Microorganisms have been widely studied as biological control agents of parasites of medical and veterinary importance. Coprophagous arthropods, bacteria and fungi are among the different organisms evaluated as potential biological control agents. Nematophagous fungi capture and digest the free forms of nematodes in the soil. Due to its zoonotic potential, Toxocara canis have been brought to the attention of researchers.
AimsThe aim of the present study was to determine whether the administration of embryonated T. canis eggs exposed to the nematophagous fungus Trichoderma virens reduces parasite infection in experimental animals.
MethodsEmbryonated T. canis eggs were exposed to T. virens mycelium for 15 days at 25°C. Subsequently, 100 fungus-exposed eggs were orally administered to 20 Swiss mice. As a positive control, another 20 mice received 100 embryonated eggs that were not exposed to the fungus. After 48h, the animals were killed, and heart, lungs and liver were harvested for the recovery of larvae.
ResultsThe organs of the animals that received embryonated T. canis eggs exposed to the fungus showed a lower mean larval recovery when compared with the animals that received embryonated eggs without fungus exposure (p<0.05).
ConclusionsThe exposure of T. canis eggs to T. virens reduces the experimental infection, demonstrating the potential of this nematophagous fungus as a biocontrol agent.
Algunos microorganismos han sido ampliamente estudiados como agentes de control biológico de parásitos de importancia médica y veterinaria. Los artrópodos coprófagos, las bacterias y los hongos están entre los diferentes organismos que sirven como agentes para el control con potencial biológico. Los hongos nematófagos capturan y digieren las formas libres de nematodos en el suelo. Toxocara canis, debido a su potencial zoonótico, ha captado la atención de los investigadores en estos estudios.
ObjetivosEl objetivo del presente estudio fue evaluar si la exposición de huevos embrionados de T. canis al hongo nematófago Trichoderma virens reduce la infección parasítica en un modelo experimental animal.
MétodosLos huevos embrionados de T. canis fueron expuestos al micelio de T. virens durante 15días a 25°C. Posteriormente, 100huevos de T. canis expuestos al hongo fueron administrados por vía oral a un grupo de 20ratones Swiss. Como control positivo se usó otro grupo de 20ratones que recibieron 100huevos embrionados no expuestos al hongo. Después de 48h, los animales fueron sacrificados y corazón, pulmones e hígado fueron extraídos para la posterior obtención de larvas.
ResultadosEl número de larvas obtenidas en los diferentes órganos fue menor en el grupo de animales que fueron infectados con los huevos embrionados de T. canis expuestos al hongo en comparación con el grupo de animales que recibieron huevos embrionados sin la exposición al hongo (p<0,05).
ConclusionesLa exposición de los huevos de T. canis a T. virens reduce la infección experimental, lo que demuestra el potencial de este hongo nematófago como agente para el control biológico.
Toxocariasis is a disease caused by the nematode Toxocara canis and different clinical forms have been observed, including visceral larva migrans, ocular larva migrans and occult or subclinical toxocariasis.18 Infections in paratenic hosts primarily occur through the accidental ingestion of the embryonated eggs of the parasite, most frequently affecting children of up to five years of age due to increased contact with contaminated soil,12 geophagy habit21 and onychophagy.2
The use of microorganisms as biological agents acting on eggs and larvae of nematodes has been widely used in recent years as an alternative control method for nematodes. Thus, the nematophagous fungi are the microorganisms most studied for this purpose. These fungi live in the soil organic matter, develop parasitic or predatory relationships with nematodes, and are classified as ovicides, endoparasites and predators.14
Due to the problems caused by chemical control, mainly the prejudicial effects on human health and environment, the development of alternative control methods has become increasingly important.21 Thus, biological control is a natural tool and an ecological alternative for the control of parasites of medical and veterinary importance. According to Araújo et al.,4 biological control reduces infections caused by gastrointestinal helminth parasites, reflecting the use of living organisms as natural antagonists in the environment. Coprophagous arthropods, bacteria and fungi are among the different organisms evaluated as potential biological control agents. Nematophagous fungi capture and digest the free forms of nematodes in the soil.20
Among several genera of fungi evaluated for the biological control of gastrointestinal nematodes, Pochonia chlamydosporia and Paecilomyces lilacinus have exhibited ovicidal activity on T. canis.6,11 Nevertheless, the genus Trichoderma has also shown ovicidal activity in vitro in T. canis eggs.7,8,16 Additionally, this genus has been extensively studied and promising results, in vitro and in vivo, have been observed in the biological control of plant parasitic nematodes.10,22
The aim of the present study was to verify whether the exposition of embryonated T. canis eggs to the nematophagous fungus T. virens reduces the infection by this parasite in experimental animals.
Materials and methodsFungal isolateThe fungal isolate used in the present study was obtained from the Mycology Laboratory of the Department of Microbiology and Parasitology at Universidade Federal de Pelotas (UFPel), Brazil. This fungus is an autochthonous isolate previously identified as T. virens based on morphological and molecular characteristics.
Obtention of T. canis eggsT. canis eggs were obtained through hysterectomies performed on parasite females according to Maia Filho et al.16 Subsequently, the eggs were maintained in a formalin solution (2%) containing streptomycin sulfate (0.05%) and chloramphenicol (0.01%). The eggs were embryonated after incubation at 25°C/15 days with daily aeration.
Exposition of T. canis eggs to T. virensOne 4mm-disk of fungal culture was transferred to Erlenmeyer flask containing 150ml of modified minimal culture medium [NH4NO3 (0.4g/l); MgSO4·7H2O (0.12g/l); Na2HPO4·7H2O (3.18g/l), KH2PO4 (0.26g/l), and yeast extract (0.3g/l)]. A total of 5 flasks were inoculated with T. virens and incubated at 25°C under gentle manual stirring twice a day during 15 days. On the 15th day, 500 embryonated T. canis eggs were added to each flask with the T. virens mycelium, and returned to incubation in the same conditions for further 15 days. Additionally, in the same day, 500 embryonated T. canis eggs were added to 5 flasks containing 150ml of modified minimal culture medium, without T. virens, and were incubated in the same conditions previously described. Subsequently, the culture medium was centrifuged at 2000rpm/5min. The supernatant was discarded, and the pellet was suspended in 1ml of 0.01M phosphate buffer solution, pH 7.4 (PBS). To count and evaluate the eggs and viability, 10μl of this solution were placed onto a slide, coverslipped and examined under a 40× objective. The eggs were considered viable when there was larvae inside, as described by Rey.19
Inoculation of experimental animalsForty Swiss mice females (Mus musculus) of 4 weeks old were acquired from the animal facility at UFPel. The animals were maintained in appropriate cages at 25°C, with water and food ad libitum. The animals were divided into two groups of 20 animals each: in group 1 (control) animals were infected by gavage feeding with 0.2ml PBS containing 100 T. canis eggs, and in group 2 (fungus-exposed eggs) mice were infected by gavage feeding with 0.2ml PBS containing 100 T. canis eggs exposed to T. virens. Forty-eight hours after the infection, the mice were killed by cervical dislocation. The liver, lungs and heart were harvested to recover the larvae. The organs were macerated and digested overnight in 50ml of 1% hydrochloric acid solution and 1% pepsin at 37°C with constant shaking at 120rpm. Subsequently, the digested organs were centrifuged at 2000rpm/5min. The supernatant was discarded, and the total sediment of each organ was evaluated on glass slides using optical microscopy (10× and 40× lens) to count the larvae.24
All animal procedures were approved by the Ethics Committee on Animal Experimentation/UFPel.
Statistical analysisThe data for larval counting from the digested organs in both groups (group 1 and group 2) were submitted to a normality test using Shapiro–Wilk, Kolmogorov–Smirnov, Cramer–von Mises and Anderson–Darling tests. As the response variable did not show normality, data were subjected to the non-parametric chi-square test. In addition, data were submitted to analysis of measures of position and frequency distribution. The analysis was performed using SAS statistical software (version 9.4), with a 5% significance level.
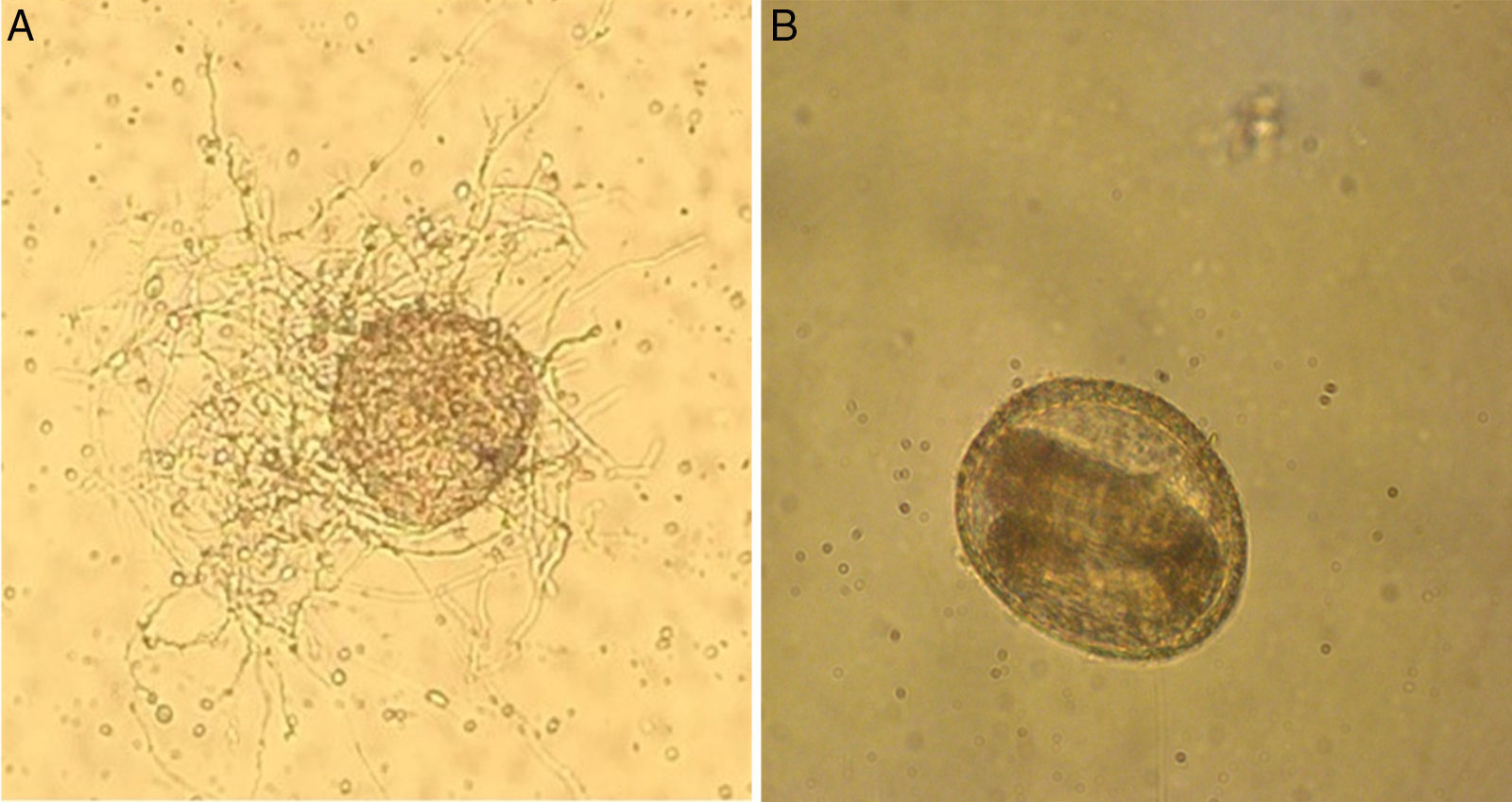
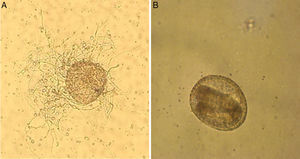
ResultsThe microscopic analyses of the eggs without fungal exposure (used in the control group) showed that these eggs were embryonated and maintained their structural integrity. T. canis eggs incubated with T. virens were also embryonated but showed colonization with fungal hyphae on the surface (Fig. 1).
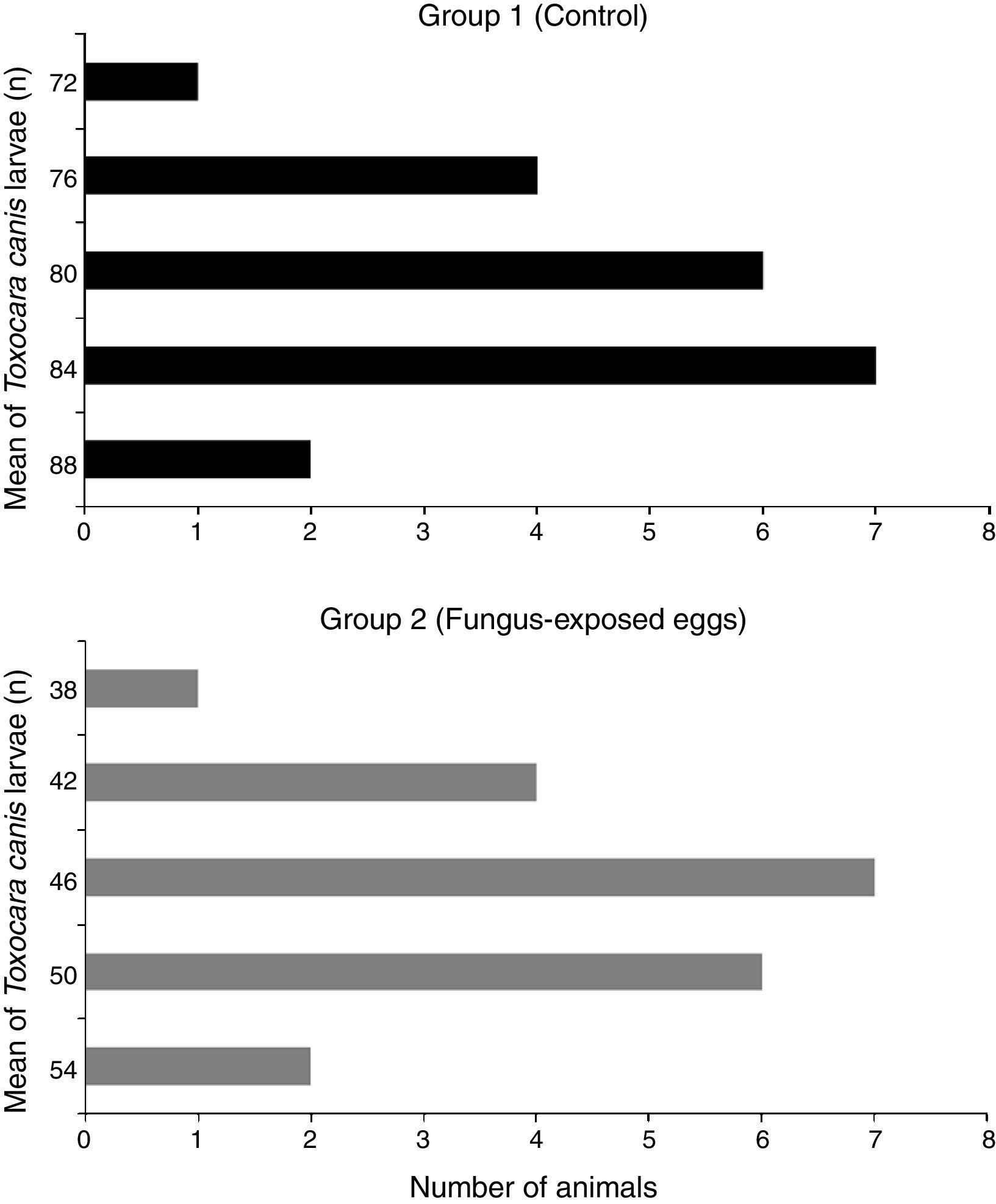
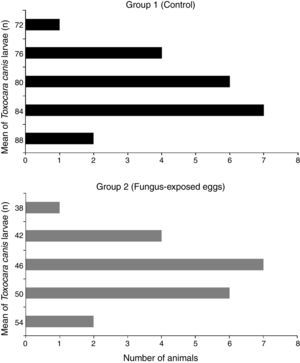
The larvae were recovered from different organs (heart, lungs and liver) in both groups. The organs of the animals that received embryonated T. canis eggs exposed to the fungus showed a lower mean larval recovery (46.3) (p<0.05) compared with the organs of animals infected with embryonated eggs not exposed to the fungus (80.6). Moreover, the reduction of larvae in the group that received embryonated T. canis eggs exposed to the fungus was 57.4%. The frequency distribution analysis demonstrated difference between the groups evaluated (p<0.05). It was observed that 100% of the animals’ organs in the control group (group 1) showed larvae count above 70. On the other hand, in the fungus-exposed eggs group (group 2), 85% of the organs evaluated showed larvae count below 50. The median values for group 1 and 2 were 80.5 and 46, respectively (Fig. 2).
DiscussionNematophagous fungi have been widely used for biological control, reflecting an ability to capture and infect nematodes. In vitro studies evaluating the use of ovicidal nematophagous fungi including Pochonia chlamydosporia, Paecilomyces lilacinus and Fusarium pallidoroseum suggest that the use of these biocontrol agents in T. canis is an ecological and viable alternative, representing a natural tool for biocontrol.3,6–8,11,13 In contrast with previous in vitro studies, the present study evaluated the recovery of T. canis larvae from mice experimentally infected with embryonated T. canis eggs previously exposed to the nematophagous fungi T. virens. The recovery of larvae from the tissues of mice infected with T. canis eggs previously exposed to the fungus T. virens was significantly lower (p<0.05) than that in the animals infected with embryonated eggs without fungus exposure. These results suggest that the eggs colonized by the fungus could exhibit reduced viability due to structural damages on the eggs and/or damage on larvae development. According to Lysek,15 the ovicidal activity of nematophagous fungi occurs by the destruction of the egg's layers, leading to the exposure and death of the embryo or by the fungal damage on the embryo's development avoiding a successful infection.
Trichoderma has been used as bionematicide for the control of plant-parasitic nematodes, particularly of the Meloidogyne genus, with excellent results, both in vitro and in vivo.1,10,17,22 Ciarmela et al.7,8 and Maia Filho et al.16 also demonstrated the ovicidal activity in vitro of these fungi against T. canis. Elgorban et al.9 suggested that the probable mechanisms of Trichoderma spp. in nematode control involved the direct parasitism of eggs and larvae, and also increased proteolytic and chitinolytic enzyme activity. Additionally, Srivastava et al.23 reported that the synthesis of proteases, chitinases, glucanases, tubulin, cell adhesion proteins, as well as stress tolerance genes are important mechanisms involved in the biological control effect by Trichoderma. We believe that similar biocontrol mechanisms are occurring on T. canis eggs; however, additional studies are needed to verify this hypothesis.
The present study is pioneering since there are no reports evaluating the ovicidal activity of nematophagous fungi on T. canis eggs in animal models. On the other hand, only studies with predatory nematophagous fungi including the genera Duddingtonia, Monacrosporium and Arthrobotrys have been used to evaluate the experimental passage of fungi through the gastrointestinal tract of domestic animals.5
ConclusionsExperimental animals that received embryonated T. canis eggs exposed to the fungus T. virens showed a lower mean of larval recovery. This result evidences the ovicidal activity of T. virens and suggests that this fungus is a potential candidate for the biological control of T. canis. However, further studies are needed to evaluate biocontrol mechanisms, as well as the interaction of biotic and abiotic factors of T. virens in environmental conditions.
FundingThis study was supported through resources provided by Programa de Pós-graduação em Parasitologia/UFPel.
Conflict of interestNone of the authors of this manuscript has a financial or personal relationship with individuals or organizations that could inappropriately influence the content of this work.
The authors would like to thank the CNPq (National Council for Scientific and Technological Development) of Brazil and CAPES of Brazil for student and researcher scholarships.