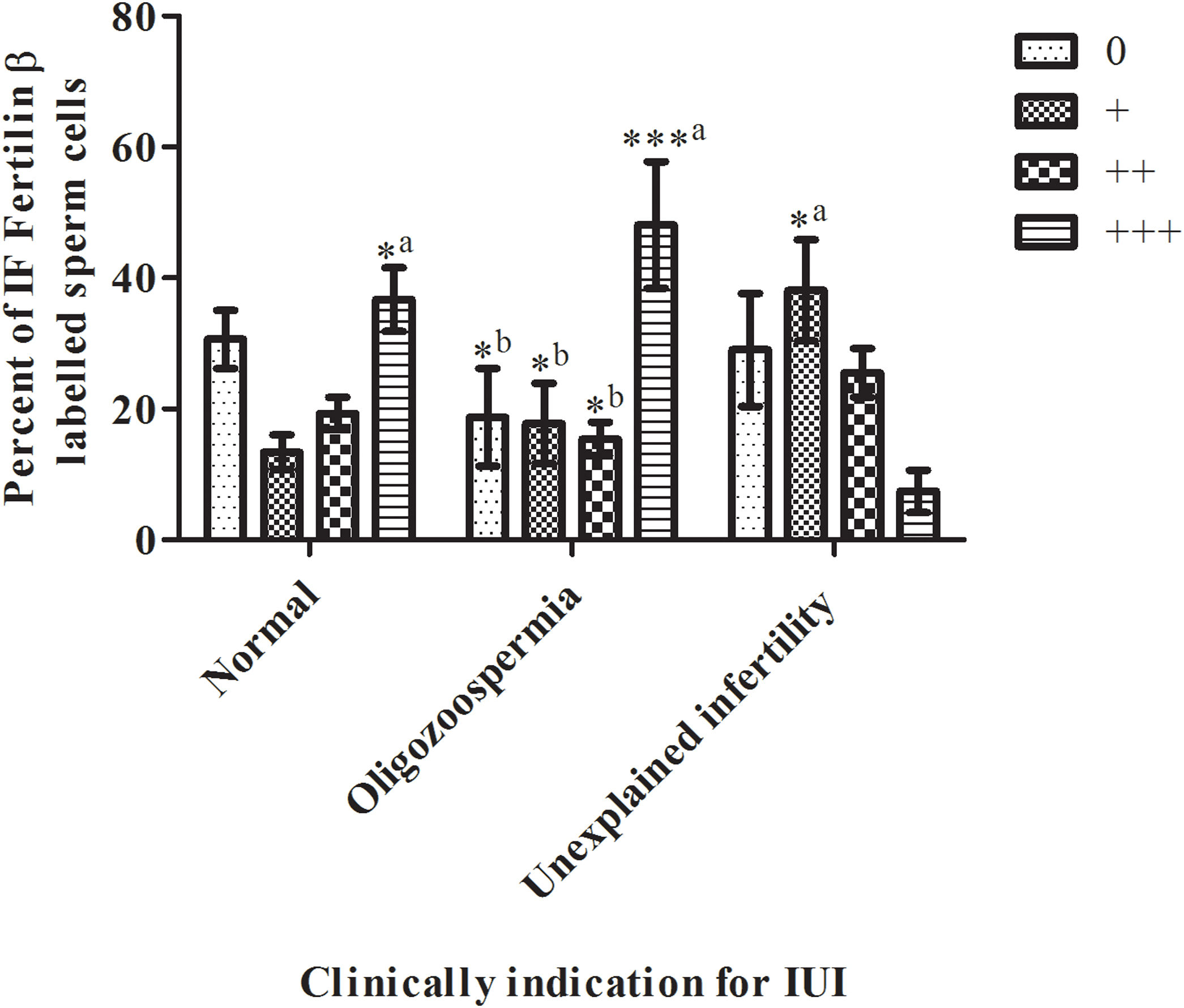
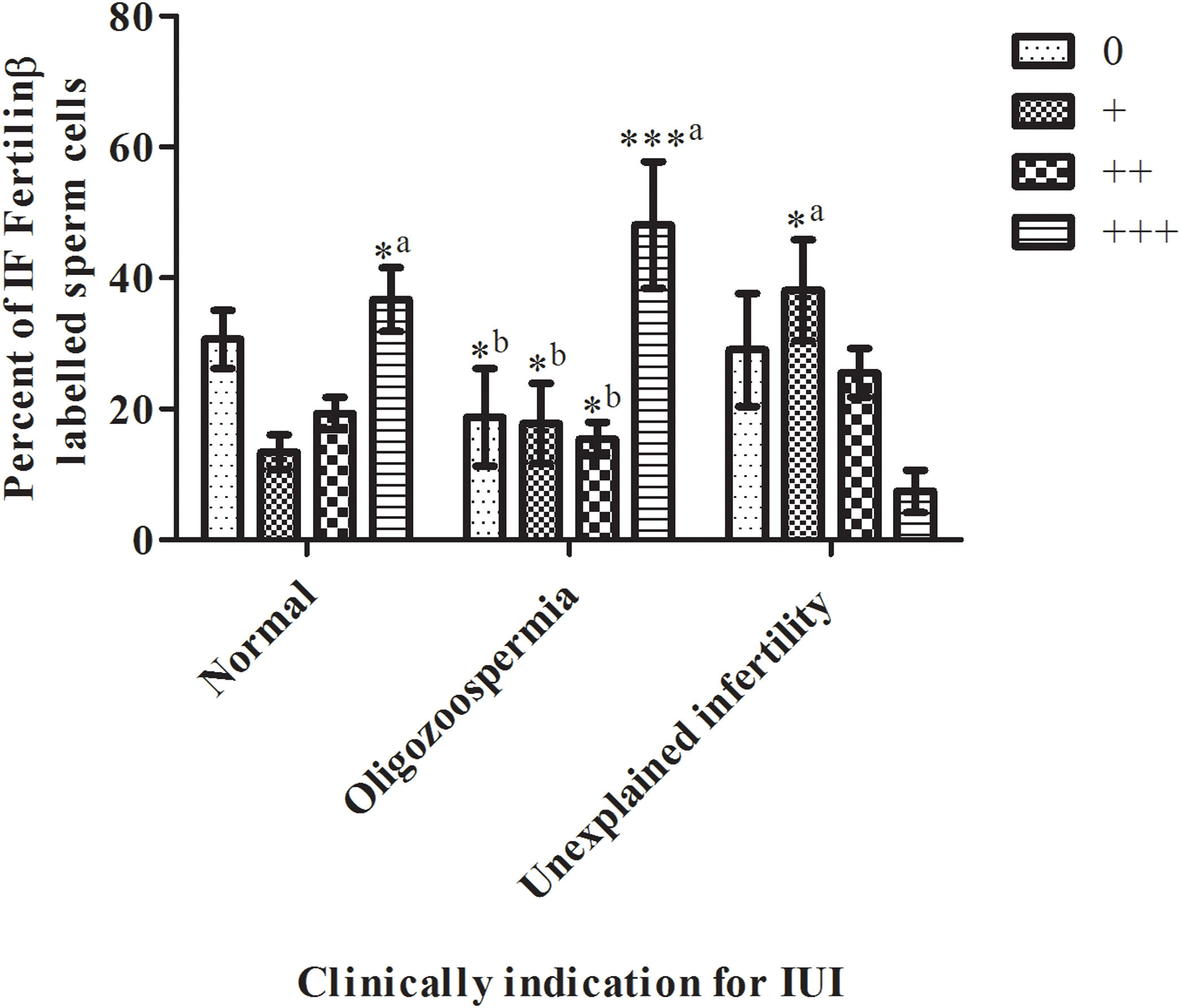
Scales according to the distribution of fluorescence labelWe tried to reliably generate the sperm antibody test rating in four categories as a scale of 0 to 3 based on fluorescence distribution (R2=0.2967, P<0.0001). As shown in Fig. 3 on the normal diagnosed group, there was no statistical significance between scale categories by the percent of immunofluorescence (IF) fertilin β labeled sperm cells (normal diagnosed group; the scale of 0 by 30.6±14.11%, the scale of + by 13.4±8.32%, scale of ++ by 19.3±7.85%, scale of +++ by 36.7±15.44%, P<0.05). All scale categories of the oligospermia group were compared with each other. The oligospermia diagnosed group's +++ scale category percent was significantly high then the 0,+, and ++ scale categories cells (oligospermia diagnosed group; scale of 0 by 18.7±23.59%, scale of + by 17.8±19.3%, scale of ++ by 15.4±8.03%, scale of +++ by 48.1±30.61%, ±SD, P<0.0001). Then, the scale categories of unexplained infertility diagnosed group were compared between each other. Only the scale of + was significantly high then scale of +++ (unexplained infertility diagnosed group; scale of 0 by 29±27.25%, scale of + by 38.1±24.42%, scale of ++ by 25.5±11.73%, scale of +++ by 7.4±10.32%, P<0.0001). In contrast, as shown in Fig. 3 unexplained infertility diagnosed group's scale of +++ percent was significantly low than normal, and oligospermia diagnosed group (P<0.0001).
Scales of 0 to +++ immunofluorescence (IF) distribution results in sperm samples of donors diagnosed with normal, oligozoospermia, and unexplained infertility (IUI: intrauterine insemination). The sperm scale scores are presented as the mean±SD. a: of the group diagnosed with oligospermia; b: indicates a significant difference compared to the +++ percentage scale of IF fertilin labeled sperm cells of the group diagnosed with infertility (P<0.0001). *, *** P<0.0001 indicates a significant difference between the compared groups of IF fertilin β labeled sperm cells. n=10 (the number of samples in each group).
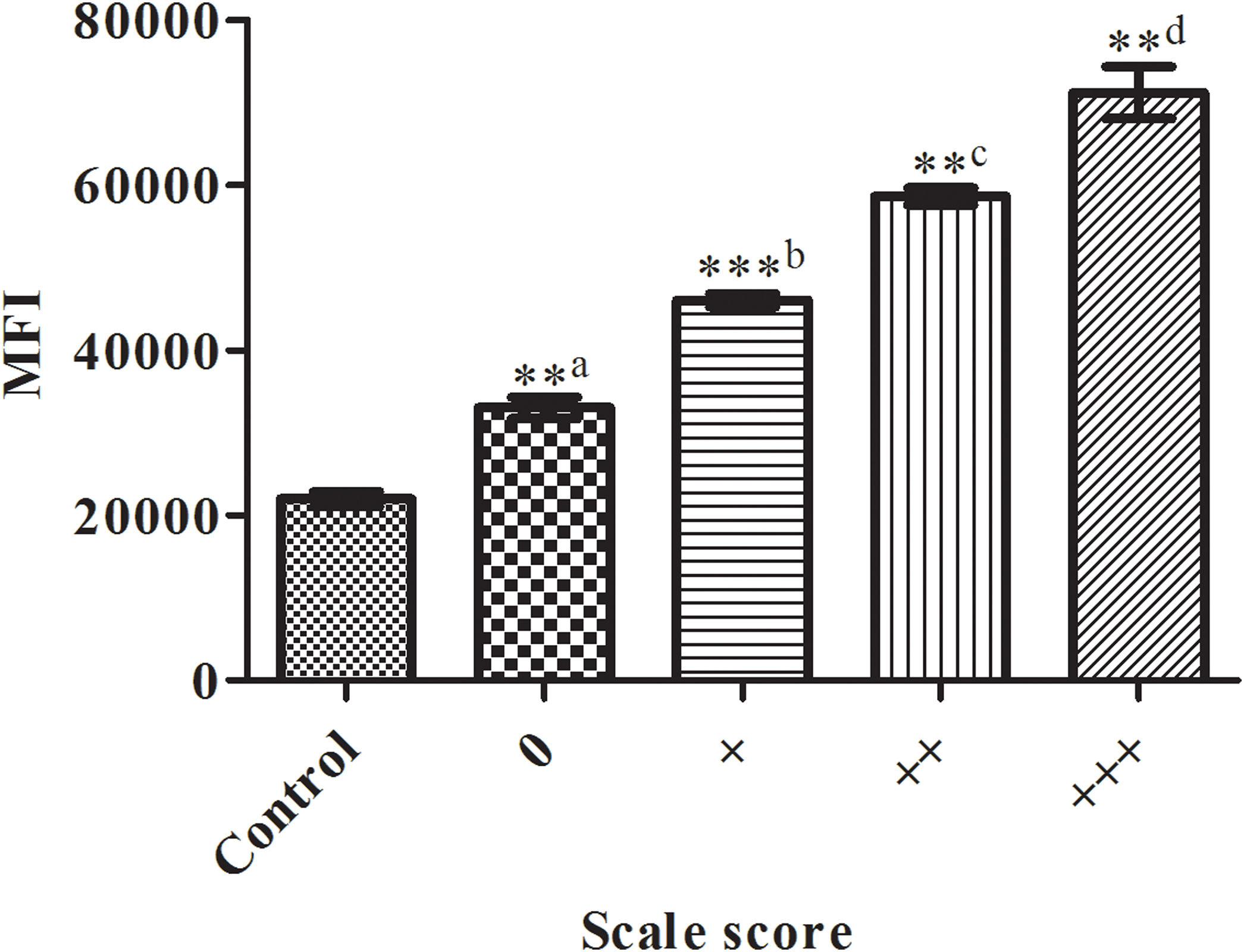
Next, we studied the changes in the fluorescence intensity of labeled sperm cells. Comparison of the mean fluorescence intensities (MFI) of spermatozoa with the sperm of donors according to the distribution of the fluorescence label by visual scoring from a scale of 0 to +++ in sperm samples is shown in Fig. 4. There were significant differences between the related mean fluorescence intensities (P<0.0001). For fertilin β assessment, the MFI was significantly (P<0.0001) lower in the control, as unstained spermatozoa, incubated with the secondary antibody (sc-2024) only (MFI by 21,942±1522, R2=0.9908), regardless of the primary antibody used.
Comparison of the mean fluorescence intensities (MFI) of spermatozoa positively labeled using a commercial antibody against fertilin β for sperm of donors according to the distribution of the fluorescence label by visual scoring from a scale of 0 to +++ in sperm samples. The bars represent the average±standard deviation of three independent experiments. a: the control group; b: the 0 group; c: the + group; d: indicates significant difference compared with the MFI of the ++ group (P<0.0001). *, **, *** P<0.0001 indicates a significant difference level compared within groups of sperm.
Thus the signification, we compared the difference in fluorescently labeled cells using the immunofluorescence staining protocol as explained above. Approximately 100 spermatozoa for fertilin β were analyzed using ImageJ (http://imagej.nih.gov/ij/, accessed 11 November 2020) and the regions of interest tool. Integrated density was calculated for the mean level of fluorescence and the area of each sperm head. Fertilin β expression exhibited a wide range of values, and there was considerable variation in MFI values and the percentage of positive scale scored (+, ++, +++) spermatozoa among clinically diagnosed groups. As shown in Table 2, our findings demonstrated that groups showed different fluorescence intensity for fertilin β (R2=0.3121).
The percentage of sperm cells with different degrees of fluorescence intensities for fertilin β (MFI: mean fluorescence intensities) (×103 is arbitrary units).
| MFI≥25×103 | MFI≥40×103 | MFI≥55×103 | MFI≥65×103 | |
|---|---|---|---|---|
| Normal | 29.8±11.5 | 14±7.775 | 19.4±7.56 | 36.8±18.43*a |
| Oligozoospermia | 19.2±23.56*b | 17.8±19.36*b | 15±7.5*b | 48±30.18***a |
| Unexplained infertility | 31±27.68 | 38.1±23.35*a | 25.5±12.05 | 5.4±5.72 |
Indicates a significant difference compared with unexplained infertility diagnosed group's scale of MFI≥65×103 percent of IF fertilinβ labeled sperm cells (P<0.0001). *, *** P<0.0001 indicates a significant difference between the compared groups of IF fertilin β labeled sperm cells (n=10, the number of samples in each group).
On the other hand, we detected rich fluorescence intensity in the unexplained infertility group but the lowest percentage of %fertilin β as mean fluorescence intensities (MFI) results (MFI by 5.4±5, P<0.0001).
ReproducibilityThe corrected total cell fluorescence (CTCF) for the Fertilin β image was calculated, normalized to background fluorescence,22 and then calculated as a percentage of the CTCF for the corresponding circled head of the sperm (Gonzalez-Castro et al., 2019). The scales according to the distribution of fluorescence label results were highly correlated with the measurement (CTCF) based on fluorescence intensity (Rp=0.9972, P<0.05). We suggest that the relationship between infertility and fertilin β protein may be due to the distribution of this protein on the sperm surface.
DiscussionThe addition and removal of various proteins during epididymal maturation and ejaculation play an important role in sperm capacitation and egg fertilization. Ellis23 reported that the location of Fertilin β on the sperm surface differs in species. Although Fertilin β is located in the anterior region of rat sperm, it is only detected in the posterior region of the equatorial region of guinea pig sperm, while it is detected in the equatorial region of mouse sperm.10 Ding et al.15 found that Fertilin β localization on human sperm was changed due to capacitation and acrosome reaction. During capacitation, fertilin β is restricted to the anterior. Then, during the acrosome reaction, the expression and localization of fertilin β changed greatly on the anterior and finally confined to the outside of the posterior head.
Fertilin−/− mice decreased fertility significantly, but these mice were healthy in terms of vital functions.1,8 As a result of functional analysis of fertilin−/− mice spermatozoa; it has been demonstrated that the inability to cross the oviduct, inadequate binding to zona pellucida, a strong reduction in binding (adhesion) to the oocyte plasma membrane were observed. For this reason, it is stated that fertilin has critical functions in several different steps in the spermatozoon–oocyte encounter.1,8 It is claimed that fertilinα is a hydrophobic fusion peptide, but it has been shown in experiments that this structure is not necessary for spermatozoa–oocyte fusion. Despite the Fertilinα−/− spermatozoa's deficiency, spermatozoon–oocyte fusion was achieved.24 The role of fertilin in spermatozoon–oocyte fusion was tested using peptide analogs that fit potential integrin binding sites of the fertilin β subunit. It has been reported that fertilineβ can bind to the oocyte plasma membrane with peptide analogs containing TDE series in the disintegrin region and spermatozoon oocyte fusion is strongly inhibited. These results have shown that the disintegrin region of fertilin is bound with the oocyte plasma membrane and is required for membrane fusion.6 Nonetheless, the expression and distribution of fertilin β protein in human sperm has not been carefully examined.
In the above information, we want to compare the possible differences of Fertilin β on donors who have normal, oligozoospermia, and unexplained infertility as the clinically indication for IUI by using the “indirect immunofluorescence technique” staining assay. Our findings indicate that our research groups’ Fertilin β has different signs in terms of density and distribution. Immune marking is very strong and can be distinguished easily according to the marking type. We tried to reliably generate 4 categories of sperm antibody test levels based on the fluorescence distribution, ranging from 0 to 3. Although our results are related to visual scored samples based on fluorescence intensity. It was determined that the percentage of fertilin β staining at +++ degree in spermatozoa of individuals with normal spermatogenic parameters was higher than the group diagnosed with unexplained infertility (P<0.0001). Therefore, we think the +++ dyeing degree is ideal. A large number of studies have been conducted on the causes of failed fertilization in men with oligozoospermia and unexplained infertility. However, to our knowledge, fertilin β deficiency in these patient groups has not been investigated as a possible factor for fertilization failure. We show for the first time that there is a reduction in the distribution of fertilin β protein in unexplained individuals compared to fertile controls. It is difficult to directly diagnose infertile individuals because of fertilin β deficiency. The reasons for this are the low number of individuals in our experimental groups, the fact that the samples of those who have been diagnosed with unexplained infertility are included in the group, and some of them have not done the second repeat in insemination. However, due to the lack of molecular evidence, we cannot gain insight into the underlying molecular mechanisms and pathways. We cannot provide mechanical evidence to support our descriptive findings. To diagnose infertility due to β deficiency of the fertile, we need to take samples from more unsuccessful individuals who have undergone 3rd insemination and conduct more comprehensive experiments. In these comprehensive experiments, we need to examine the deficiencies of not only fertilin β but also other spermatozoa surface proteins. In this study, we presented reproducible data to support our observations on the state of the Fertilin β in the donors who have normal, oligozoospermia, and unexplained infertility as the clinically indication for IUI. There was a statistically significant difference in scale +++ between samples in spermatozoa of individuals with normal spermatogenic parameters and the group diagnosed with unexplained infertility. Considering the difference in fertilin β distribution of unexplained infertility diagnosed individuals, we suggest that fertilin β can be used as fertility markers. We know that the fusion mechanism of oocyte spermatozoa should be studied using advanced techniques in terms of surface proteins. As a candidate for these studies, we hope that the use of fertilin β as a control for its distribution can increase the success in intrauterine insemination applications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by the Scientific Research Projects Coordination Unit of Istanbul University. Project number: 21093
Conflict of interestNone.
We thank Prof. Dr. Hamdi Özkara for their help with sperm collection. Special thanks to Teresa Taşkıran for their help on Spanish.