Visceral toxocariasis is a parasitic zoonosis caused by Toxocara canis. The prevalence of this parasite in dogs, soil contamination and the resistance of eggs increase human exposure to the disease. Moreover, the difficulties of the control measures justify the need for alternative ones.
AimsThe objective of this study was to evaluate the in vitro ovicidal activity of fungi isolated from soils from public places in the city of Pelotas, Rio Grande do Sul, Brazil, on Toxocara canis.
MethodsSamples of soil from ten localities were inoculated onto Petri dishes with 2% water–agar (WA) that contained antibiotics, and incubated at 25°C/21 days. Isolated fungi were tested in vitro for ovicidal activity, with five replicates. One mL of an embryonated Toxocara canis egg suspension (103 eggs) was poured over the fungal cultures after 10 days of growth. At intervals of 7, 14 and 21 days, 100 eggs were removed from each plaque and evaluated by optical microscopy.
ResultsAcremonium, Aspergillus, Bipolaris, Fusarium, Gliocladium, Mucor and Trichoderma were isolated from the soil. A significant ovicidal type 3 effect was observed in Trichoderma, Fusarium solani complex and Acremonium. Those isolates from the genus Trichoderma showed their ovicidal effect on the 14th day of fungus–egg interaction. The other fungal genera tested showed a type 2 effect.
ConclusionsThese results suggest that the use of Trichoderma and Fusarium solani complex in biological control of T. canis is promising; however, further studies should be performed.
La toxocariasis visceral es una zoonosis parasitaria causada por Toxocara canis. La prevalencia del parásito en perros, la contaminación del suelo y la resistencia de los huevos aumentan la exposición del ser humano a la infección. Además, las dificultades de las medidas de control justifican la búsqueda de otras alternativas.
ObjetivosEl objetivo de este estudio fue evaluar la actividad ovicida in vitro frente a T. canis de hongos aislados del suelo de lugares públicos en la ciudad de Pelotas, Rio Grande do Sul, Brasil.
MétodosLas muestras de suelo de 10 lugares diferentes se inocularon en placas de Petri con un 2% de agar/agua que contenía antibióticos y se incubaron a 25 ¿C durante 21 días. Se evaluó la actividad ovicida in vitro de los hongos aislados por quintuplicado. Después de 10 días de crecimiento, se vertió 1ml (103 huevos) de una suspensión de huevos embrionarios de T. canis en los cultivos de hongos. A intervalos de 7, 14 y 21 días, se retiraron 100 huevos de cada placa y se evaluaron mediante microscopia óptica.
ResultadosDel suelo se aislaron especies de los géneros Acremonium, Aspergillus, Bipolaris, Fusarium, Gliocladium, Mucor y Trichoderma. Se observó un efecto ovicida significativo de tipo 3 en Trichoderma, el complejo de Fusarium solani y Acremonium. En hongos del grupo Trichoderma se evidenció un efecto ovicida el día 14 de la interacción hongo-huevo. Los otros géneros de hongos examinados mostraron un efecto de tipo 2.
ConclusionesLos resultados del presente estudio sugieren un uso prometedor de Trichoderma y Fusarium solani para el control biológico de T. canis, pero deben realizarse estudios adicionales.
Visceral toxocariasis, also called the syndrome of visceral larva migrans (VLM), is a parasitic zoonosis, and it is the result of the migration and persistence of helminthic larva in uncommon hosts.3 Several species of helminthes can cause VLM; however, Toxocara canis is the nematode most frequently associated with the disease.10 The high prevalence of this parasite in dogs, the observed frequent contamination of the environment, and the resistance of the parasite's eggs in the soil all increase human exposure to toxocariasis and make this disease a public health problem throughout the world.28 The prevalence data regarding child toxocariasis from some countries demonstrate quite variable rates of infection.14,31,37 In Brazil, studies have reported a prevalence that varies from 8.7% to 54.8%. 5,17,33 Studies evaluating contamination by the intestinal parasites of dogs in different Brazilian cities have shown that these animals are contaminated by various species of parasites with zoonotic potential.22,23
The difficulties inherent in implementing control measures, the high resistance of the eggs in the environment, and the problems inherent in chemical control justify the need for alternative measures that would help to decontaminate the soil.2 Nematophagous fungi are natural enemies of nematodes. They possess activities directed to the parasitism of the eggs and larvae of the geohelminthes living outside of hosts and are increasingly popular candidates reducing the level of environmental contamination.4 These fungi live in the organic matter of the soil, where they develop a parasitic or predator relationship with the nematodes and are classified as toxic, opportunistic (ovicidal), endoparasites and predators.26 Those fungi parasiting eggs do not depend on the presence of nematodes in the soil for their survival. Due to this characteristic feature, they establish themselves more easily than predator fungi.16 Their ovicidal activity is characterized by the penetration of haustorial hyphae into the egg shell through the pores of the vitelline layer; this alters the permeability of the shell and the expansion of its volume.28 Among these fungi, Pochonia chlamydosporia and Paecilomyces lilacinus stand out and are extensively studied with regard to their ovicidal activity on T. canis.1,6,18
The aim of this study is to investigate and evaluate the ovicidal activity of fungi isolated from soil in southern Brazil on T. canis eggs.
Materials and methodsCollection of soil samplesSoil was collected from public areas in the municipality of Pelotas, State of Rio Grande do Sul, Brazil. The municipality is located at latitude 31°46′19″S and longitude 52°20′33″W. It is seven meters above sea level, and has a damp, subtropical climate with an annual average temperature of 17.5°C, 1379mm of rain per year, and an average relative humidity of 80%.21 The areas selected for the soil collection (10 localities; 3 equidistant places per locality) were chosen based on previous works that had been performed in the region and had shown soil contamination with Toxocara eggs.19,38 Samples of approximately 500g of soil were obtained at a depth of 10cm. The dead leaves and other residual organic matter of the top layer were ignored. After the collection, the samples were packed in plastic bags, identified, and immediately transported to the Mycology Laboratory of the Biology Institute/Federal University of Pelotas (UFPel) for processing.
Collection of T. canis eggsThe eggs were obtained directly from the uterine tubes of adult females and were washed 10 times with distilled sterile water and centrifuged at 1000rpm for 5min. Next, they were incubated at 25°C for 14 days in a solution containing formalin at 0.05%, streptomycin sulfate at 0.05%, and chloramphenicol at 0.01%. The embryonated eggs had been previously analyzed morphologically to verify their integrity.
Isolation of the fungi from the soilThe technique for the isolation of fungi was based on that of Duddington 12 and adapted for the research on fungi with ovicidal activity described by Gortari et al.20 The 2% water/agar (WA), which contained added streptomycin 5mg/1L and chloramphenicol 5mg/1L, was inoculated at the surface with a 1ml suspension of T. canis eggs (approximately 103 eggs) and immediately seeded with 0.5g of the soil, previously homogenized, in the form of a cross. The Petri dishes were incubated at 25°C and observed daily for 21 days. The identification of the fungi was based on macro- and micro-morphological characteristics9,11 and determined to the lowest possible taxonomic level.
In vitro activity of the fungal isolatesThe Petri dishes of isolates that contained Potato dextrose agar (PDA) were selected and incubated at 25°C for 10 days. From these cultures, 4mm disks were transferred to Petri dishes containing 2% WA. All the dishes were incubated at 25°C for 10 days. A 1mL suspension of embryonated T. canis eggs (103/mL) was poured over each of the fungal cultures, as well as over the surface of Petri dishes containing only 2% WA (without fungus) as a control. Five replicates were performed for each fungal isolate analyzed. At intervals of 7, 14 and 21 days, 100 eggs were removed from each dish, according to the technique described by Araújo et al.,1 colored with Aman-blue at 1% and evaluated in microscopy of light (object lense 40×), according to the parameters established by Lysek24: effect type 1, physiological, biochemical effect, without morphological damage to the egg shell, on which hyphae can be observed adhered to the shell; effect type 2, lithic effect with alterations in the morphology of the shell and embryo of the egg, without penetration of the hyphae through the shell; effect type 3, lithic effect, with morphological alteration of the shell and the embryo, as well as penetration of the hyphae and internal colonization of the egg.
Statistical analysisThe data obtained were submitted to the non-parametric Friedman test with a significance level of 1%. The fungi that differ their effects by Friedman test also underwent regression analysis up to the second order regarding the dates of assessment. In regression analysis, the choice model was based on the significance of linear and quadratic coefficient by using the Student test at 5% probability. The analyses were performed using the SAS software package.32
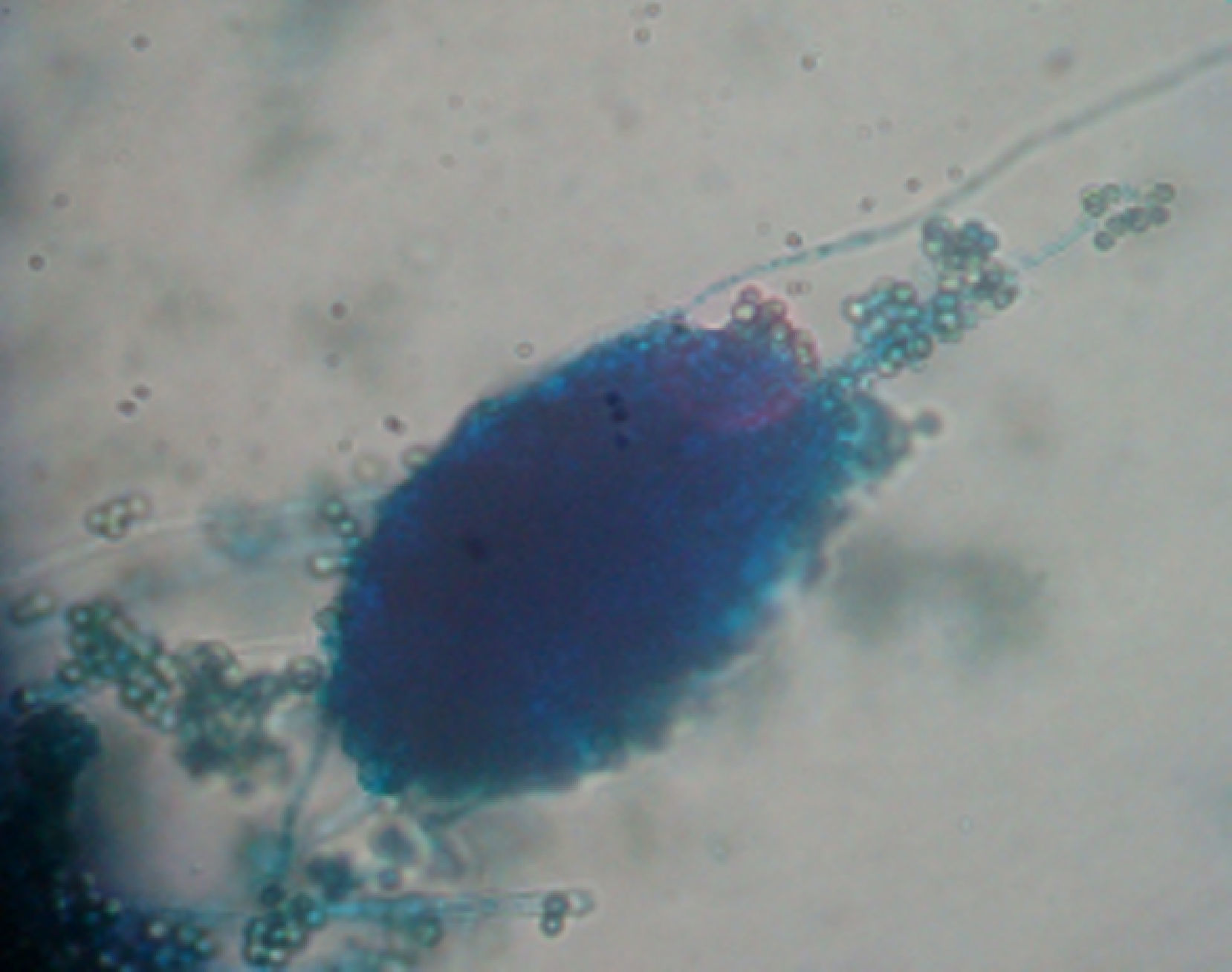
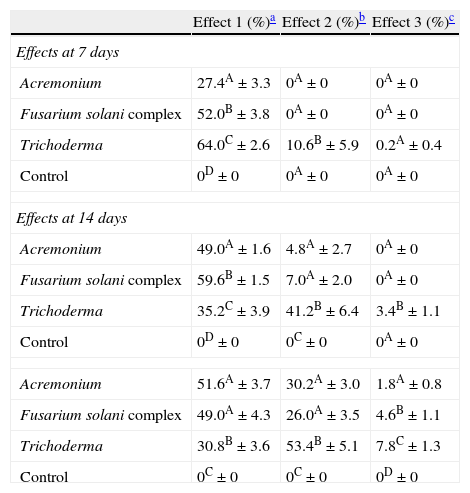
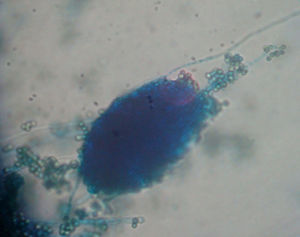
ResultsIsolates from the genera Aspergillus, Acremonium, Bipolaris, Fusarium oxysporum complex, Fusarium solani complex, Gliocladium, Mucor and Trichoderma were recovered from the soil. All the fungal isolates showed some degree of fungus–egg interaction. However, the isolates of Acremonium, Fusarium solani complex and Trichoderma presented a significant ovicidal effect type 3 on the embryonated T. canis eggs when submitted to the non-parametric Friedman test. The average results for the type 1, 2, and 3 effects at 7, 14, and 21 days for the groups treated with the fungi Acremonium, Fusarium solani complex, and Trichoderma and the control group are detailed in Table 1. In the control group, no fungal growth was detected. The ovicidal activity of the Trichoderma isolate was observed on the 14th day of interaction (Fig. 1). With the Acremonium and Fusarium solani complex isolates, the same effect was observed on the 21st day.
Percentages and standard deviation of ovicide activity of the fungi Acremonium, Fusarium solani complex, Trichoderma and control group, on Toxocara canis eggs at 7, 14 and 21 days of fungus–egg interaction.
| Effect 1 (%)a | Effect 2 (%)b | Effect 3 (%)c | |
| Effects at 7 days | |||
| Acremonium | 27.4A±3.3 | 0A±0 | 0A±0 |
| Fusarium solani complex | 52.0B±3.8 | 0A±0 | 0A±0 |
| Trichoderma | 64.0C±2.6 | 10.6B±5.9 | 0.2A±0.4 |
| Control | 0D±0 | 0A±0 | 0A±0 |
| Effects at 14 days | |||
| Acremonium | 49.0A±1.6 | 4.8A±2.7 | 0A±0 |
| Fusarium solani complex | 59.6B±1.5 | 7.0A±2.0 | 0A±0 |
| Trichoderma | 35.2C±3.9 | 41.2B±6.4 | 3.4B±1.1 |
| Control | 0D±0 | 0C±0 | 0A±0 |
| Acremonium | 51.6A±3.7 | 30.2A±3.0 | 1.8A±0.8 |
| Fusarium solani complex | 49.0A±4.3 | 26.0A±3.5 | 4.6B±1.1 |
| Trichoderma | 30.8B±3.6 | 53.4B±5.1 | 7.8C±1.3 |
| Control | 0C±0 | 0C±0 | 0D±0 |
Percentage followed by a different capital letter in the column statistically differs (p<0.01) by the Friedman test.
Effect type 1, physiological, biochemical effect, without morphological damage to the egg shell, on which hyphae can be observed adhered to the shell.
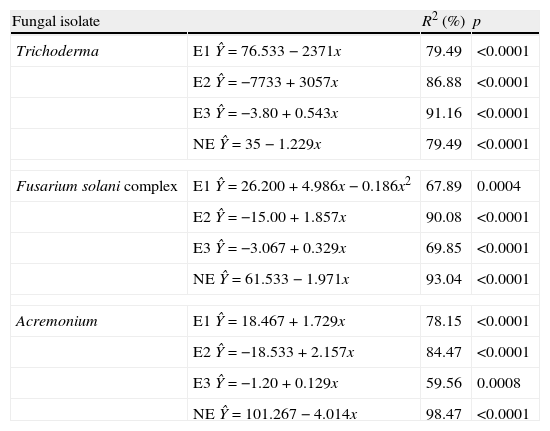
The regression analysis showed variations in effects 1, 2 and 3 for each fungal strain evaluated in connection to the time of exposure of T. canis eggs to the fungi (Table 2). The analysis revealed that independently of the fungus, type 2 and 3 effects presented increasing linear response. In Trichoderma it was observed that type 1 effect decreased linearly (2.371 every day). When evaluated type 2 and 3 effects, it was verified that, although both had evidenced increasing linear response, they differ (p<0.0001): an increase of 3.057 and 0.543 every day, respectively, was observed. The type 1 effect of Fusarium complex solani presented a quadratic response with the highest value on the 13th day after the initiation of treatment. Yet the type 2 effects showed increase of linear response with increase of 1.857 every day, as well as type 3 effect which increased 0.32 every day. Finally, the type 1 and 2 effect of Acremonium spp. demonstrated increasing linear response and similar, increasing 1.729 and 2.157 each day, respectively (p>0.05). Nevertheless, the type 3 effect showed different response (p>0.05) with an increase of 0.129 every day.
Regression equations of the effects of the fungi Trichoderma spp., Fusarium solani complex and Acremonium spp. on Toxocara canis eggs.
| Fungal isolate | R2 (%) | p | |
| Trichoderma | E1 Ŷ=76.533−2371x | 79.49 | <0.0001 |
| E2 Ŷ=−7733+3057x | 86.88 | <0.0001 | |
| E3 Ŷ=−3.80+0.543x | 91.16 | <0.0001 | |
| NE Ŷ=35−1.229x | 79.49 | <0.0001 | |
| Fusarium solani complex | E1 Ŷ=26.200+4.986x−0.186x2 | 67.89 | 0.0004 |
| E2 Ŷ=−15.00+1.857x | 90.08 | <0.0001 | |
| E3 Ŷ=−3.067+0.329x | 69.85 | <0.0001 | |
| NE Ŷ=61.533−1.971x | 93.04 | <0.0001 | |
| Acremonium | E1 Ŷ=18.467+1.729x | 78.15 | <0.0001 |
| E2 Ŷ=−18.533+2.157x | 84.47 | <0.0001 | |
| E3 Ŷ=−1.20+0.129x | 59.56 | 0.0008 | |
| NE Ŷ=101.267−4.014x | 98.47 | <0.0001 | |
E1, effect type 1; E2, effect type 2; E3, effect type 3; NE, no effect
Although the remaining evaluated fungi did not show a significant type 3 effect, a type 2 effect was observed on the 21st day in the Aspergillus (55.2%), Bipolaris (52.4%), Fusarium complex oxysporum (46.8%), Mucor (42.0%) and Gliocladium (39.2%) isolates.
DiscussionThis study is the first that researches parasitic fungi for reduction of T. canis eggs in the soil of the southern region of Brazil. Isolates from Trichoderma, Fusarium solani complex and Acremonium were notable for their promising ovicidal activities. According to Lysek,24 a fungus with ovicidal potential is one that demonstrates a large lithic effect, causing morphological alteration of the shell and embryo with penetration of hyphae and internal colonization of the egg (type 3 effect). Among the fungi evaluated, Trichoderma spp. stood out, as it presented a significant type 3 effect at the 14th day of fungus–egg interaction. This result is relevant because Overgaauw29 reported that T. canis eggs become infectious after approximately 2–6 weeks. Hence, this fungus could render the eggs inactive and thus reduce the level of environmental contamination. Several studies have evaluated the ovicidal action of different fungi on T. canis eggs. 1,7,8,18,20 Only Ciarmela et al.8 evaluated the activity of Trichoderma harzianum. This genus has been extensively researched in the biological control of numerous phytopathogenic fungi15,25,30 and in the control of phytonematodes. 16,35,36 Surveys have shown that Trichoderma spp. have the capacity for parasiting the eggs of different nematode species from the species Meloidogyne exigua,16Meloidogyne incognita,13,34Meloidogyne arenaria39 and Meloidogyne javanica.35,36 Mechanisms that have been suggested to explain the activity of Trichoderma against phytopathogenic fungi include antibiosis, competition, micro-paratisism and production of enzymes (chitinases, glucanases and proteases). All of these mechanisms, with the exception of competition, could potentially be involved in the process of biological nematode control.35 Santin,34 when assessing the potential of Trichoderma on M. incognita control, suggested that the mechanisms used by the fungus consist of the production of both inhibiting volatile metabolites and lithic enzymes that degrade the chitin of the eggs. Morton27 stated that the chitinolytical activity is likely the most relevant for the lesion on the sheath of the egg. This suggests that the ovicidal activity of the observed Trichoderma spp. on T. canis eggs in the current study may be due to some of the aforementioned mechanisms. However, further research is needed to determine the exact ovicidal mechanism used by the fungus. Although our study has not identified the species of Trichoderma isolated, our results differ from Ciarmela et al.,8 who found that T. harzianum does not affect the viability of T. canis eggs.
In Argentina, Ciarmela et al.7,8 evaluated ovicidal activity of isolated fungi in soil of public localities in the city of La Plata and showed the activity of Fusarium pallidoroseum, Fusarium oxysporum, Fusarium sulphureum and Fusarium moniliforme on T. canis eggs. In a similar study, Gortari et al.20 demonstrated the efficiency of various fungal genera on eggs of the same parasite, among which were Acremonium, Aspergillus, Fusarium, Mucor, Paecilomyces, and others. The observed ovicidal effect of the genus Fusarium in our study and in those of Ciarmela et al.7,8 and Gortari et al.20 indicate the potential of these fungi as agents of biological control of helminthic eggs. Larger investigations are necessary.
The most studied nematophagous fungi, Paecilomyces lilacinus and Pochonia chlamydosporia, stand out for their ovicidal activity. In vitro studies have shown the efficiency of these fungi on embryonated T. canis eggs.1,6,18 Although they are the species most often cited as ovicides, Pochonia chlamydosporia and Paecilomyces lilacinus were not isolated from the soil in this study.
ConclusionThe results of this study verify the presence of fungi parasitic to T. canis eggs in the soil from the Southern region of Brazil and highlight the ovicidal activity of Trichoderma and Fusarium solani complex. However, more studies to prove the biological potential of fungi evaluated, as well as their mechanisms of ovicidal action, are required. We believe that further research aimed at the search for autochthonous fungi with nematophagous potential is important, since the adaptation of the fungus to different regional conditions is a key factor to the biological control of parasites.
Conflict of interestThe authors declare that there is no conflict of interest.
Financial supportCoordination of Improvement of Higher Education Personnel (CAPES).