The epidemiology of candidemia has changed over the last decades and varies widely among geographic areas.
AimsWe examined in children (aged 0–14) with candidemia the trends in the incidence rate of this infection, as well as the clinical characteristics of the patients, in order to optimize the prognosis and the control measures of this serious disease.
MethodsA retrospective cohort study of candidemia in the period 2011–2018 in the neonatal intensive care unit (NICU), pediatric ICU (PICU) and pediatric wards of a tertiary hospital, was conducted. The clinical course, Candida species isolated, antifungal susceptibility, outcome and incidence rates were analyzed and compared.
ResultsWe diagnosed 68 episodes of candidemia in 62 children, 48% occurred in the NICU, 31% in the PICU and 21% in pediatric wards. Candida albicans was the most frequent species isolated in NICU infants (53%), and Candida parapsilosis predominated among PICU patients (59%) and pediatric wards (50%). One third of NICU infants had invasive candidiasis (IC), most of them having extremely low birth weight (ELBW) (35%). All isolates were susceptible to the antifungal administered. Over time, the incidence of candidemia decreased in the PICU (from 2.2 to 0.3 episodes/1000 patient-days, OR=0.6; 95%CI 0.5–0.8), whereas in the NICU and in the wards remained stable. Mortality occurred mostly in NICU patients (26%), predominated in ELBW infants and did not change over time.
ConclusionsThe higher incidence and mortality of candidemia and IC observed in preterm infants requires a continuous evaluation of practices and diagnostic methods which will allow improving the prognosis of this most vulnerable population.
La epidemiología de la candidemia varía con el tiempo y entre las áreas geográficas.
ObjetivosSe ha estudiado en niños (0-14 años) con candidemia la evolución de la tasa de incidencia y las características clínicas de los pacientes para optimizar el pronóstico y las medidas de control de esta grave enfermedad.
MétodosSe llevó a cabo un estudio de cohorte retrospectivo de los casos de candidemia en la unidad de cuidados intensivos neonatales (UCIN), UCI pediátrica (UCIP) y salas pediátricas de un hospital terciario, entre los años 2011 y 2018. Se compara el curso clínico, las especies de Candida, la sensibilidad antifúngica y las tasas de incidencia.
ResultadosSe diagnosticaron 68 episodios de candidemia en 62 niños; el 48% de ellos tuvieron lugar en UCIN, el 31% en UCIP y el 21% en salas pediátricas. Candida albicans fue la especie más frecuente en UCIN (53%), y Candida parapsilosis predominó en UCIN (59%) y salas pediátricas (50%). Un tercio de los bebés de la UCIN tenía candidiasis invasora (CI) y la mayoría presentaba extremado bajo peso al nacimiento (EBPN) (35%). Con el tiempo, la incidencia de candidemia disminuyó en la UCIP (de 2,2 a 0,3 episodios/1.000 días/paciente, OR: 0,6; IC 95%: 0,5-0,8), mientras que en la UCIN y en las salas permaneció estable. La mortalidad se produjo principalmente en pacientes de UCIN (26%), predominó en lactantes EBPN y no cambió con el tiempo.
ConclusionesLa mayor incidencia y mortalidad de la candidemia y CI observadas en lactantes prematuros requiere una evaluación continua de prácticas y métodos de diagnóstico que permitan mejorar el pronóstico de esta población más vulnerable.
The true incidence of candidemia in children is unknown because the diagnosis is based on blood cultures (BC), which are insensitive, detecting around half of the cases of candidemia, or even less while receiving antifungals.
The epidemiology of invasive candidiasis (IC) evolves across time and depends on the type of population, underlying pathology, the use of broad-spectrum antibiotics and antifungal prophylaxis, the infection control measures and the study design.1,9,13,18,24,30 In children, candidemia occurs mainly in very low birth weight (VLBW) infants (<1500g), in patients admitted to the neonatal and pediatric intensive care units (NICU and PICU, respectively) and in those with malignancies.23 Different multicenter and local studies have been published in the past,7,10,27,28,30,33 but little current epidemiological data are available.4,10 Therefore, it is essential to know the epidemiology of candidemia at local level to optimize treatment, outcome and to implement measures for preventing the disease. The present study was carried out to examine changes in epidemiology, as well as the clinical and microbiological features in children with candidemia over an 8-year period.
Patients and methodsWe performed a retrospective cohort study of candidemia in pediatric patients (aged 0–14 years) admitted to La Fe University Hospital, a tertiary care teaching center with 200 pediatric beds; the study period included the period between 2011 and 2018.
Cases of candidemia were diagnosed by means of microbiology laboratory procedures. Candidemia was diagnosed when having a positive BC for any Candida species, and breakthrough-candidemia was diagnosed when the infection occurred while the patient was receiving antifungals for at least 3 days before the first positive BC.22 Invasive candidiasis was defined as a positive Candida culture from cerebrospinal fluid or peritoneum, or if any of the following were present: endocarditis with echocardiographic evidence of a valvular vegetation, or solid organ involvement with lesions on diagnostic imaging. Mortality was attributed to this entity when death occurred as a direct consequence of candidemia or from candidemia-associated complications.36 Two study investigators (AP and AG) deemed death to be attributable to candidemia based on the clinical data.
Relevant data at the time of candidemia collected from the electronic medical records included age, sex, underlying disease, birth weight (BW) and gestational age (GA) for NICU patients, bacterial infection (positive culture from normally sterile body fluid), neutropenia (neutrophil count <500mm–3), endotracheal intubation, parenteral nutrition, central venous catheter (CVC), broad-spectrum antibiotics and antifungals (therapeutic or prophylactic) in the 7 days preceding the infection, and antifungal treatment start when BC became positive. Patients were categorized as neonates admitted in the NICU and children (older than 28 days and younger than 15 years) admitted in the PICU or in pediatric wards.
The identification of the microorganisms was done in the Microbiology Department of the hospital. BCs were processed by automated culture systems (Bactec; Becton Dickinson, USA, and Bact/Alert; Organon Teknika, USA), and yeasts were identified by standard biochemical and microbiological procedures, including VITEK® MS system (bioMérieux, France) that uses Matrix Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) technology.37 In vitro susceptibility to echinocandins, fluconazole and voriconazole was determined by the microdilution colorimetric Sensititre YeastOne SYO-09 panel (TREK Diagnostic Systems, Oakwood Village, USA), and CLSI breakpoints were employed.11,26 For amphotericin B (AmB), isolates inhibited by ≤1μg/ml were considered susceptible.25 For less prevalent Candida species, clinical breakpoints are undefined; therefore, those isolates showing minimum inhibitory concentration values (MIC) higher than the epidemiologic cutoff value were considered resistant.15,16,31
Statistical analysisCategorical data were expressed as absolute numbers and proportions, and continuous variables as the median and interquartile range (IQR).
Beta regression models were created to assess trends over time, calculating the odds ratio (OR) with the corresponding 95% confidence interval (IC95%). To explore the correlation between the age of the patient and the year of the infection, the Spearman and Pearson correlation coefficient was used. All analyses were performed using R software version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
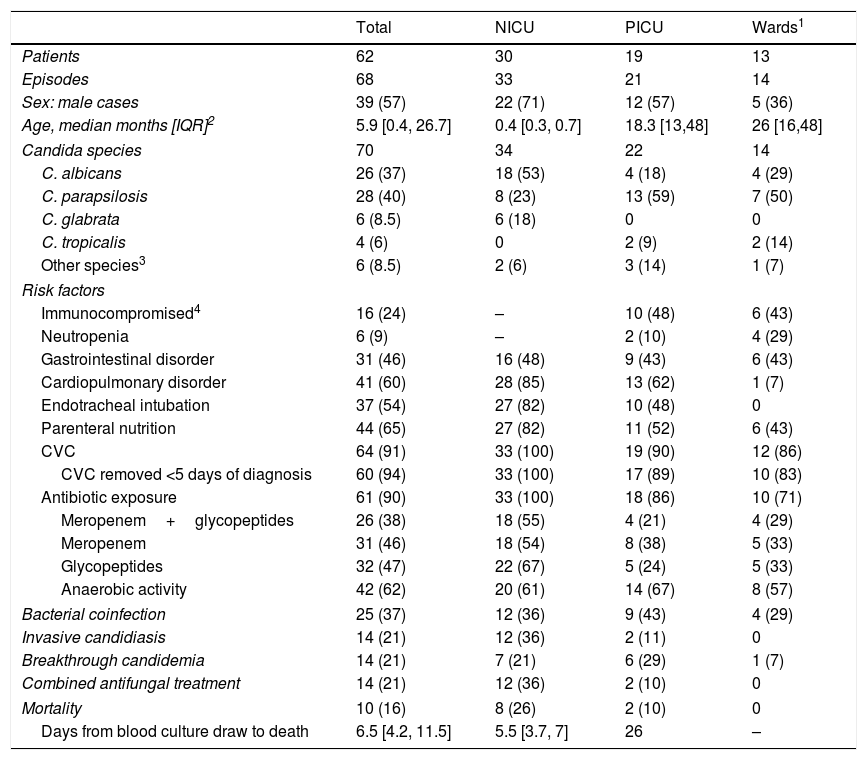
ResultsClinical and epidemiological characteristicsDuring 2011–2018, 68 episodes of candidemia in 62 children were diagnosed, with 48% of them taking place in the NICU, 31% in the PICU and 21% in pediatric wards (Table 1). Overall, 60% of the patients were younger than one year and 90% younger than 3 years. Among neonates, the median GA was 25 weeks [IQR 24, 30], 89% were preterm, 79% were extremely preterm (<28 weeks GA) and 82% had VLBW. Candida albicans was the most frequent species in NICU infants (53%), followed by Candida parapsilosis (23%). Conversely, among PICU patients and in wards, C. parapsilosis predominated (59% and 50% respectively). Excluding NICU infants, 46% episodes occurred in immunocompromised children (Table 1). In this population, C. parapsilosis was the prevalent species (59%), 37% of the children were neutropenic and the proportion of comorbid conditions, IC, and breakthrough-candidemia was similar to that of immunocompetent patients (data not shown). Underlying conditions in PICU patients included cardio-pulmonary disorders (13 cases), malignancy (4 cases), liver transplant (2 cases), primary immunodeficiency (3 cases), chronic gastrointestinal disorders (8 cases) renal insufficiency (4 cases), major burns (1 case), and carotid dissection (1 case). Most of the patients (90%) received systemic antibiotics, principally glycopeptides (32 cases), carbapenems (30 cases) or both (26 cases). The use of these antibiotics in the NICU doubled that of the PICU.
Clinical characteristics and outcome of the patients with candidemia. Figures show absolute cases and (%).
| Total | NICU | PICU | Wards1 | |
|---|---|---|---|---|
| Patients | 62 | 30 | 19 | 13 |
| Episodes | 68 | 33 | 21 | 14 |
| Sex: male cases | 39 (57) | 22 (71) | 12 (57) | 5 (36) |
| Age, median months [IQR]2 | 5.9 [0.4, 26.7] | 0.4 [0.3, 0.7] | 18.3 [13,48] | 26 [16,48] |
| Candida species | 70 | 34 | 22 | 14 |
| C. albicans | 26 (37) | 18 (53) | 4 (18) | 4 (29) |
| C. parapsilosis | 28 (40) | 8 (23) | 13 (59) | 7 (50) |
| C. glabrata | 6 (8.5) | 6 (18) | 0 | 0 |
| C. tropicalis | 4 (6) | 0 | 2 (9) | 2 (14) |
| Other species3 | 6 (8.5) | 2 (6) | 3 (14) | 1 (7) |
| Risk factors | ||||
| Immunocompromised4 | 16 (24) | – | 10 (48) | 6 (43) |
| Neutropenia | 6 (9) | – | 2 (10) | 4 (29) |
| Gastrointestinal disorder | 31 (46) | 16 (48) | 9 (43) | 6 (43) |
| Cardiopulmonary disorder | 41 (60) | 28 (85) | 13 (62) | 1 (7) |
| Endotracheal intubation | 37 (54) | 27 (82) | 10 (48) | 0 |
| Parenteral nutrition | 44 (65) | 27 (82) | 11 (52) | 6 (43) |
| CVC | 64 (91) | 33 (100) | 19 (90) | 12 (86) |
| CVC removed <5 days of diagnosis | 60 (94) | 33 (100) | 17 (89) | 10 (83) |
| Antibiotic exposure | 61 (90) | 33 (100) | 18 (86) | 10 (71) |
| Meropenem+glycopeptides | 26 (38) | 18 (55) | 4 (21) | 4 (29) |
| Meropenem | 31 (46) | 18 (54) | 8 (38) | 5 (33) |
| Glycopeptides | 32 (47) | 22 (67) | 5 (24) | 5 (33) |
| Anaerobic activity | 42 (62) | 20 (61) | 14 (67) | 8 (57) |
| Bacterial coinfection | 25 (37) | 12 (36) | 9 (43) | 4 (29) |
| Invasive candidiasis | 14 (21) | 12 (36) | 2 (11) | 0 |
| Breakthrough candidemia | 14 (21) | 7 (21) | 6 (29) | 1 (7) |
| Combined antifungal treatment | 14 (21) | 12 (36) | 2 (10) | 0 |
| Mortality | 10 (16) | 8 (26) | 2 (10) | 0 |
| Days from blood culture draw to death | 6.5 [4.2, 11.5] | 5.5 [3.7, 7] | 26 | – |
1Patients were under the supervision of the following services: Oncology (5 patients), Gastroenterogy (4 patients), General Pediatrics (2 patients), Rheumatology (1 patient) and Urology (1 patient); 2IQR: interquartile range; 3Other species include C. krusei (2), C. lusitaniae (2), C. famata (1) and C. dubliniensis (1); 4Solid tumor (4 patients), hematopoietic stem cell transplantation (4 patients), liver transplantation (3 patients), primary immunodeficiency (3 patients), acute lymphoid leukemia (1 patient), polyarteritis nodosa (1 patient).
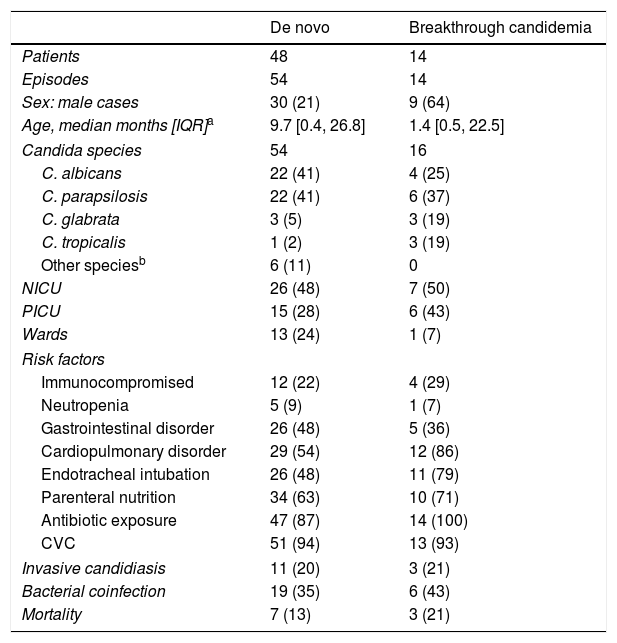
Candidemia was diagnosed in concomitance with a bacterial infection in 25 episodes (37%): 17 episodes were associated with Gram-positive bacteremia, 6 episodes with Gram-negative bacteremia, and 2 episodes with mixed bacteremia. Invasive candidiasis occurred mainly in NICU infants (36%), in whom C. albicans prevailed (8 out of 12 cases), and extremely low BW (ELBW) infants were affected the most (7 cases). Renal fungal balls and peritonitis were the most frequent IC observed (5 cases each). Other IC localizations included brain (3 cases), musculoskeletal system (2 cases), eye (1 case) and heart (1 case). The clinical features and mortality rate of patients with breakthrough-candidemia were comparable with those of patients suffering the novo candidemia (Table 2). However, 79% of breakthrough-candidemia episodes occurred in patients with some sort of immunosuppression: 6 neonates, 2 children with primary immunodeficiency, 1 case of liver transplant and 1 case of hematopoietic stem cell transplantation. Breakthrough-candidemia developed in 10 patients receiving fluconazole, liposomal AmB (LAmB, 1 case), voriconazole (1 case) and LAmB plus caspofungin for an Aspergillus infection (1 case). Two patients with breakthrough-candidemia had mixed Candida infections (C. albicans+Candida glabrata, and C. parapsilosis+Candida tropicalis).
Characteristics and risk factors of patients with candidemia de novo versus breakthrough-candidemia. Figures show absolute cases and (%).
| De novo | Breakthrough candidemia | |
|---|---|---|
| Patients | 48 | 14 |
| Episodes | 54 | 14 |
| Sex: male cases | 30 (21) | 9 (64) |
| Age, median months [IQR]a | 9.7 [0.4, 26.8] | 1.4 [0.5, 22.5] |
| Candida species | 54 | 16 |
| C. albicans | 22 (41) | 4 (25) |
| C. parapsilosis | 22 (41) | 6 (37) |
| C. glabrata | 3 (5) | 3 (19) |
| C. tropicalis | 1 (2) | 3 (19) |
| Other speciesb | 6 (11) | 0 |
| NICU | 26 (48) | 7 (50) |
| PICU | 15 (28) | 6 (43) |
| Wards | 13 (24) | 1 (7) |
| Risk factors | ||
| Immunocompromised | 12 (22) | 4 (29) |
| Neutropenia | 5 (9) | 1 (7) |
| Gastrointestinal disorder | 26 (48) | 5 (36) |
| Cardiopulmonary disorder | 29 (54) | 12 (86) |
| Endotracheal intubation | 26 (48) | 11 (79) |
| Parenteral nutrition | 34 (63) | 10 (71) |
| Antibiotic exposure | 47 (87) | 14 (100) |
| CVC | 51 (94) | 13 (93) |
| Invasive candidiasis | 11 (20) | 3 (21) |
| Bacterial coinfection | 19 (35) | 6 (43) |
| Mortality | 7 (13) | 3 (21) |
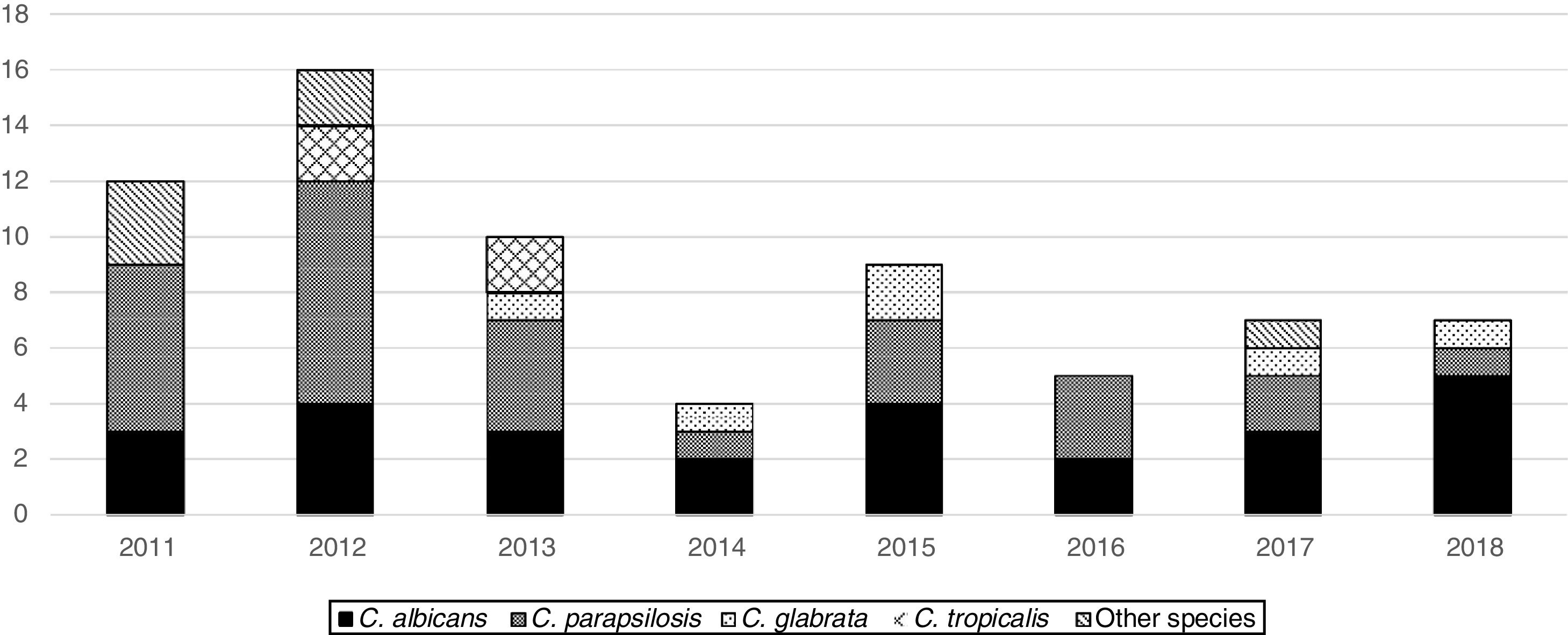
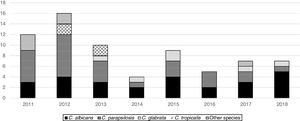
From 2011 to 2018, the overall trend of candidemia significantly decreased (from 0.3 to 0.2 episodes/1000 patient-days, OR=0.9, 95%CI 0.8–1, p=0.017). The cause was the sharp decline in the PICU incidence (from 2.2 to 0.3, OR=0.6, 95%CI 0.5–0.8, p=0.001), whereas in the NICU and in the wards the incidence trend remained unchanged (from 0.6 to 1.1, OR=1, 95%CI 1.1–0.8, p=0.8, and from 0 to 0.03, OR=1, 95%CI 0.9–1.2, p=0.7, respectively). In consequence, the analysis of the age-specific trend showed that the age of presentation of candidemia decreased over the study period (Pearson correlation=−0.21, Spearman correlation=−0.33). Candida parapsilosis frequency significantly decreased over the study period (OR=0.8, 95%CI 0.7–0.9, p=0.002), being 50% of the isolates recovered in 2011 and 2012 and the other 50% within the following 6 years (Fig. 1). Nonetheless, the frequency of C. albicans isolates remained rather unchanged over the study period.
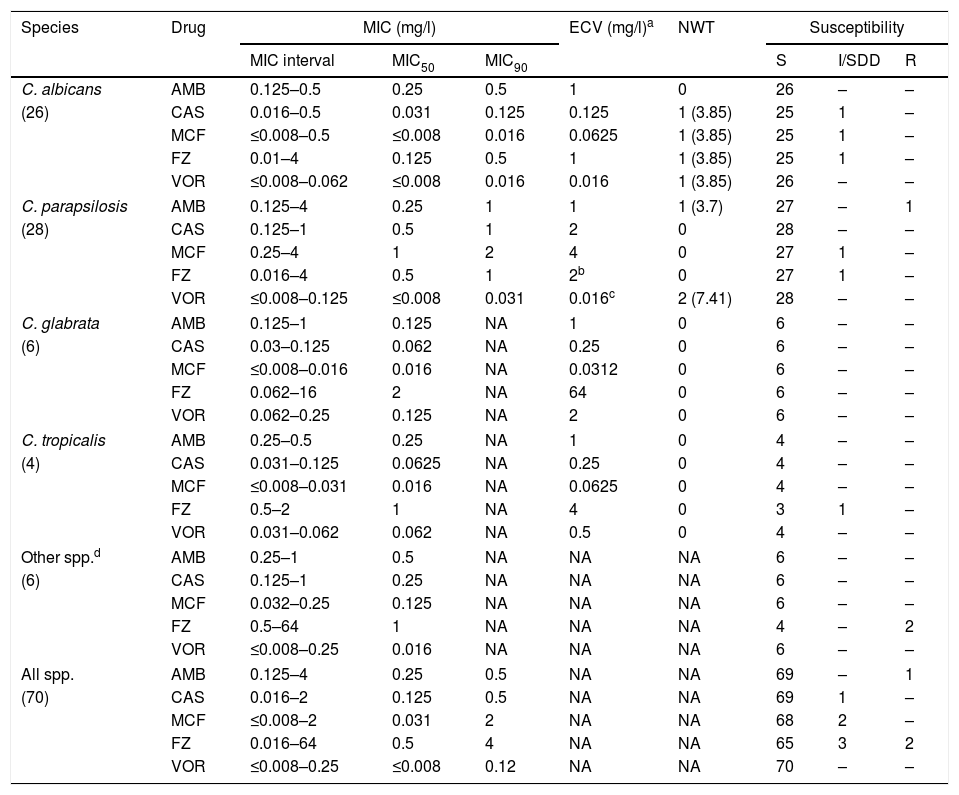
Antifungal susceptibilityCaspofungin and micafungin showed excellent activity against most Candida species, including C. parapsilosis isolates, with only 2 isolates with intermediate susceptibility (Table 3). One C. parapsilosis isolate from a NICU infant not previously treated with antifungals was resistant to LAmB (MIC 4μg/ml). Fluconazole was active against most isolates (93%), excepting 2 C. krusei inherent resistant isolates, and 3 isolates susceptible-dose-dependent (C. albicans, C. parapsilosis and C. tropicalis). Multi-resistance was not found in the tested isolates. In addition, no differences in the antifungal MICs of the C. albicans and C. parapsilosis isolates recovered from the different pediatric units, patients with breakthrough-candidemia or with de novo candidemia were found, nor between patients who died or survived (data not shown).
In vitro susceptibilities of Candida species.
| Species | Drug | MIC (mg/l) | ECV (mg/l)a | NWT | Susceptibility | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC interval | MIC50 | MIC90 | S | I/SDD | R | ||||
| C. albicans | AMB | 0.125–0.5 | 0.25 | 0.5 | 1 | 0 | 26 | – | – |
| (26) | CAS | 0.016–0.5 | 0.031 | 0.125 | 0.125 | 1 (3.85) | 25 | 1 | – |
| MCF | ≤0.008–0.5 | ≤0.008 | 0.016 | 0.0625 | 1 (3.85) | 25 | 1 | – | |
| FZ | 0.01–4 | 0.125 | 0.5 | 1 | 1 (3.85) | 25 | 1 | – | |
| VOR | ≤0.008–0.062 | ≤0.008 | 0.016 | 0.016 | 1 (3.85) | 26 | – | – | |
| C. parapsilosis | AMB | 0.125–4 | 0.25 | 1 | 1 | 1 (3.7) | 27 | – | 1 |
| (28) | CAS | 0.125–1 | 0.5 | 1 | 2 | 0 | 28 | – | – |
| MCF | 0.25–4 | 1 | 2 | 4 | 0 | 27 | 1 | – | |
| FZ | 0.016–4 | 0.5 | 1 | 2b | 0 | 27 | 1 | – | |
| VOR | ≤0.008–0.125 | ≤0.008 | 0.031 | 0.016c | 2 (7.41) | 28 | – | – | |
| C. glabrata | AMB | 0.125–1 | 0.125 | NA | 1 | 0 | 6 | – | – |
| (6) | CAS | 0.03–0.125 | 0.062 | NA | 0.25 | 0 | 6 | – | – |
| MCF | ≤0.008–0.016 | 0.016 | NA | 0.0312 | 0 | 6 | – | – | |
| FZ | 0.062–16 | 2 | NA | 64 | 0 | 6 | – | – | |
| VOR | 0.062–0.25 | 0.125 | NA | 2 | 0 | 6 | – | – | |
| C. tropicalis | AMB | 0.25–0.5 | 0.25 | NA | 1 | 0 | 4 | – | – |
| (4) | CAS | 0.031–0.125 | 0.0625 | NA | 0.25 | 0 | 4 | – | – |
| MCF | ≤0.008–0.031 | 0.016 | NA | 0.0625 | 0 | 4 | – | – | |
| FZ | 0.5–2 | 1 | NA | 4 | 0 | 3 | 1 | – | |
| VOR | 0.031–0.062 | 0.062 | NA | 0.5 | 0 | 4 | – | – | |
| Other spp.d | AMB | 0.25–1 | 0.5 | NA | NA | NA | 6 | – | – |
| (6) | CAS | 0.125–1 | 0.25 | NA | NA | NA | 6 | – | – |
| MCF | 0.032–0.25 | 0.125 | NA | NA | NA | 6 | – | – | |
| FZ | 0.5–64 | 1 | NA | NA | NA | 4 | – | 2 | |
| VOR | ≤0.008–0.25 | 0.016 | NA | NA | NA | 6 | – | – | |
| All spp. | AMB | 0.125–4 | 0.25 | 0.5 | NA | NA | 69 | – | 1 |
| (70) | CAS | 0.016–2 | 0.125 | 0.5 | NA | NA | 69 | 1 | – |
| MCF | ≤0.008–2 | 0.031 | 2 | NA | NA | 68 | 2 | – | |
| FZ | 0.016–64 | 0.5 | 4 | NA | NA | 65 | 3 | 2 | |
| VOR | ≤0.008–0.25 | ≤0.008 | 0.12 | NA | NA | 70 | – | – | |
AMB: amphotericin B; CAS: caspofungin; MCF: micafungin; FZ: fluconazole; VOR: voriconazole; NWT: non wild type; S: susceptible; SDD: susceptible dose dependent; I: intermediate; R: resistant.
All episodes of NICU candidemia except one, a neonate who died before receiving antifungals, were treated with LAmB, with 12 cases in combination with fluconazole. In children, 17 episodes (49%) were treated with fluconazole (10 in the PICU and 7 in the wards), and other 17 episodes were treated with LAmB, including one case treated with LAmB and fluconazole, one case was treated with LAmB and voriconazole, and another one with LAmB and caspofungin. Over the study period, only one episode of candidemia was treated with micafungin.
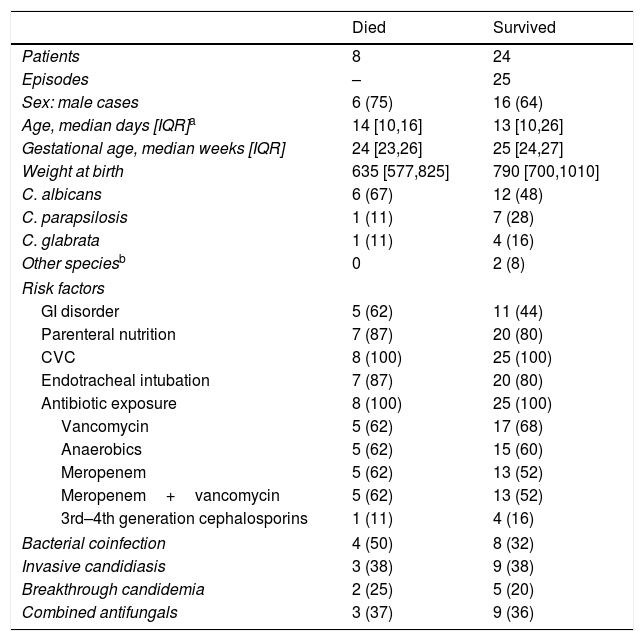
Candidemia-related death predominated in NICU infants with a median GA of 24 weeks [IQR 23, 26], 75% cases of IC and 62% of C. albicans infection (Table 4). Furthermore, mortality in NICU infants did not change over the study period, whereas the only 2 deaths in the PICU (9%) occurred in 2012.
Comparison of the clinical characteristics of the NICU patients who survived and those who died. Figures show absolute cases and (%).
| Died | Survived | |
|---|---|---|
| Patients | 8 | 24 |
| Episodes | – | 25 |
| Sex: male cases | 6 (75) | 16 (64) |
| Age, median days [IQR]a | 14 [10,16] | 13 [10,26] |
| Gestational age, median weeks [IQR] | 24 [23,26] | 25 [24,27] |
| Weight at birth | 635 [577,825] | 790 [700,1010] |
| C. albicans | 6 (67) | 12 (48) |
| C. parapsilosis | 1 (11) | 7 (28) |
| C. glabrata | 1 (11) | 4 (16) |
| Other speciesb | 0 | 2 (8) |
| Risk factors | ||
| GI disorder | 5 (62) | 11 (44) |
| Parenteral nutrition | 7 (87) | 20 (80) |
| CVC | 8 (100) | 25 (100) |
| Endotracheal intubation | 7 (87) | 20 (80) |
| Antibiotic exposure | 8 (100) | 25 (100) |
| Vancomycin | 5 (62) | 17 (68) |
| Anaerobics | 5 (62) | 15 (60) |
| Meropenem | 5 (62) | 13 (52) |
| Meropenem+vancomycin | 5 (62) | 13 (52) |
| 3rd–4th generation cephalosporins | 1 (11) | 4 (16) |
| Bacterial coinfection | 4 (50) | 8 (32) |
| Invasive candidiasis | 3 (38) | 9 (38) |
| Breakthrough candidemia | 2 (25) | 5 (20) |
| Combined antifungals | 3 (37) | 9 (36) |
Our study clearly illustrates that infants admitted in the NICU had different infecting species, incidence trend, management and outcome when compared to those admitted in the PICU and the wards. Overall, candidemia predominated in young infants, with a trend toward an earlier age of presentation over the study period. In the NICU, the incidence of candidemia remained unchanged over time and occurred most frequently in VLBW. However, we observed a substantial declining trend of candidemia in PICU patients that correlated with a marked decrease in C. parapsilosis isolates. Improving infection control practices may have contributed to this declining trend in the PICU, as it has been reported in other centers.2,4,10,27 Furthermore, C. parapsilosis is particularly acquired from exogenous sources and, therefore, it is more susceptible to be kept under control through CVC management bundles improvement and hand-washing.
Despite the extreme severity of patients with candidemia in both the NICU and the PICU, extreme prematurity may be a determining factor in the prognosis. The steady trend in the NICU, as previously described,4,9,34 reflects the prolonged hospitalization and increased survival of preterm infants, who have impaired cellular immunity, and immaturity of the skin and gastrointestinal barriers that predispose to Candida translocation.17C. albicans was more prevalent in NICU infants and those with IC, consistent with other studies.4,34C. albicans seems to be more virulent than other Candida species3 and frequently arises from endogenous sources (translocation from gastrointestinal and genital tract), which are less affected by infection control measures. Therefore, the source of infection differed in NICU and PICU patients. Source control is a determining factor in the outcome of patients with candidemia. In our study, all NICU infants and most of PICU patients underwent removal of CVC as well as adequate drainage of infected material and surgical correction, in an effort to achieve an adequate source control; when the source is endogenous, as seems to happen in most NICU infants, control measures become more limited and ineffective. Prophylaxis is generally preferred in NICUs with IC rates of 2–5%.14,21 However, if the IC rate is low, prophylaxis might not cut the mortality or the incidence.5 Since 2013 targeted antifungal prophylaxis is used in our infants when fulfilling two major criteria (abdominal surgery; parenteral nutrition ≥7 days; high-grade Candida colonization), or one major criteria and two minor criteria (BW<1000g or GA<26 weeks; CVC≥7 days; broad-spectrum antibiotics >3 days; necrotizing enterocolitis; systemic steroids). Consequently, candidiasis in ELBW decreased from 4.4% to 2.9%, which represents a relatively low rate. The significant outbreak of Candida auris we are suffering since 2016 in our institution in the adult intensive care units did not affect the pediatric units. To date, published data on C. auris infection is limited to adult population.32
A large number of patients had most of the risk factors for candidemia described in the literature such as the presence of CVC, mechanical ventilation, parenteral nutrition and prolonged treatment with antibiotics.8,38 However, the role of those risk factors could not be assessed because we did not include a control group. Furthermore, it is difficult to determine if they are the cause of candidemia or simply reflect how severely ill the patients are. Almost all patients were extremely ill and had been treated with broad-spectrum antibiotics when candidemia occurred, which is the most consistently identified risk factor associated with candidemia, including the duration of the antibiotic therapy and the use of more than one antibiotic.6 In our study, there was substantial variation in antibiotic exposures among the different units; in the NICU, the use of carbapenems and glycopeptides doubled that of the PICU, despite the fact that there was no difference in the number of previously documented bacterial infections among units. However, smaller volumes of blood obtained in sick infants or lower-level bacteremia may also contribute to the lesser number of demonstrated bacterial infections in NICU infants. A decrease in the use of antibiotics has been associated with a greatest decrease in neonatal candidiasis.2 It is possible that with the enforcement of the hospital antibiotic policy the treatments select patient microbiota, which in turn determines Candida infection susceptibility and species. In retrospect, we were unable to assess the appropriateness of the antibiotics used. However, avoiding prolonged treatment with broad-spectrum antimicrobials, via antimicrobial stewardship programs, especially carbapenem antibiotics, when culture results are negative may reduce ecologic opportunities for candidal overgrowth.
The low percentages of antifungal resistance and the few C. glabrata and C. krusei infections diagnosed in our study, with no signs of emergence over the 8-year period, may be related to the infrequent use of azole prophylaxis in children. Over the study period, we found a decrease in breakthrough-candidemia, since all episodes occurred before 2016. Breakthrough-candidemia was more frequent in immunocompromised patients, and comorbid conditions and outcome were similar to the novo candidemia, similar to a multicenter study in adults that includes our hospital.12 All breakthrough-candidemia isolates were susceptible to the antifungal agent used in the moment that candidemia occurred. A rapid identification and control of a focus of infection is a key element of treatment for candidemia; poor candidemia clearance may promote a higher translocation burden and, therefore, a persisting risk for breakthrough-candidemia.
In our study, a considerable number of children with candidemia were successfully treated with fluconazole. A major finding of this study is that since 2012 mortality entirely ceased in non-neonate children. Comparing children receiving an echinocandin and LAmB, no differences in mortality and efficacy have been observed,8 although current guidelines recommend echinocandins as empirical treatment for candidemia in non-neonate children.29,35 Mortality rates in the NICU did not improve over the 8 years and was higher in ELBW infants with IC. Mortality of candidemia is related to the underlying condition, the susceptibility and virulence of the organism and, particularly, to the delay in the diagnosis and treatment.3 Perhaps more aggressive initial antifungal doses may help to achieve a rapid control of the focus of infection.
Our study has some limitations. As a retrospective study from a single tertiary care hospital, the results may not be extrapolated to children receiving care in other settings. We were unable to document detailed clinical information, including the time in which the antifungal treatment was started, severity of the illness, changes in clinical practices, and in fluconazole prophylaxis. The small number of some groups or species limits the performance of statistical analysis and, therefore, the inference of differences. Despite these limitations, this study provides useful epidemiological information on the species distribution and antifungal susceptibility profiles in candidemia, which are essential to optimize empirical antifungal therapy. Lastly, we included information on all patient ages, not only limited to critical care settings, which allowed us to see a broader picture of pediatric candidemia demographics and incidence trends.
In conclusion, extremely preterm infants are currently the highest risk group for C. albicans IC and candidemia-related mortality. Since C. albicans source is generally endogenous and, therefore, less affected by infection control measures, preserving balanced resident microbiota and reducing antibiotic exposure, whenever possible, are essential to improve the prognosis of candidemia in this most vulnerable population. Finally, faster and more sensitive Candida identification methods than conventional culture techniques, such as T2Candida19 or next generation sequencing (NGS),20 could provide information about Candida species, resistance and origins of candidemia, allowing targeted antifungal therapy, which should result in a decrease in Candida-associated morbidity and mortality.
Ethical approvalThis article does not contain any studies with human participants or animals performed by any of the authors.
FundingThere is no funding source.
Conflict of interestThe authors declare that they have no conflict of interest.