The colonization of the surfaces of dental prostheses by Candida albicans is associated with the development of denture stomatitis. In this context, the use of fluconazole has been proposed, but its disadvantage is microbial resistance. Meanwhile, the oil of Allium sativum has shown an effect in controlling biofilm formation by C. albicans.
AimsThe objective of this study was to determine the antifungal activities of the essential oil of A. sativum and fluconazole against clinical isolates of Candida species obtained from rigid, acrylic-based partial or total dentures and to compare these agents’ effects on both biofilm and planktonic cells.
MethodsA total of 48 clinical isolates obtained from the acrylic surface of partial or complete dentures were examined, and the following species were identified: C. albicans, Candida glabrata, Candida tropicalis, and Candida krusei. For each isolate, the antifungal activities of the essential oil of A. sativum and fluconazole against both biofilm and planktonic cells were evaluated using the Clinical & Laboratory Standards Institute (CLSI) M27-A3 method. The isolates were also evaluated by semiquantitative XTT reduction.
ResultsAll planktonic Candida isolates were susceptible to the essential oil of A. sativum, whereas 4.2% were resistant to fluconazole. Regarding susceptibilities in biofilms, 43.8% of biofilms were resistant to A. sativum oil, and 91.7% were resistant to fluconazole.
ConclusionsAll planktonic cells of the different Candida species tested are susceptible to <1mg/ml A. sativum oil, and the majority are susceptible to fluconazole. Susceptibility decreases in biofilm cells, with increased resistance to fluconazole compared with A. sativum oil. The essential oil of A. sativum is thus active against clinical isolates of Candida species obtained from dentures, with effects on both biofilm and planktonic cells in vitro.
La colonización por parte de Candida albicans de las superficies de las prótesis dentales se asocia con el desarrollo de estomatitis. Se ha propuesto el uso de fluconazol, pero su desventaja es la resistencia microbiana. El aceite de Allium sativum ha mostrado su efectividad al controlar la formación de biopelícula de objetivos.
ObjetivosDeterminar la sensibilidad de cepas clínicas de especies de Candida, obtenidas de prótesis dentales parciales o totales rígidas de base acrílica, al aceite esencial de A. sativum y comparar su efecto en células planctónicas y en biopelícula.
MétodosSe incluyeron 48 cepas clínicas de la superficie acrílica de prótesis dentales totales o parciales, identificadas entre las siguientes especies: C. albicans, Candida glabrata, Candida tropicalis y Candida krusei. Se evaluó la sensibilidad de cada una al aceite esencial de A. sativum y al fluconazol mediante la metodología M27-A3 del CLSI, tanto sobre células planctónicas como en biopelícula, y mediante el método semicuantitativo de la reducción de XTT en el último caso.
ResultadosTodas las cepas planctónicas de Candida fueron sensibles al aceite esencial de A.sativum, mientras que el 4,2% fue resistente al fluconazol. En cuanto a su sensibilidad en biopelícula, el 43,8% fue resistente a A. sativum y el 91,7% lo fue al fluconazol.
ConclusionesTodas las cepas en forma planctónica de las diferentes especies de Candida fueron sensibles a concentraciones inferiores a 1mg/ml del aceite esencial de A. sativum y en menor proporción a fluconazol. La sensibilidad disminuyó en las células en biopelícula, con mayor resistencia al fluconazol en comparación con el aceite esencial de A. sativum. Por tanto, el aceite esencial de A. sativum es activo frente a cepas clínicas de diferentes especies de Candida, obtenidas de dentaduras, con efectos en biopelícula y células planctónicas in vitro.
The incidence and prevalence of fungal infections have increased considerably in recent years. The main fungal cause of these infections is the genus Candida,20 comprising 17 species of medical interest that have been associated with infections in humans, and particularly Candida albicans, Candida tropicalis, Candida parapsilosis, and Candida glabrata.10,33
Wearing complete dentures is a risk factor because these prostheses can promote Candida biofilm formation and oral candidiasis.31Candida infections are commonly associated with biofilms on both the mucosa and the plastic surfaces of indwelling devices. These biofilms consist of matrix-enclosed micro-colonies of yeast, hyphae, and pseudohyphae arranged in a complex structure18 and are inherently resistant to antifungals, so affected devices generally need to be removed.34,35
Candida pathogenicity has been attributed to several virulence factors, including adhesion to host cells or medical devices; biofilm formation; and the secretion of hydrolytic enzymes such as proteases, phospholipases, and hemolysins.14,42 Among clinical Candida strains, biofilm formation is variable and depends on the species.25,28
Colonization of and biofilm formation on the surfaces of dental prostheses comprise important risk factors for the development of denture stomatitis because they support diverse microbial species, promoting the mechanisms that confer resistance and increase pathogenicity in Candida in particular. In fact, Bilhan et al. (in 2009) and De la Rosa et al. (in 2012) reported a significant association between the presence of C. albicans on prosthetic surfaces and the development of denture stomatitis.5,17
Regarding the use and care of dental prostheses, hygienic techniques and the use of medicines, antiseptics, and nanomaterials, among other approaches, have been proposed to control biofilm development and possible secondary conditions associated with the microorganisms present in these prostheses.21,38 However, among the disadvantages of these proposed approaches are the emergence of strains with antimicrobial resistance and alterations in the prosthetic structure. Therefore, studies of alternative treatments that offer the fewest secondary effects are required. Alternative medicine includes several natural compounds that are effective against certain pathogenic species of interest in dentistry, as in other medical fields.27,30
The essential oil of Allium sativum comprises one of the natural alternatives most robustly proven to exhibit activity against diverse microorganisms. Previous works report that allicin is one of the active components of A. sativum oil and exerts a significant effect in inhibiting the growth of Pseudomonas aeruginosa biofilms.30 In 2011, Khodavandi et al. reported that the oil of A. sativum has a similar effect in controlling biofilms of C. albicans American Type Culture Collection (ATCC) strains in comparison with fluconazole.27
The purpose of the present study was to determine the antifungal activity of the essential oil of A. sativum and to compare it with that of fluconazole in the context of clinical isolates of Candida species obtained from dental prostheses.
Materials and methodsStudy design and clinical isolatesA cross-sectional study approved by the institutional bioethics committee was conducted. A total of 56 patients aged ≥40 years with total or partial bilateral, rigid, acrylic-based dental prostheses (16 maxillary, 12 mandibular and 28 both maxillary/mandibular) and with a minimum of 6 months of use were included in our study. When the patients had both types of prostheses (maxillary/mandibular), only one sample was taken. All of them had requested diagnostic dental care during the period of July to December 2014.
Patients with flexible dental prostheses were excluded. Those who agreed to participate were given a brief explanation of the protocol and were then asked for authorization by signing an informed consent form.
The patients were asked to remove their dental prosthesis from their oral cavity in order to undergo a clinical examination of the oral mucosa with artificial light and a dental mirror. Any pathological lesions observed were registered. If a patient was diagnosed with sub-prosthetic stomatitis, exfoliative cytology was performed, and the sample was stained using the Gram technique to discern whether yeast, hyphae, and/or pseudohyphae were present. The cytology result was reported to the patient, and the case was then followed until remission.
Samples were taken from the internal surfaces of the acrylic prosthesis basis with a sterile swab (Protec®; México, D.F., México); in particular, after the prosthesis was removed, scraping of all support surfaces that were in contact with the palate and the alveolar ridges was immediately performed. After being obtained, the swabs were suspended in 500μL of 0.9% saline solution (PiSA, Jalisco, Mexico) and were then immediately taken to the microbiology laboratory for processing.
Microbiological assessment and identification of isolatesEach sample collected was mixed for 20s with a vortex, after which 100μl of the suspension were plated on CHROMagar Candida (CHROMagar®; Paris, France) and incubated at 36°C for 2 days. To ensure that seemingly negative samples were in fact negative, these samples were incubated for an additional 7-day period at a temperature of 30±1°C. Using chromogenic cultures, presumptive identification of Candida species was performed according to the colorimetric characteristics described by the manufacturer of the agar for each species (colonies green in color=C. albicans, mauve in color=C. glabrata, pink in color with a curly texture=C. krusei, and blue in color=C. tropicalis). The use of this medium allowed the separation of two or more strains from the overgrowth of different species from the same sample. Cultures positive for one or more species and purified cultures were reseeded and purified on Sabouraud glucose agar plates (Difco®; Detroit, MI, USA) by incubating the cultures at 36±1°C for 2 days and confirming the species’ presence using an optical microscope. Filamentous morphology was observed on cornmeal agar (Difco®). Subsequently, the yeast species were identified using the carbohydrate assimilation ID 32C AUX system and the Apiweb™ database (bioMérieux®; Marcy l’Etoile, France). The phenotypic isolates were preserved in yeast peptone dextrose (YPD) broth (1% w/v yeast extract, 2% w/v peptone, and 2% w/v dextrose; Difco®) with 50% glycerol and frozen for later use.
Growth conditionsAll organisms were stored in vials of distilled water at room temperature. Later, each strain was grown in YPD medium (yeast extract 1%, w/v, peptone 2%, w/v dextrose 2%, w/v) in threaded centrifuge capped-cone tubes (Falcon # 2095, 17,120mm, Becton Dickinson, Franklin Lakes, NJ, USA), and incubated overnight on an orbital shaker (Labnet, NJ, USA) at 36°C. Each culture grew in the budding-yeast phase under these conditions. The cells were harvested on three occasions and washed in sterile phosphate buffer (2.7mM potassium chloride and 137mM sodium chloride, pH 7.4; Sigma–Aldrich®; St. Louis, MO, USA). Next, the cells were resuspended in RPMI-1640 supplemented with l-glutamine (Sigma–Aldrich®), buffered with morpholino-propane sulfonic acid (MOPS) (Sigma–Aldrich®) and adjusted to the desired cellular density, which was equivalent to 1–1.5×106 colony-forming units (CFU) per ml. Cellular density was determined by counting the cells in a hemocytometer (Propper Manufacturing Company, Inc., NY, USA). This cell concentration was selected because previous researchers have demonstrated that optimal biofilm formation occurs at this particular density.27 The standardized cell suspension was used immediately.
Biofilm assaysFor biofilm and antifungal activity assays, each experiment was performed in triplicate to confirm the results. The standard deviation (SD) was calculated for three independent experiments. We used three strains (C. albicans ATCC 90028, Candida krusei ATCC 6258 and C. parapsilosis ATCC 96142) as controls in each experiment.
Biofilm assays were performed as previously described,35 but pre-sterilized, polystyrene, flat-bottomed, 96-well microtiter plates (Costar®, EIA/RIA plate, with a low evaporation lid and high binding; Corning, NY, USA) were used. Biofilms were formed by pipetting standardized cell suspensions (100μl of a suspension containing 106 cells/ml in RPMI-1640) into selected wells of microtiter plates and incubated for 48h at 37°C. After biofilm formation, the medium was aspirated, and non-adherent cells were removed by thoroughly washing the biofilms three times in sterile phosphate-buffered solution (PBS) (137mM NaCl, 12mM KCl, 2.7mM Na2HPO4, and 2.7mM KH2PO4, pH 7.4; Sigma–Aldrich®).
Measurement of biofilm formationA semi-quantitative measurement of biofilm formation was performed using an XTT (2,3-bis (2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide) (Sigma–Aldrich®; St. Louis, MO, USA) reduction assay, essentially as described previously.38 Briefly, XTT was prepared as a saturated solution at a concentration of 0.5g/l in Ringer's lactate (PiSA, Jalisco, Mexico). This solution was filter sterilized through a filter with a 0.22-μm pore size, aliquoted, and then stored at −80°C. Prior to each assay, an aliquot of XTT stock was thawed, and menadione (Sigma–Aldrich®; 10nM, prepared in acetone) was dispensed into the aliquot at a final concentration of 1μM. A 100-μl aliquot of the XTT/menadione was then added to each pre-washed biofilm to measure metabolic activity levels. Specifically, samples were incubated in the dark for 1h at 37°C, and the colorimetric change (a reflection of the biofilm's metabolic activity) was measured using a microplate reader system (Thermo Fisher Scientific, Shanghai, China) at 492nm.
Microscopic examinations of the biofilms that formed on microtiter plates were conducted by light microscopy using an inverted microscope (Zeiss, Oberkochen, Germany).
Antifungal activity testingOne clinically utilized antifungal agent, i.e., fluconazole (Pfizer, Toluca, Mexico), and one alternative agent, i.e., the essential oil of A. sativum, were used in this study. Fluconazole was prepared at a stock concentration of 1280μg/ml in RPMI-1640 (Sigma). The essential oil of A. sativum was obtained by hydrodistillation at low pressure and low temperature and GC-MS, as described previously6; the oil distillation was performed by a distillation company (Javier Morales-López, distiller). Final concentrations of fluconazole and the essential oil of A. sativum, ranging from 0.5 to 128μg/ml and from 7.8 to 1000μg/ml, respectively, were tested. Prior to susceptibility testing, each isolate was sub-cultured on Sabouraud dextrose agar and CHROMagar Candida to ensure purity and viability. Susceptibility to fluconazole and the essential oil of A. sativum was tested via a broth microdilution assay according to M27-A3 using updates provided by M27-S4 of the Clinical & Laboratory Standards Institute (CLSI).12,13
RPMI-1640 medium without bicarbonate was prepared with L-glutamine and buffered at pH 7.0 (with 0.165M MOPS). Each yeast inoculum suspension was prepared using a spectrophotometer to obtain a final concentration of 0.5–2.5×103cells/ml. The trays were incubated in air at 35°C, and minimal inhibitory concentration (MIC) endpoints were read visually after 24h. To eliminate the effects of trailing growth, in cases with low growth, spectrophotometric endpoints for fluconazole were also determined at 24h after stirring with wooden sticks, and readings were performed at 48h. The visual and spectrophotometric endpoints were defined as the lowest drug concentrations that resulted in a prominent decrease in growth and a 50% reduction in optical density (OD), respectively, when compared with the data for a drug-free growth-control well. Quality control was performed by testing the Candida strains recommended by the CLSI, which included C. parapsilosis ATCC 22019 and C. krusei ATCC 6258.
The interpretive susceptibility criteria employed for fluconazole were the same as those specified by the CLSI in the M27-S4 document13 and considered the following three categories: S=susceptible, SDD=susceptible dose-dependent, and R=resistant. Isolates of C. krusei are considered resistant to fluconazole, irrespective of the MIC.
Biofilms were washed thoroughly three times with sterile PBS prior to the addition of the antifungal agent, and free wells were included to serve as controls. Biofilm MICs were determined at 50% inhibition (MIC50Biofilm) compared with drug-free control wells by utilizing the XTT reduction assay described previously. To evaluate the susceptibility of planktonic and biofilm Candida species to both the essential oil of A. sativum and fluconazole, the following performance criteria were considered: in the case of fluconazole, the breakpoints were those described by the CLSI in the M27-S4 document, and in the case of the oil of A. sativum, as there were no previously described cutoffs, the MIC50 was considered as being reached when 50% inhibition was determined at the concentrations tested (1–1000μg/ml).
Statistical analysisA descriptive analysis was performed, categorizing the study population into the following three age groups: (I) 60–69 years, (II) 70–79 years, and (III) ≥80 years. Mann–Whitney U tests were performed to assess whether the age of the prosthesis generated differences with respect to the presence of denture stomatitis and filamentation. Kruskal–Wallis tests were also performed to analyze the categorized species of Candida and the MIC50 of fluconazole.
The Spearman test was used to determine whether there was a correlation between prosthetic age and biofilm formation and between the fluconazole planktonic MIC50 and the oil of A. sativum planktonic MIC50. For all tests, a significance level of p≤0.05 was considered. Differences between categorical variables were evaluated using the chi-square test. Comparisons of the species distribution and MIC distribution were additionally performed using the chi-square test for categorical variables. For all statistical analyses, we used the statistical software SPSS ver. 20.0 for Windows (IBM; Chicago, IL, USA).
ResultsIn total, 56 patients were studied, with 66% (n=37) female (average age, 61.2 years) and 34% (n=19) male (average age, 62.6 years). In all, 39.2% (n=22) had denture stomatitis, with 32% (n=18) of cases in the palate and 7.2% (n=4) in both the palate and the mandibular alveolar ridges. A total of 37 prostheses were colonized by one or more species of Candida, including 8 maxillary prostheses, 9 mandibular prostheses, and 20 maxillary/mandibular prostheses, from which 48 isolates were obtained. In all, 41 samples were total dentures, and 15 were partial dentures with acrylic bilateral support. No significant differences were observed in the analysis by type; however, a complete analysis of all prosthetic surfaces is presented. The most common species was C. albicans, with 25 isolates, followed by C. glabrata, with 11 isolates; C. tropicalis, with 10 isolates; and C. krusei, with two isolates.
The biofilm-forming capacity was evaluated through semi-quantitative spectrophotometric measurement of the reducing salt XTT at a wavelength of 492nm, which provides OD values proportional to the number of viable cells forming biofilms. Based on the previously proposed classification,39 it was determined that all isolates were strong producers of biofilms.
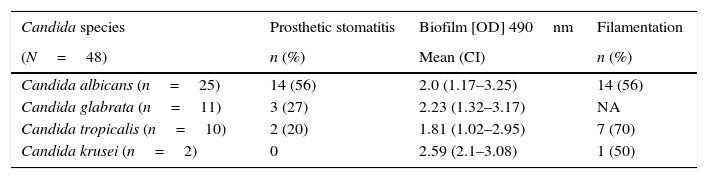
Considering the specific species, C. krusei had the highest values for biofilm formation, with an average OD at 490nm of 2.59. C. glabrata was in second place, with the greatest capacity and an average OD at 490nm of 2.23. Finally, C. albicans showed an average OD at 490nm of 2.0, and C. tropicalis had an average OD at 490nm of 1.81 (Table 1). Regarding the ability of the isolates to exhibit filamentation, there were no significant differences (p=0.14, chi2 test) according to their clinical origin.
Prosthetic stomatitis frequency, biofilm formation (mean optical density [OD]490), and filamentation capacity among Candida species isolates.
| Candida species | Prosthetic stomatitis | Biofilm [OD] 490nm | Filamentation |
|---|---|---|---|
| (N=48) | n (%) | Mean (CI) | n (%) |
| Candida albicans (n=25) | 14 (56) | 2.0 (1.17–3.25) | 14 (56) |
| Candida glabrata (n=11) | 3 (27) | 2.23 (1.32–3.17) | NA |
| Candida tropicalis (n=10) | 2 (20) | 1.81 (1.02–2.95) | 7 (70) |
| Candida krusei (n=2) | 0 | 2.59 (2.1–3.08) | 1 (50) |
NA=not applicable. CI=confidence interval.
The characterization of the components of the essential oil of A. sativum is presented in Table 2. The MIC50 values for the essential oil of A. sativum and fluconazole when applied to the clinical isolates of Candida in the planktonic cell and biofilm states were determined.
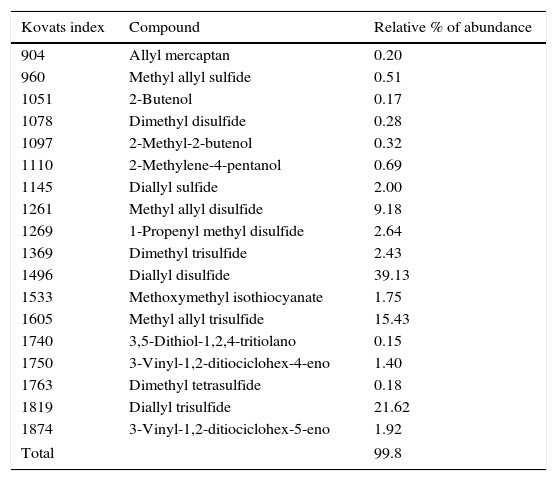
Chemical composition of the essential oil used.
| Kovats index | Compound | Relative % of abundance |
|---|---|---|
| 904 | Allyl mercaptan | 0.20 |
| 960 | Methyl allyl sulfide | 0.51 |
| 1051 | 2-Butenol | 0.17 |
| 1078 | Dimethyl disulfide | 0.28 |
| 1097 | 2-Methyl-2-butenol | 0.32 |
| 1110 | 2-Methylene-4-pentanol | 0.69 |
| 1145 | Diallyl sulfide | 2.00 |
| 1261 | Methyl allyl disulfide | 9.18 |
| 1269 | 1-Propenyl methyl disulfide | 2.64 |
| 1369 | Dimethyl trisulfide | 2.43 |
| 1496 | Diallyl disulfide | 39.13 |
| 1533 | Methoxymethyl isothiocyanate | 1.75 |
| 1605 | Methyl allyl trisulfide | 15.43 |
| 1740 | 3,5-Dithiol-1,2,4-tritiolano | 0.15 |
| 1750 | 3-Vinyl-1,2-ditiociclohex-4-eno | 1.40 |
| 1763 | Dimethyl tetrasulfide | 0.18 |
| 1819 | Diallyl trisulfide | 21.62 |
| 1874 | 3-Vinyl-1,2-ditiociclohex-5-eno | 1.92 |
| Total | 99.8 | |
The MIC50 for all isolates studied in planktonic growth was between 31.2 and 1000μg/ml. The MIC50 values by species were as follows: 188μg/ml for C. tropicalis, 236.2μg/ml for C. albicans, 321μg/ml for C. glabrata and 503μg/ml for C. krusei. There were no essential-oil-resistant isolates at ≥1000g/ml.
Biofilm cellsIn the biofilm state, by species, the MIC50 values were significantly higher: a MIC50Biofilm of 603.1μg/ml was determined for C. albicans, followed by MIC50Biofilm values of 640.6 and 667μg/ml for C. glabrata and C. tropicalis, respectively. For C. krusei, the MIC50Biofilm was >1000μg/ml. Among all species, cases of resistance to the essential oil of A. sativum occurred, as evidenced by insensitivity to the highest concentration evaluated; accordingly, 24% of all isolates of C. albicans were resistant, and 54.5% of C. glabrata, 70% of C. tropicalis, and 100% of C. krusei isolates were resistant.
Antifungal activity of fluconazolePlanktonic cellsThe fluconazole MIC50 in the planktonic state (or suspension) was 8.92μg/ml for C. albicans, which was the species most susceptible to fluconazole. The second most sensitive species was C. tropicalis, with a MIC50 of 11.1μg/ml, and the least sensitive species were C. glabrata and C. krusei, with MIC50 values of 13.6 and 19μg/ml, respectively. In total, 60% of all C. albicans isolates and 18% of C. glabrata isolates were fluconazole resistant, and 82% of C. glabrata isolates were SDD. Additionally, C. tropicalis was resistant in 30% of cases and SDD in 20% of cases, and overall C. krusei was resistant.
Biofilm cellsThe fluconazole MIC50Biofilm for biofilm isolates was achieved only in two isolates of C. albicans, in one C. glabrata isolate, and in one C. krusei isolate; in all cases, the MIC50Biofilm was 64μg/ml. According to the fluconazole breakpoints for Candida described by the CLSI in the M27-S4 document (the only benchmarks described), 100% resistance was observed for all species.
The essential oil of A. sativum compared with fluconazoleWhen comparing the percentages of sensitivity or resistance to the essential oil of A. sativum and fluconazole among the planktonic states of the different Candida species, a difference was determined without observing statistical significance in the chi-square test (p=0.12), whereas a significant difference was observed in the biofilm state by species (p<0.01). However, the susceptibility of the isolates to both antifungal agents was observed as a decrease in biofilm growth.
By comparing the concentrations required to produce inhibition of at least 50% of the growth of Candida in the planktonic and biofilm states, it was determined that the required concentration of the essential oil of A. sativum may be up to 300% higher for cells in biofilms. By contrast, the fluconazole concentration required to inhibit biofilms of Candida cells may be up to be 700% higher. The analysis of this difference is presented in Table 3.
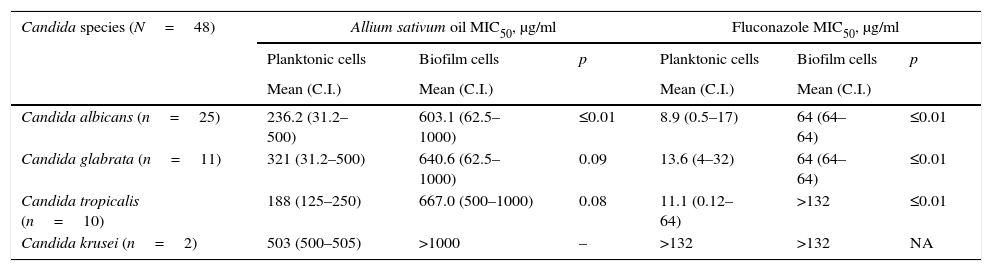
Comparison of the antifungal activities of Allium sativum essential oil and fluconazole in biofilm and planktonic cells.
| Candida species (N=48) | Allium sativum oil MIC50, μg/ml | Fluconazole MIC50, μg/ml | ||||
|---|---|---|---|---|---|---|
| Planktonic cells | Biofilm cells | p | Planktonic cells | Biofilm cells | p | |
| Mean (C.I.) | Mean (C.I.) | Mean (C.I.) | Mean (C.I.) | |||
| Candida albicans (n=25) | 236.2 (31.2–500) | 603.1 (62.5–1000) | ≤0.01 | 8.9 (0.5–17) | 64 (64–64) | ≤0.01 |
| Candida glabrata (n=11) | 321 (31.2–500) | 640.6 (62.5–1000) | 0.09 | 13.6 (4–32) | 64 (64–64) | ≤0.01 |
| Candida tropicalis (n=10) | 188 (125–250) | 667.0 (500–1000) | 0.08 | 11.1 (0.12–64) | >132 | ≤0.01 |
| Candida krusei (n=2) | 503 (500–505) | >1000 | – | >132 | >132 | NA |
NA=not applicable. C.I.=confidence interval.
There was also a significant difference (p<0.01) observed between the MIC50 and the MIC50Biofilm of the A. sativum oil in the analysis by species, and a significant difference was observed for C. albicans compared with the other species. Fluconazole exhibited no significant difference in its inhibition of C. albicans in biofilm or planktonic cells, but among the remaining Candida species, it was not possible to evaluate whether a difference was present due to the high resistance observed.
Although the range of denture use duration was broad, namely, <1 year to >20 years, when assessing whether the prosthetic age generated differences in the presence of stomatitis (p=0.33) and filamentation (p=0.79), no significant differences were found. With respect to the categorized Candida species (p=0.91) and the MIC50 of fluconazole (p=0.86), no differences were found either. The Spearman correlation revealed that in our sample, there was no correlation of age with biofilm formation (p=0.21), the MIC50 of fluconazole in planktonic cells (p=0.75) or the MIC50 of the essential oil of A. sativum in planktonic cells (p=0.49).
DiscussionIn this study, the in vitro susceptibility of different Candida species obtained from isolates of total dentures or partial dentures with acrylic bilateral support to both the oil of A. sativum and fluconazole was evaluated. Initially, when the Candida species were isolated from the surfaces of the dentures and typed, a prevalence of 52% was found for C. albicans, followed by 23% for C. glabrata, 21% for C. tropicalis, and 4% for C. krusei. These prevalence values were similar to those reported in the literature: in 2012, Ribeiro et al. specifically reported a prevalence of 64.4% for C. albicans, 14.4% for C. glabrata, and 3.3% for C. tropicalis when examining colonization of the surfaces of dental prostheses.37
Moreover, in 2013, Cavaleiro et al. reported the following frequencies of Candida colonizing the oral mucosa of healthy patients: 66% for C. albicans, 9% for C. glabrata, and 4.5% for C. tropicalis.8 It has been noted that the Candida species that colonize prosthetic surfaces usually correspond to those found colonizing the oral mucosa of patients.8
Once a dental prosthesis is set in place, the microorganisms present in the mouth colonize its surface and eventually form a biofilm on the acrylic surface. Interestingly, in the current study, the presence of more than one species was observed in 17.8% of the sampled patients, and 30% of these exhibited sub-prosthetic stomatitis. According to de la Rosa-García et al. (2013), the presence of more than one species increases the risk of developing infections.16
Meanwhile, the percentage of patients with sub-prosthetic stomatitis was 39.2%; this frequency is low compared with other reports. In particular, De la Rosa-García et al. (2013) reported a frequency of 56.6% in a population of healthy subjects and patients with diabetes (n=99).16 Moreover, in the present study, it was found that the percentage of prostheses colonized by Candida species was 66%, similar to other reports: De la Rosa et al. (2012) found a 62.2% frequency of Candida colonization in prostheses.17 When risk factors associated with the presence of sub-prosthetic stomatitis were analyzed, no statistical associations with a poor fit of the prosthesis (p=0.66), colonization of the prosthesis (p=0.24), or high consumption of carbohydrates (p=0.14) were found. However, poor hygiene and nocturnal use of dental prostheses were factors that were significantly associated with the presence of sub-prosthetic stomatitis (p<0.01 and p=0.01, respectively). The microenvironment associated with poor hygiene at the contact surface of dental prostheses specifically promotes the development of Candida in addition to inhibiting the continuous flow of saliva, thereby favoring food accumulation and Candida adhesion to the surface. In addition, not removing prostheses at night does not allow the palatal epithelium and alveolar edges to recover from the trauma and maceration that exists under the prostheses. In this respect, our results are consistent with those previously reported in the literature.43,44
Regarding the biofilm formation capacity of Candida species in dental prostheses, “strong production” of biofilms was observed for all clinical isolates tested. In a previous study,39 it was determined that for C. albicans and C. tropicalis isolated from dental prostheses, the average capacity to produce biofilms at a time point of 24h was characterized by “moderate production”, whereas in a similar time frame, C. glabrata was classified as a “strong producer” of biofilms.
In that work, a comparison was performed between isolates from the prostheses and the buccal mucosa of patients with diabetes mellitus, and slight differences regarding biofilm production between the isolates from the two groups were mentioned.39 The difference in the ability of the biofilm-forming isolates previously reported and the ability observed in the present study might be due to the differing origins of the isolates. It has been noted that isolates recovered from dental prostheses are found in biofilms, which increases their adhesiveness. In addition, the condition and age of prostheses are decisive in activating microorganisms’ virulence factors and their capacity for survival in a complex ecosystem. In the present study, biofilms were quantified after 48h of formation; this production time was higher than the previously reported time of 24h.
For each of the clinical isolates in the present study, the MIC50 of the essential oil of A. sativum was determined in both planktonic and biofilm cells. The MIC50 of the A. sativum oil in planktonic growth of C. albicans was 236.2μg/ml; for C. glabrata, it was 321μg/ml; for C. tropicalis, it was 188μg/ml; and for C. krusei, it was 503μg/ml. Previous reports related to A. sativum refer to extracts of fresh garlic in aqueous medium and suspensions of garlic powder.1,23,26,29,41
Few reports have examined the concentrations of A. sativum extract required for a fungistatic or fungicidal effect on Candida species. Moreover, in the studies conducted to date, there is no consensus regarding MIC determination or the method of producing the garlic extract. In 2006, Shams-Ghahfarokhi et al. reported that the MIC50 of an aqueous extract of A. sativum in planktonic isolates of C. albicans, C. glabrata, and C. tropicalis was 31.2μg/ml.41 In 2002, Lemar et al. conducted a study of a C. albicans strain and noted that its growth was inhibited by fresh garlic extract at a MIC50 of 290μg/ml.29 Ghannoum reported a MIC50 for an aqueous extract of A. sativum within a range of 800–1600μg/ml for ATCC strains of C. albicans and C. tropicalis,23 with these being the effective concentrations of the A. sativum essential oil obtained by hydrodistillation.6 These values were determined in the studies by the method of microdilution and were able to affect clinical isolates obtained from biofilms developed on dental prostheses.
Other studies have evaluated uncharacterized extracts and employed different methodologies. In the present study, the MIC50 for C. albicans was found to occur at a greater concentration than that reported by Lemar et al. in 2002.29 However, those authors used YPD culture medium, whereas in the present study, we used RPMI-1640, which is the medium recommended by the standardized CLSI M27-A3 method.13 In addition, in the present study, a characterized essential oil of A. sativum was evaluated, in contrast to the aqueous extract employed in the previous work by Lemar et al. (2002)29; differences in the components of the two substances remain to be analyzed. In the current study, an A. sativum essential oil obtained by a hydrodistillation process, in which 47 compounds were detected, was evaluated. Of the compounds, sulfur compounds prevailed in the composition, and these have been reported to possess therapeutic effects.6 With respect to the biologically active components of A. sativum and their mechanism, allicin is a substance formed by exposing the organism's tissue to the oxygen in the air. This exposure releases the alliinase enzyme localized in the cell membrane, which acts on the substrate denominated alliin, subsequently releasing allicin. Allicin is an unstable substance that is a precursor of tri- and di-diallyl sulfide, the most abundant compounds in the essential oil.2,3
In the current study, the MIC50 of the essential oil of A. sativum in C. albicans biofilm growth was 603.1μg/ml; for C. glabrata, it was 640.6μg/ml; and for C. tropicalis, it was 667μg/ml. Furthermore, C. krusei demonstrated resistance to the concentrations employed. To our knowledge, there are no reports on the susceptibility of Candida species in biofilms to the essential oil of A. sativum. However, studies have examined the MICs of specific, active elements of garlic oil.40 In the present study, the specific concentration of each of the active compounds in the essential oil of A. sativum was not determined, but the MIC50 was clearly increased when cells were in the biofilm state because the structure of the biofilm delayed the flow of the compounds in question, together with virulence factors expressed by Candida when within a biofilm.15,19,24,40
When the MIC50 of fluconazole in isolates in planktonic form was determined, the average MIC50 of fluconazole for C. albicans was 8.92μg/ml; for C. glabrata, it was 13.6μg/ml; and for C. tropicalis, it was 11.11μg/ml. The MICs of these Candida strains can be interpreted as follows based on the cutoffs proposed by the CLSI12: C. albicans was fluconazole resistant in 60% of cases and SDD in 16% of cases, the susceptibility of C. glabrata was dose dependent (SDD) in 82% of cases and resistant in 18% of cases, and C. tropicalis was resistant in 30% of cases and SDD in 20% of cases according to the latest CLSI report (2012). The CLSI does not consider fluconazole cutoffs for C. krusei because this species is considered intrinsically resistant to the antifungal agent. Ben-Ami et al. reported a resistance to fluconazole of 1.6% for C. glabrata in their total samples4; in the current study, fluconazole resistance was found in 46% of cases, with 31% SDD for this species in particular. This difference in the percentage of resistance was probably due to the characteristics of the isolated strains, associated with the site of sample collection: our samples were specifically taken from the surfaces of dental prostheses. It is likely that species isolated from a biofilm express genes that enhance their resistance and virulence due to the different types of stresses that occur in the medium in which they grow.7
In 2012, Cleveland et al. conducted a study in which two populations from different regions of the U.S. were sampled. The authors reported that there were differences in the percentages of resistance of Candida species isolated from the different regions; however, the percentage of resistance reported for C. albicans was 2% in both populations. Meanwhile, the percentages of resistance reported for C. glabrata, C. tropicalis, and C. krusei were 11 and 13%, 4 and 9%, and 100% and 100%, respectively, for the two populations.11 As mentioned previously, the differences in the percentages of resistance may be due to differences in the sampling site and changes in the cutoffs recently proposed by the CLSI. In the present study, the samples were taken from patients at risk for candidemia, similarly to what was performed in the report by Cleveland et al., who reported differences in resistance between the populations of different geographical areas, but the percentage of difference is not large.11
Regarding the MIC50 of fluconazole for Candida species in biofilms, the MIC50 was 64μg/ml and was only effective against isolates of C. albicans and C. glabrata. The percentages of resistance were markedly higher: all species showed fluconazole resistance of 100%. The MIC50 necessary to reach and inhibit Candida species in sessile growth is up to 7 times higher than the MIC50 necessary to inhibit planktonic growth. In 2001, Chandra et al. reported that resistance to fluconazole is related to the phases of biofilm development.9 In the current study, the phase in which we applied the antifungal agent was during early maturation. In 2003, Mukherjee et al. reported that in certain species of Candida, the MIC can decrease during the first phase of biofilm formation (the first 6h)32; this may explain why the MIC50 values were so high in our study. In 2001, Ramage et al. reported MIC50 values >1024μg/ml for an ATCC strain of C. albicans.36,39 Fu et al. (2014) reported a gradual increase in the MIC50 in relation to the time of biofilm formation: at 2 and 4h, the MIC50 was 1μg/ml; at 8h, it was 8μg/ml; and at 24h, it was >1024μg/ml.22 As previously mentioned, resistance is related to the phase of biofilm formation; according to what has been described, as the biofilm matures, higher concentrations are required to inhibit Candida, and this observed mechanism is affected by the arrangement of the drug on the cell in question.
Due to the rigorous methodology employed and the extensive validation by different authors, the present study provides important microbiological data on the in vitro sensitivity of Candida species to the essential oil of A. sativum, allowing us to determine the specific concentrations at which the species’ growth is inhibited not only as cells in suspension but also as cells in biofilms.
ConclusionThe essential oil of A. sativum is more effective than fluconazole in inhibiting both planktonic cells in vitro and biofilms of C. albicans, C. glabrata, C. tropicalis, and C. krusei isolated from dental prostheses.
Conflict of interestThere is no conflict of interest, either real or potential, for any of the authors; that is, no financial relationships, personal relationships, academic or intellectual competition is present.
The manuscript has been entirely reviewed by a native English speaker. The reviewing of the manuscript by Maggie Brunner and American Journal Experts has been particularly appreciated. We appreciate the technical support and academic collaboration of PhD Diana María Escobar García, Chemist Virginia Flores Gutierrez, Nutritionist Mónica Acebo and PhD Othir Galicia Cruz for all facilities. Dr. Josué Roberto Bermeo Escalona developed a postdoctoral fellowship 2015-2016 (PRODEP Ref. DSA/103.5/15/7823. This work was supported by Thematic Networks development of academic collaboration: “Network for the Study of virulence and resistance of Candida albicans applied to the diagnosis, treatment and prevention of oral candidiasis.” PROMEP-UASLP No. 45495-0, (SEP, MEXICO) and PFCE-UASLP 2016.