Phytophthora capsici is a major fungal plant pathogen that causes root and crown rot of pepper crops and its oospores are the most resistant propagules.
ObjectivesTo evaluate the effect of different temperature regimes and exposure times on the survival of P. capsici oospores.
MethodsThermal inactivation treatments simulated field conditions, through the use of different constant and cycling temperature regimes, in moistened sterilized soil (15-53°C) and sterilized water (45-53°C). The plasmolysis method evaluated oospore viability. Relationships between oospores viability and exposure time were statistically determined by linear regression. Interpolation was used to calculate the estimated times required to kill a determined percentage of the population.
ResultsThe required time to reduce P. capsici oospores viability decreased with increasing temperatures. Times required to kill 100% of oospores were 199-22-6.6-4.7-1.0hours at 40-45-47.5-50-53°C respectively in moistened soil and 31-1.0-0.2hours at 45-50-53°C in water. Oospores were scarcely affected at temperatures ≤ 35°C. With 1,680hours at 15-35°C, oospores survival in soil ranged from 88 to 36%. The 4 hours-40°C regime killed 100% of oospores after 28days, while the 5 hours-35°C regime after 70days killed only 75%. Time required to achieve total oospores death was remarkably shortened in water when compared with moistened soil.
ConclusionsThe developed models can be used to predict survival values at any exposure time with constant temperatures ranging from 40 to 53°C in moistened soil and from 45 to 53°C in water. The weakening of P. capsici oospores under sublethal heating, is a useful observation that can be applied for pathogen control with solarization.
Phytophthora capsici, importante fitopatógeno fúngico causante de la podredumbre radicular del pimiento, posee oosporas (los propágulos más resistentes).
ObjetivosEvaluar diferentes regímenes térmicos y tiempos en la supervivencia de las oosporas.
MétodosLa inactivación térmica simuló condiciones de campo, empleando diferentes regímenes constantes y cíclicos en suelo húmedo (15-53°C) y en agua (45-53°C) estériles. La viabilidad de las oosporas se evaluó mediante plasmólisis correlacionándola por regresión lineal con el tiempo de exposición. Los tiempos estimados necesarios para matar un determinado porcentaje de oosporas fueron calculados por interpolación.
ResultadosEl tiempo requerido para reducir la viabilidad de las oosporas disminuyó al aumentar la temperatura. Los tiempos requeridos para matar al 100% de las oosporas fueron 199-22-6,6-4,7-1,0 horas a 40-45-47,5-50-53°C respectivamente en suelo húmedo y 31-1,0-0,2 horas a 45-50-53°C en agua. Las oosporas fueron escasamente afectadas por temperaturas ≤ 35°C. Durante 1.680 horas a 15-35°C, la supervivencia de oosporas en suelo varió entre 88 y 36%. El régimen de 4 horas-40°C mató al 100% después de 28 días, mientras el régimen de 5 horas-35°C después de 70 días sólo mató al 75%. El tiempo requerido para matar al 100% de las oosporas fue acortado significativamente en agua respecto al suelo húmedo.
ConclusionesLos modelos desarrollados pueden utilizarse para predecir valores de supervivencia para cualquier tiempo de exposición a temperaturas constantes de 40-53°C en suelo y de 45-53°C en agua. El debilitamiento de las oosporas de P. capsici bajo calentamiento subletal constituye una observación útil que puede aplicarse en el control del patógeno mediante solarización.
Phytophthora spp. are important soilborne pathogenic fungi for different crops; Phytophthora capsici is a major pathogen that causes root and crown rot of pepper crops and consequently, limits its production. Depending on environmental conditions, it also can damage other plant organs such as leaves, branches, stem and fruits. P. capsici is formed namely by vegetative propagules (mycelium, sporangia and zoospores, but rarely chlamydospores), and sexual resistant spores (oospores). Vegetative propagules cause rapid development of disease under favourable conditions while oospores are the residual inoculum in soil, which under nonfavourable conditions, permit the disease persistence11.
Postplant strategies used by growers for the control of root and crown rot of pepper are mainly based on soil-water management and application of fungicides. These latter methods do not always provide successful results and therefore, fungal inactivation treatments prior to the planting are widely used, such as chemical fumigation. A drawback of chemical fumigants is that their effectiveness may be limited due to reinfestation of soil after the treatment. Environmental pollution and their danger to humans are other inconveniences that have also made alternatives necessary6.
One of these alternatives is soil solarization. Solarization is an hydrothermal process in which soil is moistened, covered with transparent plastic and exposed to sunlight to reach high temperatures capable of destroying plant pathogens. Soil solarization practices have shown satisfactory control of different types of diseases in many areas4–6,13,18–20,22–30.
The effectiveness of solarization depends on different variables, such as the soil colour and structure, soil moisture, air temperature, length of day, and intensity of sunlight6. Thus, as solarization conditions can vary greatly from site to site, in vitro laboratory thermal inactivation studies of plant pathogens are mandatory effective tools to predict the utility of solarization as a disease management practice in the particular conditions of each site.
In addition to soil solarization, other heat treatments can be used to justify the interest of in vitro thermal inactivation assays: soil steaming disinfestation, thermotherapy of infected seedlings by pathogens before planting, and residue composting of infected crops2,3.
Furthermore, little information is available concerning the exposure times necessary to kill soilborne plant pathogens at temperatures below 45°C. These temperatures are most frequently found in solarized soils and are often considered to be “sublethal”. Spare data with bacteria and fungi have also shown that temperatures below 45°C can be lethal if maintained for long periods7.
The aim of this in vitro study is to evaluate the effect of different temperature regimes and exposure times on the survival of P. capsici oospores.
Material and methodsIn vitro production of oosporesP. capsici 02/155 (pepper isolate from Villanueva de Gállego, Zaragoza, Spain), of the A1 compatibility type, was crossed with isolate P. capsici 59 (from the INRA collection, Antibes, France) of the A2 compatibility type. A1 and A2 isolates were paired in Petri dishes (90-mm diameter) containing 15ml of V8-juice broth (without agar). The plates were sealed with parafilm and incubated in darkness at 25°C until they were used (ranging from 28 to 35days).
Oospore fixation in nylon meshOospores were extracted by blending the V8 liquid culture in 100ml of deionized water. This suspension was filtered through a 100μm nylon mesh and washed with deionized water. Sporangia and mycelium propagules remained on the filter and oospores were recovered from the filtrate. In the filtered suspension through the 100μm nylon mesh, the oospores concentration was determined by a Fuchs-Rosenthal haemocitometer. Afterwards, the filtered suspension was collected and refiltered by vacuum through 25μm nylon meshes (1cm2, CISA, Barcelona) mounted on the top of an analytical filter holder (Millipore®) connected to a membrane diaphragm vacuum pump (maximum flow: 1.9 m3/h, maximum vacuum: 80 mbar, 230V, 50/60Hz, 1.1 A; Model ME-2, Vacuubrand®, Wertheim, Germany). A droplet of oospores suspension, containing at least 1,000 oospores, was drawn into each nylon mesh using vacuum (approximately 100 mbar). Consequently, oospores with a diameter size nearly the size of the mesh pores were embedded in the interstices and smaller ones passed through the mesh21. The nylon meshes containing embedded oospores were maintained at 5°C (usually no more than three days) until they were used in the corresponding experiments.
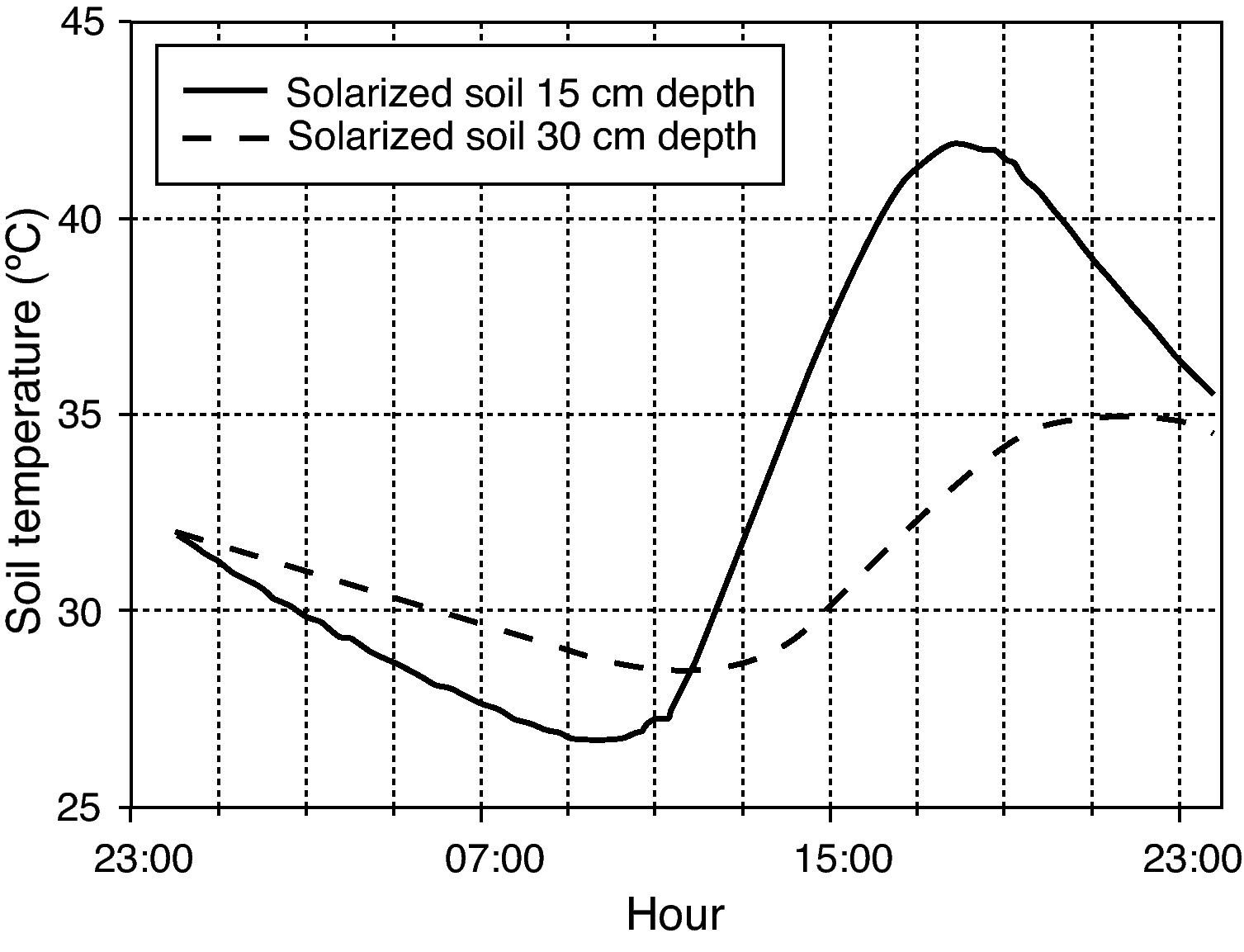
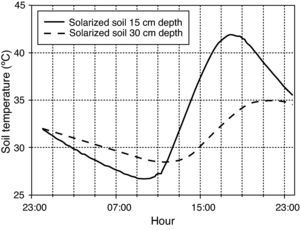
Thermal treatmentsThermal inactivation treatments were carried out by simulating field conditions. Mesh squares containing oospores were introduced into glass test tubes (25 x 150mm) filled with 30 grams of moistened sterilized soil. Soil water content was adjusted to field capacity (percent volumetric water content of 22.5% v/v). Tubes were loosely capped with plastic tops to retard water loss, but allowed for gas exchange. Tubes were left to equilibrate overnight and the next day, they were maintained at nine constant (15, 25, 30, 35, 40, 45, 47.5, 50 or 53°C) and two daily cycling (4hours at 40°C, 20hours at 25°C; 5hours at 35°C, 19hours at 30°C) temperature regimes. The tubes in the lowest range of constant temperatures (15°C) were placed in a programmable refrigerated precision temperature incubator (stability:±0.5°C; Model Hotcold-UB-2101505, Selecta®, Abrera, Barcelona, Spain). Those tubes in the range of constant temperatures above 15°C were placed in a water bath (stainless steel tank of 15cm depth x 62cm length x 50cm width) with an immersion circulation heater (stability:±0.2°C; Model Termotronic-3000389, Selecta®). The two daily cycling temperature regimes were maintained in water baths with immersion circulation heaters connected to the mains through a programmable timer. The two cycling temperature regimes were chosen to simulate daily temperature fluctuations at two soil depths (15 and 30cm) during summer solarization (6 August-22 September 2009) in a closed greenhouse located at Derio (Northern Spain), a zone characterized by a humid mild climate. The temperatures recorded during the latter period were used to build the cycling regimes and represented the optimum conditions for evaluation of solarization in that area (fig. 1). Heat assays at constant temperatures (45°, 50° and 53°C) were also carried out in a water medium instead of in moistened soil in order to compare heat effectiveness in soil and in water, and with the aim to evaluate the use of hot water as a possible thermotherapy tool.
Hourly temperatures continuously recorded in solarized (moisten) soil for depths of 15 and 30cm on 6 September 2009 in a greenhouse field experiment located at Derio (Northern Spain). Soil was tarped with 50-μm-thick (2 mil) transparent low density polyethylene plastic film from 6 August to 22 September 2009.
Exposure times were different at each temperature regime: at high temperatures rates, exposure times were less than at low levels. At each combination of temperature and exposure time, three mesh squares were removed and oospore viability percentages were measured.
Plasmolysis methodThe plasmolysis method17 was used to evaluate oospore viability. Mesh squares were introduced in 4mol l-1 sodium chloride solution for about 45min and microscopically (Olympus CH40, x200) observed. The cytoplasm of the viable oospores was contracted to form a central ball-like structure and non-viable ones did not plasmolize as they had lost the differential permeability of their cellular membrane. Oospores were classified as viable (plasmolized) and unviable (non-plasmolized).
Quantification of oosporesIn each different combination of temperature regime and exposure time, three glass test tubes (each containing one mesh square with embedded oospores) were used and 100 oospores were quantified in each mesh square. The exposed data, expressed as a percentage, were the average of three counts of 100 oospores. Each experiment was repeated once.
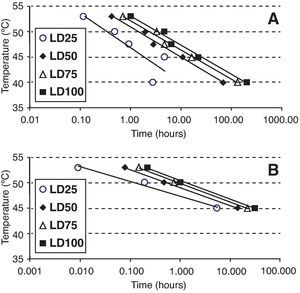
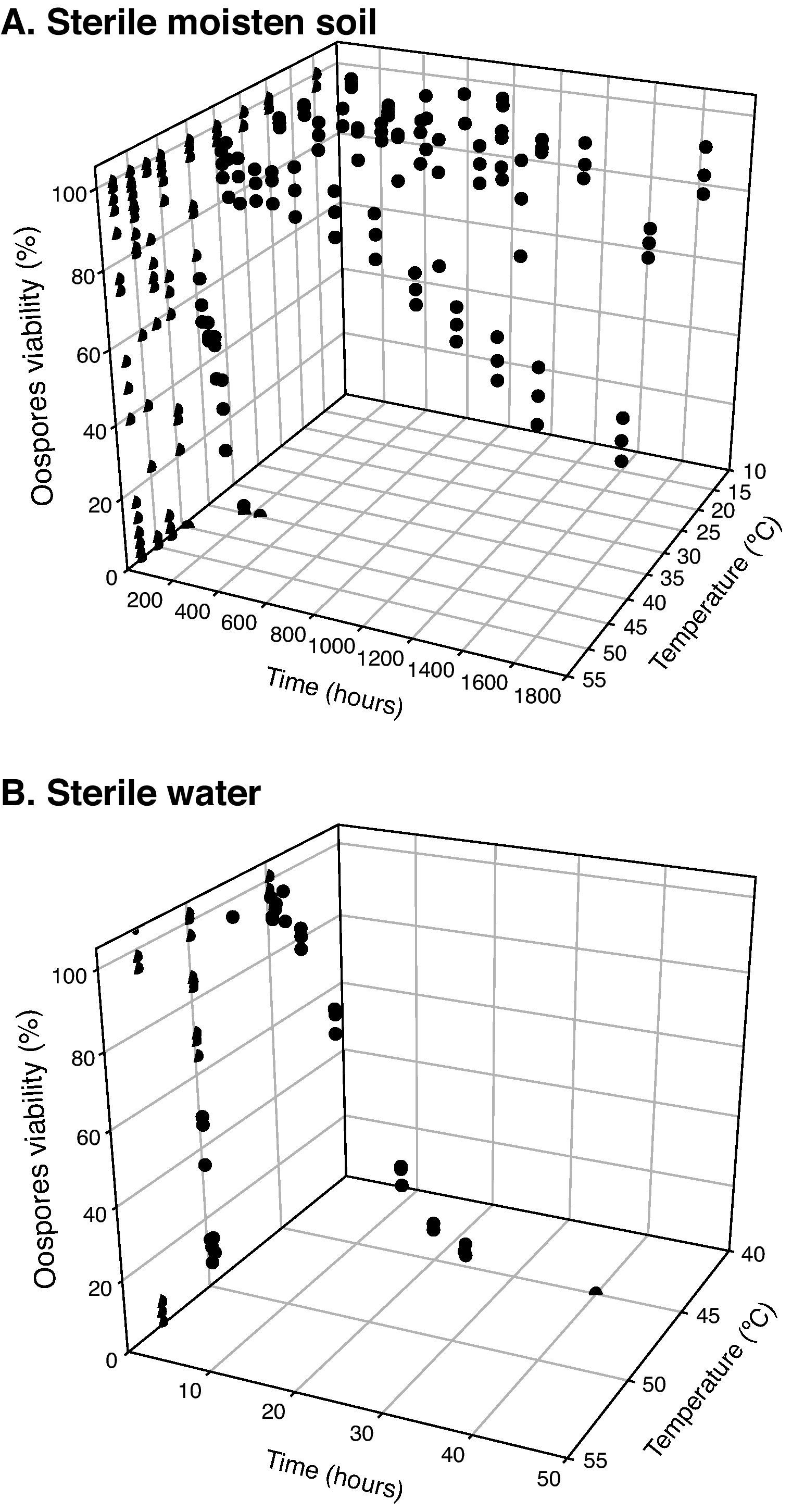
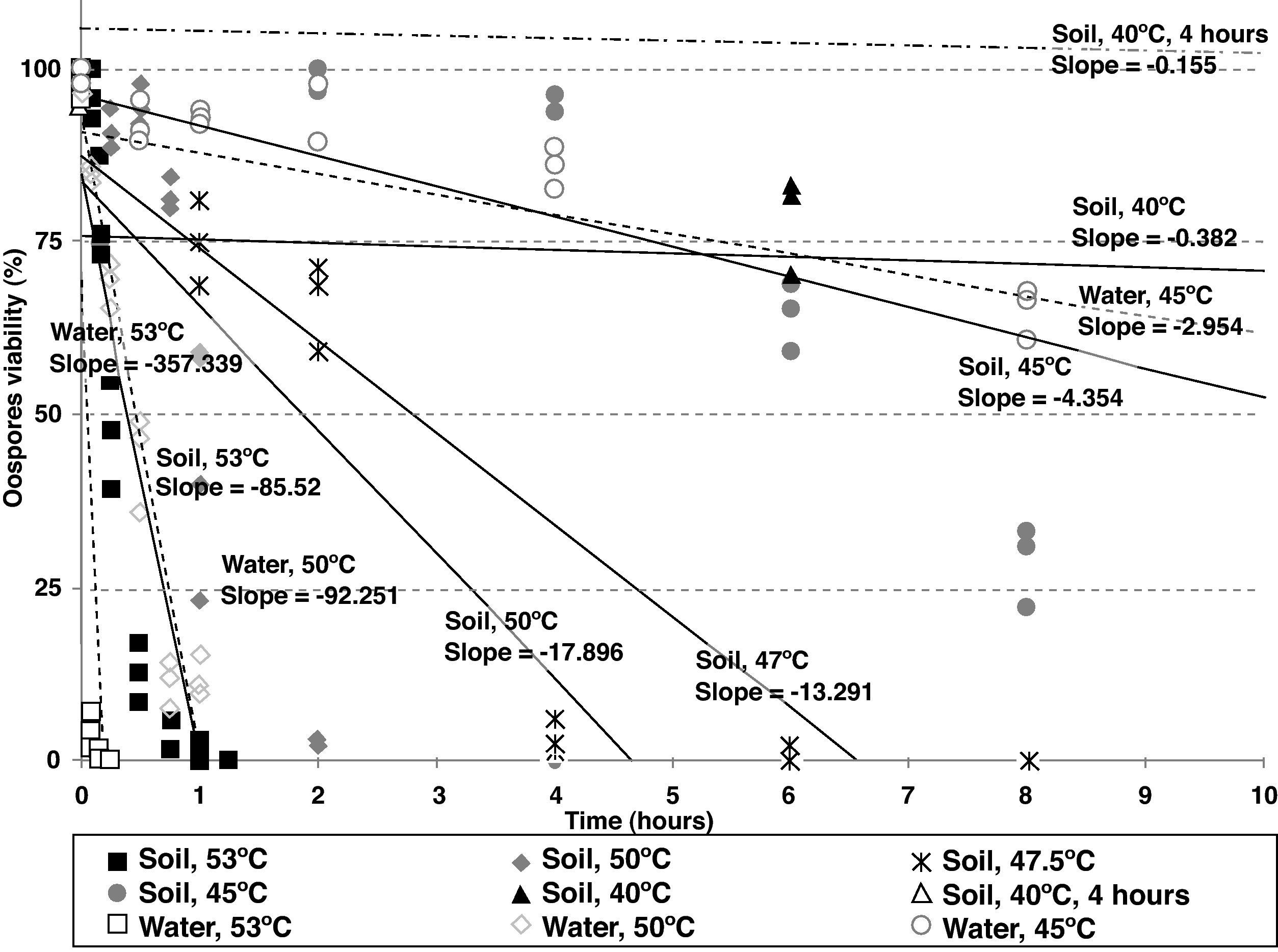
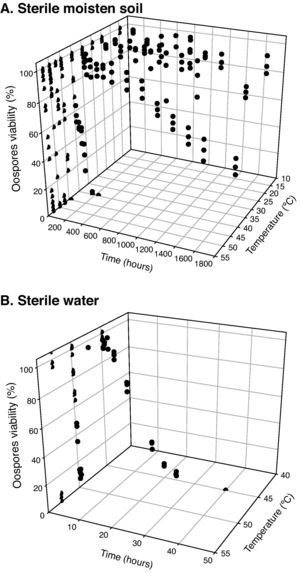
Statistical analysisOospore survival data points from constant temperature regimes in moistened soil were pooled and drawn in 3 dimensions against each exposure time for each temperature with Sigma Plot (version 10.0 for Windows) software (fig. 2). Relationships between the oospores viability percentage and the exposure time at each temperature regime were statistically determined by linear regression analysis, using the general-purpose procedure for regression (proc REG) of SAS (Statistical Analysis Software, SAS Institute, Cary, NC; release 9.1). The slopes of the regression lines for each temperature were compared with the use of an analysis of covariance, using the General linear models procedure (proc GLM). Viability percentage values were used as the dependent variable, temperature as class variable and the exposure time as covariate in a model that included temperature, exposure time and their interaction. At each temperature regime (constant or cycling), the lethal dose (LD) was defined as the exposure time required to reduce the oospores population by 25, 50, 75 or 100% (LD25, LD50, LD75 and LD100) and was determined by the intersection of regression lines and horizontal lines corresponding to oospores viability values of 75, 50, 25 and 0% respectively (fig. 3). Exposure time at which 75% of the population of oospores was killed (LD75) was plotted on a logarithmic scale against temperature. A new linear regression analysis of the data points was made according to Pullman et al27 and repeated for LD25, LD50 and LD100. This provided a different regression line for each of the four LD percentages (fig. 4).
Regression lines corresponding to Phytophthora capsici oospores viability versus exposure time (first 10hours) at different temperature regimes and media (constant temperatures in sterile moistened soil [continous lines]: 53, 50, 47.5, 45 and 40°C; cycling temperature in sterile moistened soil [two dotted single dashed lines]: 40°C for 4hours and 25°C for the remainder of each day; constant temperatures in water [dashed lines]: 53, 50 and 45°C).
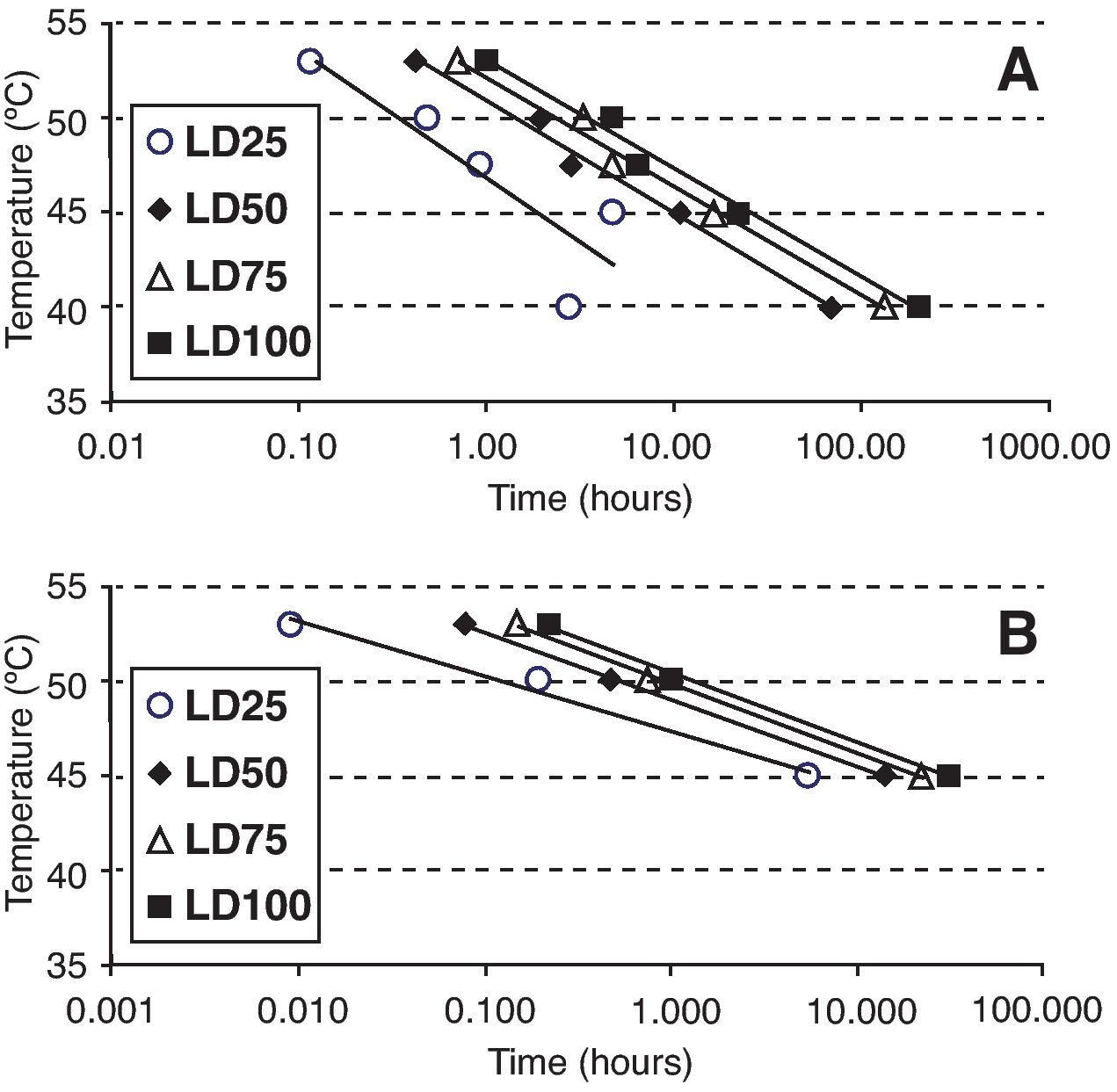
Times and temperatures exposures required to kill 25, 50, 75 and 100% (LD25, LD50, LD75 and LD100) of Phytophthora capsici oospores in different media. A. Sterile moistened soil (22.5% volumetric water content) (constant temperatures between 40 and 53°C). B. Sterile water (constant temperatures between 45 and 53°C).
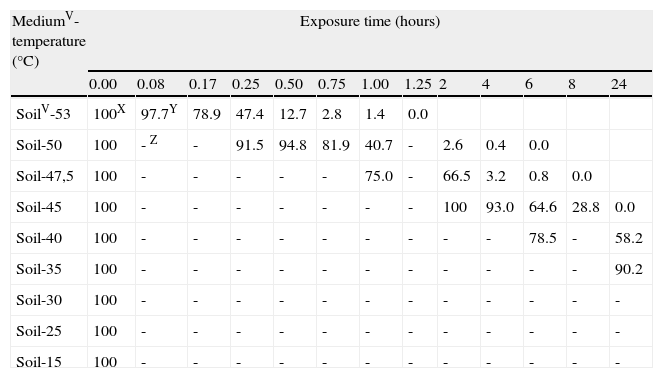
Sigmoidal curves were obtained when oospores viability percentages were plotted against exposure times for each temperature in both assays (moistened soil and water) (fig. 2). Oospores were not affected by heat at temperatures equal or below 35°C. The survival of oospores in moistened soil ranged from 88% at 15°C to 36% at 35°C (Table 1; fig. 2) for an exposure time of 70days (1,680hours). However, heat sensitivity of oospores at temperatures above 40°C was remarkable: treatments in moistened soil from 168 to 240hours at 40°C, from 8 to 24hours at 45°C, from 6 to 8hours at 47.5°C, from 4 to 6hours at 50°C and from 1 to 1.25hours at 53°C all proved to be lethal for oospores (Table 1). Of note, time required to achieve total lethality was significantly shorter in water, with values ranging from 24 to 40hours at 45°C, from 1 to 2hours at 50°C and from 0.17 to 0.25hours at 53°C (Table 1).
SurvivalU of Phytophthora capsici oospores at each exposure time and each constant temperature regimen in different mediaV.
| MediumV-temperature (°C) | Exposure time (hours) | ||||||||||||
| 0.00 | 0.08 | 0.17 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 2 | 4 | 6 | 8 | 24 | |
| SoilV-53 | 100X | 97.7Y | 78.9 | 47.4 | 12.7 | 2.8 | 1.4 | 0.0 | |||||
| Soil-50 | 100 | - Z | - | 91.5 | 94.8 | 81.9 | 40.7 | - | 2.6 | 0.4 | 0.0 | ||
| Soil-47,5 | 100 | - | - | - | - | - | 75.0 | - | 66.5 | 3.2 | 0.8 | 0.0 | |
| Soil-45 | 100 | - | - | - | - | - | - | - | 100 | 93.0 | 64.6 | 28.8 | 0.0 |
| Soil-40 | 100 | - | - | - | - | - | - | - | - | - | 78.5 | - | 58.2 |
| Soil-35 | 100 | - | - | - | - | - | - | - | - | - | - | - | 90.2 |
| Soil-30 | 100 | - | - | - | - | - | - | - | - | - | - | - | - |
| Soil-25 | 100 | - | - | - | - | - | - | - | - | - | - | - | - |
| Soil-15 | 100 | - | - | - | - | - | - | - | - | - | - | - | - |
| MediumV- temperature (°C) | Exposure time (hours) | ||||||||||||
| 48 | 72 | 96 | 168 | 240 | 336 | 504 | 672 | 840 | 1,008 | 1,176 | 1,344 | 1,680 | |
| Soil-40 | 50.0 | 44.5 | 28.1 | 0.8 | 0.0 | ||||||||
| Soil-35 | 89.8 | - | 87.1 | 86.3 | 86.7 | 86.3 | 81.0 | 73.5 | 65.9 | 58.3 | 50.7 | 43.2 | 35.6 |
| Soil-30 | - | - | - | 100 | - | 98.0 | 97.1 | 99.6 | 88.2 | 95.5 | 86.5 | - | 82.0 |
| Soil-25 | - | - | - | 100 | - | 100 | 95.7 | 97.6 | - | 98.0 | - | 95.7 | |
| Soil-15 | - | - | - | 100 | - | 93.1 | 91.0 | 98.4 | 92.7 | 88.6 | - | - | 87.8 |
| MediumV-temperature (°C) | Exposure time (hours) | |||||||||||||
| 0 | 0.08 | 0.17 | 0.25 | 0.5 | 0.75 | 1 | 2 | 4 | 8 | 16 | 20 | 24 | 40 | |
| WaterV-53 | 100 | 4.2 | 0.5 | 0.0 | ||||||||||
| Water-50 | 100 | 84.8 | - | 68.8 | 43.8 | 11.2 | 12.0 | 0.0 | ||||||
| Water-45 | 100 | - | - | - | 92.0 | - | 93.1 | 95.0 | 85.8 | 65.1 | 24.1 | 10.0 | 5.0 | 0.0 |
USurvival assessments of oospores of P. capsici were determined by the plasmolysis method (Jiang and Erwin, 1990).
VMedia: soil=sterile soil with moisture at field capacity (22.5% volumetric water content). Water=sterile water.
XAt each temperature regimen, the values of oospores viability throughout exposure time are expressed as a percentage of the initial viability value.
YPercentage values are the average of three counts of 100 oospores.
ZNo data.
This reduction in survival of P. capsici oospores was modeled using linear regression. The model selected for the comparisons among temperature regimes was Y=a+b·X, in which Y is the oospores viability percentage and X is the number of hours of exposure to the temperature regime. A lack of fit of the model to the data was observed at temperatures below 35°C with values of the coefficient of determination (R2) equal to or lower than 0.35 (Table 2).
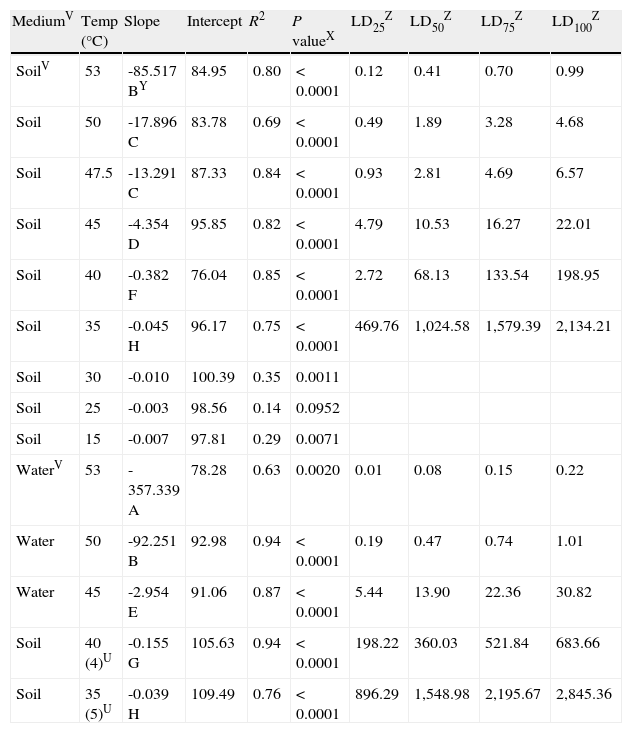
Linear regression analysisS of survivalT of oospores of Phytophthora capsici versus exposure time at each temperature regime (constant or cyclingU) in different mediaV. Regression coefficients and estimated exposure times required to kill a determined population percentage.
| MediumV | Temp (°C) | Slope | Intercept | R2 | P valueX | LD25Z | LD50Z | LD75Z | LD100Z |
| SoilV | 53 | -85.517 BY | 84.95 | 0.80 | < 0.0001 | 0.12 | 0.41 | 0.70 | 0.99 |
| Soil | 50 | -17.896 C | 83.78 | 0.69 | < 0.0001 | 0.49 | 1.89 | 3.28 | 4.68 |
| Soil | 47.5 | -13.291 C | 87.33 | 0.84 | < 0.0001 | 0.93 | 2.81 | 4.69 | 6.57 |
| Soil | 45 | -4.354 D | 95.85 | 0.82 | < 0.0001 | 4.79 | 10.53 | 16.27 | 22.01 |
| Soil | 40 | -0.382 F | 76.04 | 0.85 | < 0.0001 | 2.72 | 68.13 | 133.54 | 198.95 |
| Soil | 35 | -0.045 H | 96.17 | 0.75 | < 0.0001 | 469.76 | 1,024.58 | 1,579.39 | 2,134.21 |
| Soil | 30 | -0.010 | 100.39 | 0.35 | 0.0011 | ||||
| Soil | 25 | -0.003 | 98.56 | 0.14 | 0.0952 | ||||
| Soil | 15 | -0.007 | 97.81 | 0.29 | 0.0071 | ||||
| WaterV | 53 | -357.339 A | 78.28 | 0.63 | 0.0020 | 0.01 | 0.08 | 0.15 | 0.22 |
| Water | 50 | -92.251 B | 92.98 | 0.94 | < 0.0001 | 0.19 | 0.47 | 0.74 | 1.01 |
| Water | 45 | -2.954 E | 91.06 | 0.87 | < 0.0001 | 5.44 | 13.90 | 22.36 | 30.82 |
| Soil | 40 (4)U | -0.155 G | 105.63 | 0.94 | < 0.0001 | 198.22 | 360.03 | 521.84 | 683.66 |
| Soil | 35 (5)U | -0.039 H | 109.49 | 0.76 | < 0.0001 | 896.29 | 1,548.98 | 2,195.67 | 2,845.36 |
SThe model used for the analysis was: Viability=a+b x (hours).
TSurvival assessments of oospores were determined by the plasmolysis method (Jiang and Erwin, 1990).
UCycling temperature regimes that simulated solarization consisted of temperatures increased daily to 40 or 35°C for 4 and 5hours respectively (length of time in parenthesis in hours); the remainder of each day was maintained at 25 or 30°C respectively.
VMedium: Soil=sterile soil with moisture at field capacity (22.5% volumetric water content). Water=sterile water.
XSignificance associated to the coefficient of determination (R2).
YValues of slopes followed by the same capital letter do not differ statistically (P < 0.05) according to the analysis of covariance.
ZEstimated exposure times (hours) at each temperature regime required to kill 25, 50, 75 and 100% of the oospores population respectively.
As the temperature increased, the slope of the regression lines also showed an increase, implying that higher temperatures enhanced the rhythm of lethality. For constant temperatures in moistened soil, significant differences between the slopes of the regression lines were determined by analysis of covariance and pairwise comparisons between 53 and 50°C (P<0.0001), between 47.5 and 45°C (P<0.0001) and between 45 and 40°C (P<0.0001). Between 50 and 47.5°C, there was no significant difference (P=0.1225). The difference between the slopes of the constant and cycling exposures to 40°C in moistened soil was also significant (P<0.0001) (fig. 3; Table 2).
For constant temperatures in water (53, 50 and 45°C), the regression lines also showed significantly different slopes based on the analysis of covariance between 53 and 50°C (P=0.0001) and between 50 and 45°C (P<0.0001) (fig. 3; Table 2).
Heat treatments in water inactivated oospores more rapidly than treatments in moistened soil at all the tested temperatures (53, 50 and 45°C). These results are in agreement with the significant differences found in the slopes of the regression lines at 53°C (P=0.0004), at 50°C (P<0.0001), and at 45°C (P=0.0107). Notably, the oospores inactivation in water at 50°C and in moistened soil at 53°C showed no significant differences between the slopes (P=0.5939) (fig. 3; Table 2).
The analysis of the previous data is in accordance with the estimated exposure times required to kill a determined percentage of the oospore population: LD100 values in water were only 0.22hours at 53°C and 1.01hours at 50°C, whereas in moistened soil these values were higher (0.99 and 4.68hours respectively). However, LD100 values were closer when comparing moistened soil and water at 45°C (22.01 and 30.82hours respectively), moistened soil at 50 and 47.5°C (4.68 and 6.57hours respectively), and moistened soil at 53°C and water at 50°C (0.99 and 1.01hours respectively) (Table 2).
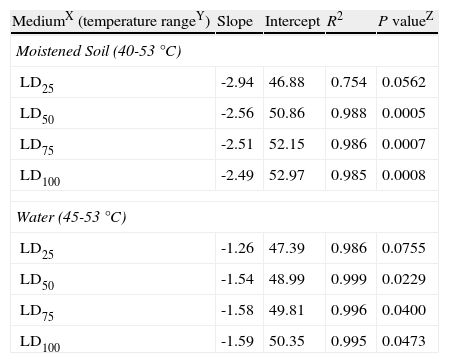
Values obtained from the intersection points between regression lines and LD25, LD50, LD75 and LD100 percentages (fig. 3) were plotted on a logarithmic exposure time scale against temperature and new regression lines were obtained (fig. 4, A and B). These new regression lines showed highly significant determination coefficients in moistened soil and water, except the LD25 parameter which was not significant (P values>0.05) (Table 3). The former models can be used to predict survival values at any exposure time with constant temperatures, ranging from 40 to 53°C in moistened soil and from 45 to 53°C in water.
Linear regression analysisV of constant temperatures versus exposure times required to kill a 25, 50, 75 or 100% population percentage (LD25, LD50, LD75 and LD100) of Phytophthora capsici oospores in moistened soil or in water.
| MediumX (temperature rangeY) | Slope | Intercept | R2 | P valueZ |
| Moistened Soil (40-53°C) | ||||
| LD25 | -2.94 | 46.88 | 0.754 | 0.0562 |
| LD50 | -2.56 | 50.86 | 0.988 | 0.0005 |
| LD75 | -2.51 | 52.15 | 0.986 | 0.0007 |
| LD100 | -2.49 | 52.97 | 0.985 | 0.0008 |
| Water (45-53°C) | ||||
| LD25 | -1.26 | 47.39 | 0.986 | 0.0755 |
| LD50 | -1.54 | 48.99 | 0.999 | 0.0229 |
| LD75 | -1.58 | 49.81 | 0.996 | 0.0400 |
| LD100 | -1.59 | 50.35 | 0.995 | 0.0473 |
VThe model used for the analysis was: Temperature=a+b x ln (hours).
XMedium: Moistened soil=sterile soil with moisture at field capacity (22.5% volumetric water content). Water=sterile water.
YConstant temperatures: 40, 45, 47.5, 50 and 53°C in moistened soil; 45, 50 and 53°C in water.
ZSignificance associated to the coefficient of determination (R2).
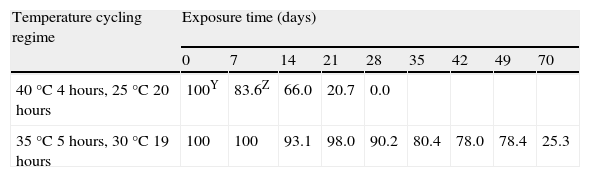
The daily cycling temperature regime, consistent of 4hours at 40°C and 20hours at 25°C, killed 100% of oospores after 28days, as opposed to the regime consistent of 5hours at 35°C and 19hours at 30°C, with which, after 70days of exposure, 25% of oospores survived (Table 4).
SurvivalV of Phytophthora capsici oospores exposed to daily cycling temperatures regimes in moistened soilX.
| Temperature cycling regime | Exposure time (days) | ||||||||
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 70 | |
| 40°C 4hours, 25°C 20 hours | 100Y | 83.6Z | 66.0 | 20.7 | 0.0 | ||||
| 35°C 5hours, 30°C 19 hours | 100 | 100 | 93.1 | 98.0 | 90.2 | 80.4 | 78.0 | 78.4 | 25.3 |
VSurvival assessments of oospores were determined by the plasmolysis method (Jiang and Erwin, 1990).
XSterile soil with moisture at field capacity (22.5% volumetric water content).
YAt each temperature regimen, the values of oospores viability throughout exposure time are expressed as a percentage of the initial viability value.
ZPercentage values are the average of three counts of 100 oospores.
Almost one century ago, heat started to be used to inactivate spores of different microorganisms, together with the implementation of the logarithmic transformation, and the application of simple linear regressions for the analysis of the results obtained2,7,27.
Our results are in line with those reported in an early study demonstrating that the logarithmic relationship between time and temperature on the survival of four phytopathogenic soilborne fungi (microsclerotia of Verticillium dahliae, oospores of Pythium ultimum, chlamydospores of Thielaviopsis basicola and moniloid cells and runner hyphae of Rhizoctonia solani) was maintained at temperatures ranging from 37 to 50°C27. Futhermore, in the latter study, it was observed that the exposure time required to kill 90% of P. ultimum oospores increased as the temperature decreased below 50°C.
Seventy years ago, an early report described the results on the exposure to intermittent heat for the control of plant pathogens14. Since then, only a limited number of assays have been published7,9,26,31. An important fact is that the tolerance to heat exposure varies among different fungal species and consequently, further studies are needed to define the effect of heat in relationship to the different fungal plant pathogens26.
Several authors have assessed laboratory thermal inactivation of different pathogens including pythiaceous fungi 2,3,7,18,27,32. However, odd data available for heat inactivation of oospores only defines lethal treatment doses, with the exception of one current report in which survival models of thermal inactivation have been established with Aphanomyces cochlioides oospores9.
In an early report on thermal laboratory inactivation3, it was observed that 5-8 week old P. capsici oospores inoculated in moistened autoclaved soil and subjected to 50°C for 30minutes were still capable of producing disease in cucumber test plants. However, if survival of oospores was assessed by the plating technique on PDA medium instead of using test plants, 50°C for 30minutes was lethal. It has been shown that Phytophthora cactorum and Phytophthora megasperma oospores are less heat resistant: 45°C for 30minutes was lethal for oospores of P. cactorum and 45°C for 20minutes was sufficient to kill P. megasperma oospores (low temperature isolate). However, a high temperature isolate of P. megasperma survived (3-6%) a heat treatment of 45°C for 30minutes18. In other heat inactivation reports involving P. ultimum, Pythium sylvaticum and P. aphanidermatum oospores, the latter species was shown to be the most resistant one, and this fact is not surprising since it is considered a hot weather pathogen3,27,32. Pythium aphanidermatum oospores after 30minutes exposure to 52.5°C in moisten autoclaved soil were still capable of producing disease in cucumber test plants. Yet if the survival of oospores was assessed by the plating technique on PDA medium instead of using test plants, 52.5°C for 30minutes was lethal3. In the same way, P. sylvaticum oospores subjected to 50°C for 30minutes were not capable of producing disease, but when the survival of oospores was assessed by the plating technique, 45°C for 30minutes was lethal3. At 37°C, the exposure time for LD90 was 25.8days for P. ultimum oospores and at 50°C the LD90 value was 27minutes in moistened natural soil27. It also has been reported that P. ultimum thin-walled oospores were killed by heat at 50°C for 30minutes, but that thick-walled oospores needed higher temperatures (70°C for 30minutes)32.
Our estimated LD50 values in moistened soil were almost similar to those reported by Dyer et al9 in water at 40, 45, 50°C with A. cochlioides oospores. Considering that heat transmission in water is greater than in moistened soil for short periods of time6, we can conclude that P. capsici oospores appear to be less heat tolerant than A. cochlioides oospores, even though the comparison between different published reports is hampered due to the different methodologies applied. Our estimated LD50 values in water at 45 and 50°C were lower than those estimated by Dyer at al9 and thus, it was reconfirmed that A. cochlioides oospores are more resistant than P. capsici oospores.
In conclusion, when comparing our results with those of other oospores of different species, such as P. cactorum, P. megasperma, P. ultimum, P. sylvaticum and P. aphanidermatum (and discussed above), P. capsici and A. cochlioides appear to need higher temperatures and/or longer exposure times to achieve eradication, and thus, their control is theoretically more difficult and complex.
In our work, using the plasmolysis method as viability test, oospores buried in moistened soil and heated at 50°C during 30minutes showed a survival rate of 95%, whereas between 4 and 6hours of exposure time was needed to kill all the oospores (table 1). At the same temperature, through the use of linear regression, 4.68hours was estimated as the necessary exposure time to achieve total lethality (table 2). Assessing oospore survival by plating techniques in selective agar media produces the problem in which germinability percentages for oospores of P. capsici are very low, ranging from 0 to 40%15, and therefore, the survival of oospores is underestimated. In a solarization trial for the control of oospores of P. capsici and Phytophthora nicotianae buried at 10 and 25cm soil depth6, no consistent results were obtained because the survival evaluation was hindered by the poor germination rates. Only approximately 1% of the oospores were germinated in the Phytophthora selective agar medium PARPH16.
In the present study, an exhaustive evaluation of temperature and time effect on oospore survival has been carried out with the aid of the plasmolysis viability test, leading to the construction of kinetic models for the study of thermal inactivation of P. capsici oospores. As in other previous reports2,27, our models were calculated (by linear regression) relating the oospores viability with exposure time at each temperature and thus achieving satisfactory fitness. Nevertheless, within this model, constant temperatures of 30, 25 and 15°C in moistened soil did not accurately describe inactivation. Instead, low determination coefficients were displayed (table 2). This could be explained by the fact that temperatures below 35°C poorly affected oospore survival. A lack of fit of the model to the data was also observed at these temperatures in agreement with Coelho et al7. At constant temperatures of 40°C or above, the required exposure times were low and the estimated models by linear regression fitted well with the data in moistened soil (R2 values between 0.69 and 0.85). Our model was also well-fitted at a constant temperature of 35°C (R2=0.75) as well as at the two daily cycling temperature regimes of 4hours at 40°C (R2=0.94) and 5hours at 35°C (R2=0.76), despite the fact that the exposure times required to kill all the oospores were very high (2,134, 684 and 2,845hours, respectively) (table 2). This is in keeping with the findings of the above mentioned study7, that reported that P. nicotianae chlamydospores were hardly affected by temperatures of 35°C. Maximum temperature for in vitro mycelium growth of P. capsici has been established between 35 and 36°C10, and consequently, it is not surprising that temperatures equal or below 35°C do not affect the survival of oospores, since these propagules are widely considered more resistant than mycelium.
For this reason, solarization treatments in field conditions for the control of P. capsici will be most successful if application time and site conditions achieve temperatures above 35°C. Our results obtained in the assay of daily cycling temperature regime (5hours at 35°C) proved that solarization would fail even if extended up to 10weeks, since 25% of the oospores were still viable. As opposed to the above regime, the daily cycling temperature regime (4hours at 40°C) stamped out disease after 4weeks (table 4).
Time and temperature data obtained in thermal inactivation studies with cycling temperatures under controlled environmental conditions may be used to develop reliable treatment guidelines for solarization under field conditions. Additionally, the cumulative number of hours at which soil temperatures exceed the threshold can be calculated to determine the duration of treatment necessary for the control of soilborne plant pathogens8.
Since each pathogen requires a specific exposure time at each temperature for its control8, thermal inactivation with cycling temperatures in controlled environmental conditions carried out in our study with P. capsici oospores can be useful in indicating the solarization effectiveness for the control of this pathogen under field conditions.
A method to relate cycling and constant temperature regimes can be very practical, because it offers the possibility to predict solarization effectiveness in each particular case based on the kinetic model of inactivation at constant temperatures.
The cumulative number of hours above 40°C in the cycling regime (4hours at 40°C and 20hours at 25°C) required to kill 25, 50, 75 and 100% of the P. capsici oospores population in moistened soil were respectively: LD25=33, LD50=60, LD75=87 and LD100=114hours. These values were obtained by dividing by six (= 24hours day/4hours at 40°C) the total number of hours of incubation time in the cycling regime of 40°C (table 2). When compared with the lethal doses estimated in the constant regime of 40°C (LD25=3, LD50=68, LD75=134 and LD100=199hours), the exposure times required in the constant regime were higher than in the cycling regime except for the LD25 value which was lower. This fact appears to indicate that once a minimum cumulative number of hours above 40°C is achieved (which in our study has been estimated close to 60hours), P. capsici oospores start to die faster in the cycling regime (when measured in the effective cumulative time). The latter observation could be caused by the weakening of oospores due to sublethal heating, which is a widespread phenomenon that has been demostrated with a variety of pathogens1,12. These authors speculate that an irreversible deterioration in viability of conidia of Fusarium oxysporun f. sp. niveum is inflicted by the heat shock, thus reaching a “point of no return” from which they can no longer recover. These authors also suggest that pathogen propagules might be affected under lower temperatures, possibly facilitating pathogen control by heating.
The results reported herein are also in agreement with Dyer et al9 who determined that survival of A. cochlioides oospores exposed to alternating high and low temperatures in water (four 24hours cycles at 45°C for 4hours and 21°C for 20hours) fitted closely to a cumulative effects model. They also confirmed that dormant oospores that survived a high temperature period remained dormant, accumulating heat-related damages.
The estimated cumulating hours above 35°C were compared between the constant and the cycling regimes at 35°C. The estimated exposure times required to kill the same percentage of oospore population were always lower in the cycling regime (LD25=187, LD50=322, LD75=457 and LD100=593hours) than in the constant regime (LD25=470, LD50=1,025, LD75=1,579 and LD100=2,134hours) as opposed to the constant and the cycling regimes at 40°C. Of note, the LD values of the cycling regime at 35°C were calculated by dividing by 4.8 (= 24hours day/5hours at 35°C) the corresponding values shown in table 2. Our results at 35°C are in agreement with Assaraf et al1 who indicated that pathogen mortality can be achieved at temperatures that are lower than those needed for immediate full control, as long as sufficient time is allowed for the delayed heat mortality. The significance of this phenomenon can be applied to solarization, where soil heating is relatively mild.
Our study closely reproduces common temperature profiles observed in soil solarization assays by using soil moisture adjusted to field capacity. These conditions are equivalent to those found in real field conditions, except for the use of sterilized soil with the aim of the exclusive evaluation of thermal effects. Sublethal effects of high temperature, such as increased susceptibility of microbial infection, can also contribute to plant pathogens control under solarization35. Time and temperature requirements developed under sterile laboratory conditions will not reflect the possible contribution of antagonistic soil organisms or the release of chemical toxicants in the heated field soils to plant pathogens mortality33,34. The models constructed under these conditions are expected to overestimate the time necessary for pathogen death8.
Consequently, the model developed in our study with sterile soils is also expected to overestimate the time needed for P. capsici oospores eradication.
Since constant and intermittent temperatures below 40°C were not effective to inactivate P. capsici oospores, other alternatives that can improve these results have to be explored in the future. One possibility could be the combined use of sublethal temperatures and organic amendments for the improvement of P. capsici control by soil solarization. This can be especially useful in temperate regions with climatic conditions and a crop season unsuitable for the application of soil solarization. Under a particular climate and a compatible season with the crop production cycle, data presented in this study may provide a helpful tool for evaluating the effectiveness of solarization in reducing the soil oospores population of P. capsici, an economically important soilborne plant pathogen worldwide.
FinancingAitzol Etxeberria was the recipient of a grant from the Basque Government. This research was supported by the National Institute for Agricultural and Food Research and Technology (INIA) of the Spanish Ministry of Agriculture, Fisheries and Food (project RTA-2008-00058-C03; PLAN NACIONAL de INVESTIGACION CIENTÍFICA, DESARROLLO e INNOVACIÓN TECNOLÓGICA) and by the Department of Agriculture, Fisheries and Food of the Basque Government (project BIOFUMI).
Conflict of interestsAuthors have no conflict of interests.





![Regression lines corresponding to Phytophthora capsici oospores viability versus exposure time (first 10hours) at different temperature regimes and media (constant temperatures in sterile moistened soil [continous lines]: 53, 50, 47.5, 45 and 40°C; cycling temperature in sterile moistened soil [two dotted single dashed lines]: 40°C for 4hours and 25°C for the remainder of each day; constant temperatures in water [dashed lines]: 53, 50 and 45°C). Regression lines corresponding to Phytophthora capsici oospores viability versus exposure time (first 10hours) at different temperature regimes and media (constant temperatures in sterile moistened soil [continous lines]: 53, 50, 47.5, 45 and 40°C; cycling temperature in sterile moistened soil [two dotted single dashed lines]: 40°C for 4hours and 25°C for the remainder of each day; constant temperatures in water [dashed lines]: 53, 50 and 45°C).](https://static.elsevier.es/multimedia/11301406/0000002800000002/v1_201305061355/S1130140611000295/v1_201305061355/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)