Malassezia species are considered opportunistic yeasts of increasing clinical importance. These lipophilic yeasts are associated with various human diseases, especially pityriasis versicolor (PV), a chronic superficial scaling dermatomycosis.
AimsThe aim of this study was to isolate, identify and analyze the distribution of the different species of Malassezia in patients with PV in Rosario city (Argentina).
MethodsA total of 264 clinical samples were studied. Isolates were identified on the basis of microscopic observation of cells, and physiological properties, such as the presence of catalase, ability to use Tween compounds, splitting of esculin, and morphology, color and precipitate production on chromogenic agar CHROMagar-Malassezia medium (CHROMM).
ResultsThe highest prevalence of PV in this study was observed in the 25- to 45-year-old group. No differences were found in the development of PV between sexes. The most affected areas of body were the trunk and face. Malassezia sympodialis (51%) was the most commonly isolated species, followed in frequency by M. globosa (40%), Malassezia furfur (7%), Malassezia obtusa (1%) and Malassezia slooffiae (1%).
ConclusionsThe success for a correct identification of these yeasts is important to improve our knowledge about their epidemiological role in PV and also to detect the appearance of strains which are resistant to the commonly used antifungal drugs.
Las especies de Malassezia son consideradas levaduras oportunistas de importancia clínica creciente. Estas levaduras lipófilas están asociadas con diversas enfermedades humanas, especialmente la pitiriasis versicolor (PV), una dermatomicosis superficial crónica.
ObjetivosEl objetivo de este estudio fue aislar, identificar y analizar la distribución de diferentes especies de Malassezia en pacientes con PV en la ciudad de Rosario, Argentina.
MétodosSe estudiaron 264 muestras clínicas y los aislamientos fueron identificados según la observación microscópica de las células, propiedades fisiológicas tales como, presencia de catalasa, habilidad para utilizar compuestos Tween, desdoblamiento de la esculina y la descripción del color, morfología y formación de precipitado empleando el medio cromógeno CHROMagar Malassezia (CHROMM).
ResultadosLa mayor prevalencia de PV, en este estudio, fue encontrada en el grupo de 25-45 años de edad, no encontrandose diferencias con respecto al sexo. Las áreas más afectadas fueron tronco y cara. Malassezia sympodialis (51%) fue la especie más aislada, seguida por Malassezia globosa (40%), Malassezia furfur (7%), Malassezia obtusa (1%) y Malassezia slooffiae (1%).
ConclusionesLa importancia de una correcta identificación de estas levaduras es necesaria para el conocimiento de su rol epidemiológico en la PV y también para detectar la presencia de cepas resistentes a los antifúngicos.
The genus Malassezia comprises lipophilic yeasts belonging to the normal cutaneous microbiota of humans and warm-blooded animals,16 and can be cultured from almost all body areas. However, they may become pathogenic under certain conditions and are associated with a broad spectrum of clinical infections as pityriasis versicolor (PV), folliculitis, seborrheic dermatitis, some forms of atopic dermatitis, confluent and reticulate papillomatosis, and even systemic infections.4,7,23,28,30,35
PV is common in late teens and young adults of both sexes, and is characterized by well-demarcated scaling patches with variable pigmentation.12 Although PV was described at the beginning of the nineteenth century; its etiological agents have been a matter of debate. This controversy may be due to various morphological features and fastidious growth requirements of Malassezia yeasts in vivo.
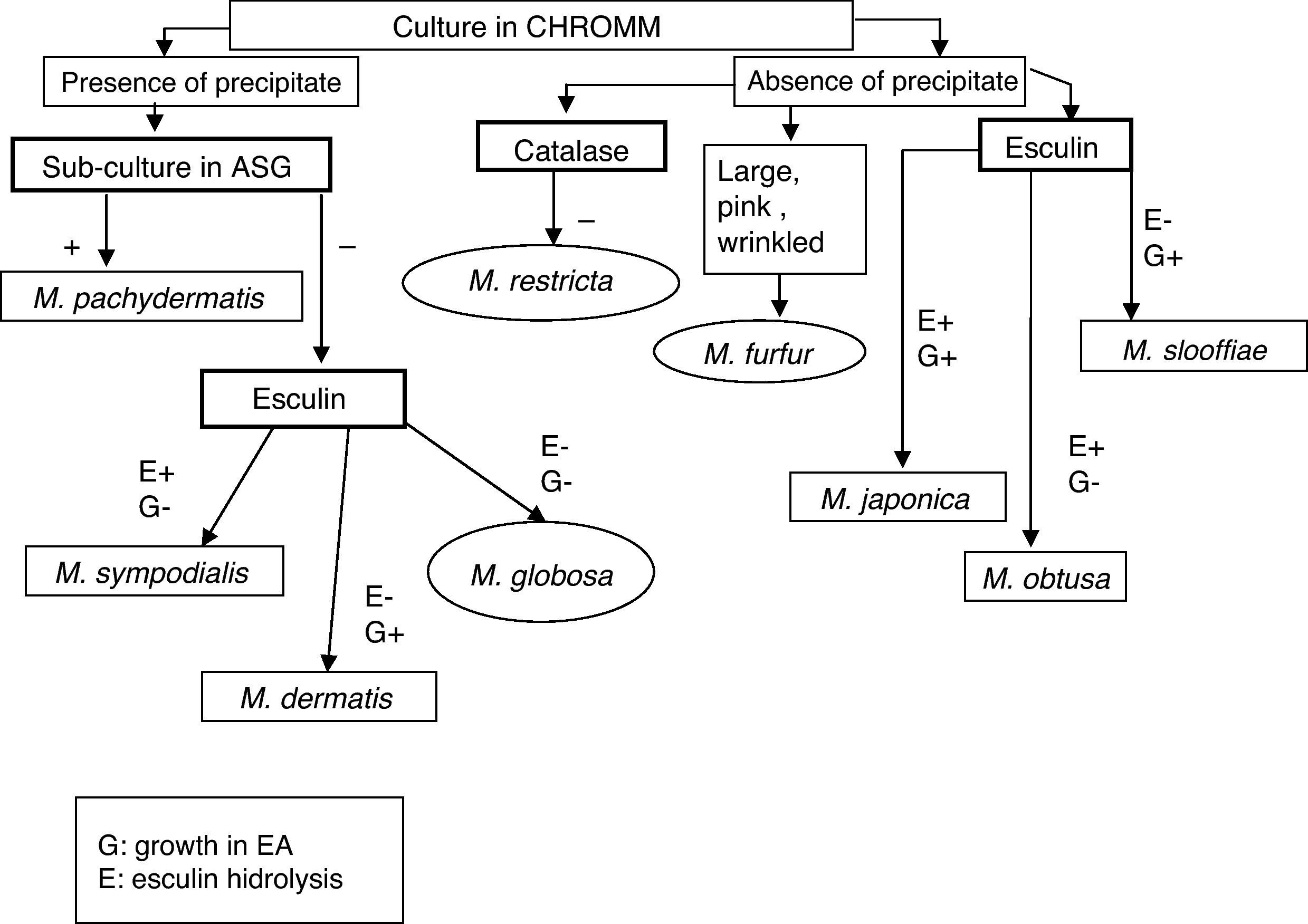
Morphology, ultrastructure, physiology (catalase reaction, splitting of esculin, lipids assimilation pattern, utilization of Cremophor EL, and thermotolerance), and molecular biology of Malassezia have been recently revised.13 This genus includes the following fourteen species: Malassezia pachydermatis, Malassezia furfur, Malassezia sympodialis, Malassezia globosa, Malassezia obtusa, Malassezia restricta, Malassezia slooffiae, Malassezia caprae,3,14,15,17,18,29,39Malassezia dermatis,41Malassezia japonica,42Malassezia equina,3Malassezia nana,24Malassezia yamatoensis43 and Malassezia cuniculi sp. nov.4M. nana, M. equina, M. caprae and Malassezia cuniculi sp. nov., have only been isolated from domestic animals.4 There is little information about the epidemiology and ecology of Malassezia species; hence, the clinical significance of these species is not fully distinguished. Some species are polymorphic, in particular M. furfur; others have fastidious requirements for growth, and their maintenance in subculture is difficult, such as M. globosa and M. restricta. Variations in susceptibility to antifungal drugs have been documented according to the different Malassezia species.22,33 The absence of rapid and simple identification methods may have serious implications in the administration of a prompt and appropriate therapy, especially when Malassezia yeasts are responsible for nosocomial bloodstream infections.6 Using the property of difference in fatty acid requirements of variants, some schemes have been devised to confirm the identity of the species by cultural methods.15,18 Nowadays, the Japanese system of identification has been proposed.25,26 It requires subculturing the isolated strain on several media as CHROMagar-Malassezia (CHROMM), Sabouraud Glucose Agar, Cremophor EL agar or Tween 60-Esculin agar.2
The aim of this research was to evaluate the prevalence of Malassezia species in the superficial infection of the stratum corneum (pityriasis versicolor) in Rosario city, and to analyze their distribution according to patients’ characteristics such as age, gender and site of lesions.
Materials and methodsPatientsA total of 264 patients (167 females and 97 male) with suspected PV diagnostic were evaluated. Their ages ranged from 5 to 60 years old. The study was conducted from April 2008 to December 2009 at the Mycology Reference Centre (CEREMIC), National University of Rosario, Santa Fe, Argentina. All patients were from Rosario city. Located in Argentina's central region, Rosario has temperate climate. A questionnaire was filled to get informative data about the family history, age, gender, location, clinical type and extent of disease in patients. The Human Ethics Committee rules were satisfied through informed consent.
Collection and culture of samplesThe samples from patients with suspected PV were obtained by scraping the patients’ lesions with scalpel and adhesive tape.34
Specimens were examined by optic microscopy with Lactophenol Blue and by observation under fluorescence microscope with Calcofluor white.36
All samples collected were inoculated on modified Dixon's Agar (mDA) medium and Sabouraud Glucose Agar (SGA) with 2–3 drops of sterile olive oil, for 10 days at 32°C.
Isolated yeastsWhen growth of Malassezia yeasts was detected on either mDA or SGA with 2–3 drops of sterile olive oil, they were selected and maintained by transfer on fresh medium (mDA) at biweekly intervals for their further identification.
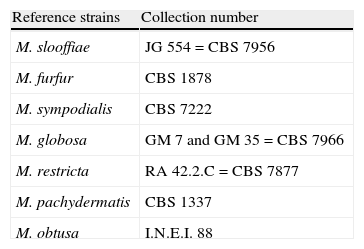
Standard strains of the genus Malassezia were always used as control in the laboratory routine. They were M. pachydermatis, M. furfur, M. sympodialis, M. globosa, M. obtusa, M. restricta and M. slooffiae, which were provided by Dr Guillot (École Nationale Véterinaire College d’Alfort, Maisons-Alfort, France) and by the Dr Carlos Malbrán Institute (INEI-Micología, Buenos Aires, Argentina). Reference strains used are shown in Table 1.
Reference strains.
| Reference strains | Collection number |
| M. slooffiae | JG 554=CBS 7956 |
| M. furfur | CBS 1878 |
| M. sympodialis | CBS 7222 |
| M. globosa | GM 7 and GM 35=CBS 7966 |
| M. restricta | RA 42.2.C=CBS 7877 |
| M. pachydermatis | CBS 1337 |
| M. obtusa | I.N.E.I. 88 |
CBS, Centraalbureau voor Schimmelcultures, Delft, The Netherlands; GM, Gillian Midgley, St Thomas’ Hospital, London, UK; JG, Jacques Guillot, École Nationale Véterinaire College d’Alfort, Maisons-Alfort, France; RA, Ruth Ashbee, Department of Microbiology, University of Leeds, Leeds, UK; INEI, Instituto Dr. Carlos G. Malbrán, Buenos Aires, Argentina.
Malassezia species were identified in the first instance carrying out the scheme proposed by Guillot et al.18 at first moment. Moreover, the new scheme proposed by Kaneko et al.26 that requires modified CHROMagar-Malassezia medium, SGA, Tween 60-Esculin agar (EA), Cremophor El agar (CrEl) and catalase reaction, was proved. The latter scheme uses the following culture media:
Modified Dixon Agar (mDA): Strains of Malassezia were cultured and maintained on mDA composed by 6g peptone (Oxoid), 36g malt extract, 20g ox bile (Oxoid), 2ml glycerol, 2ml oleic acid and 12g agar per litre.
CHROMagar-Malasseziamedium (CHROMM): Composed by 56.3g CHROMagar-Malassezia basal medium (CHROMagar, Paris, France) and 10ml Tween 40 (per litre).25
Tween 60-Esculin Agar (EA): Composed by 10g peptone, 10g glucose, 2g yeast extract, 0.5g ferric ammonium citrate, 1g esculin, 5ml of Tween 60 and 15g agar (per litre).25
Utilization of Cremophor EL (CrEL): The capacity to grow on SGA supplemented with CrEL (partially-purified plant extract containing primarily, but not exclusively, ricinoleic acid) as unique lipid source.
Catalase reaction: The presence of catalase was determined by using a drop of hydrogen peroxide (30% solution).
The strains to be identified were placed on CHROMM, SGA and EA, incubating at 32°C for 4–7 days.
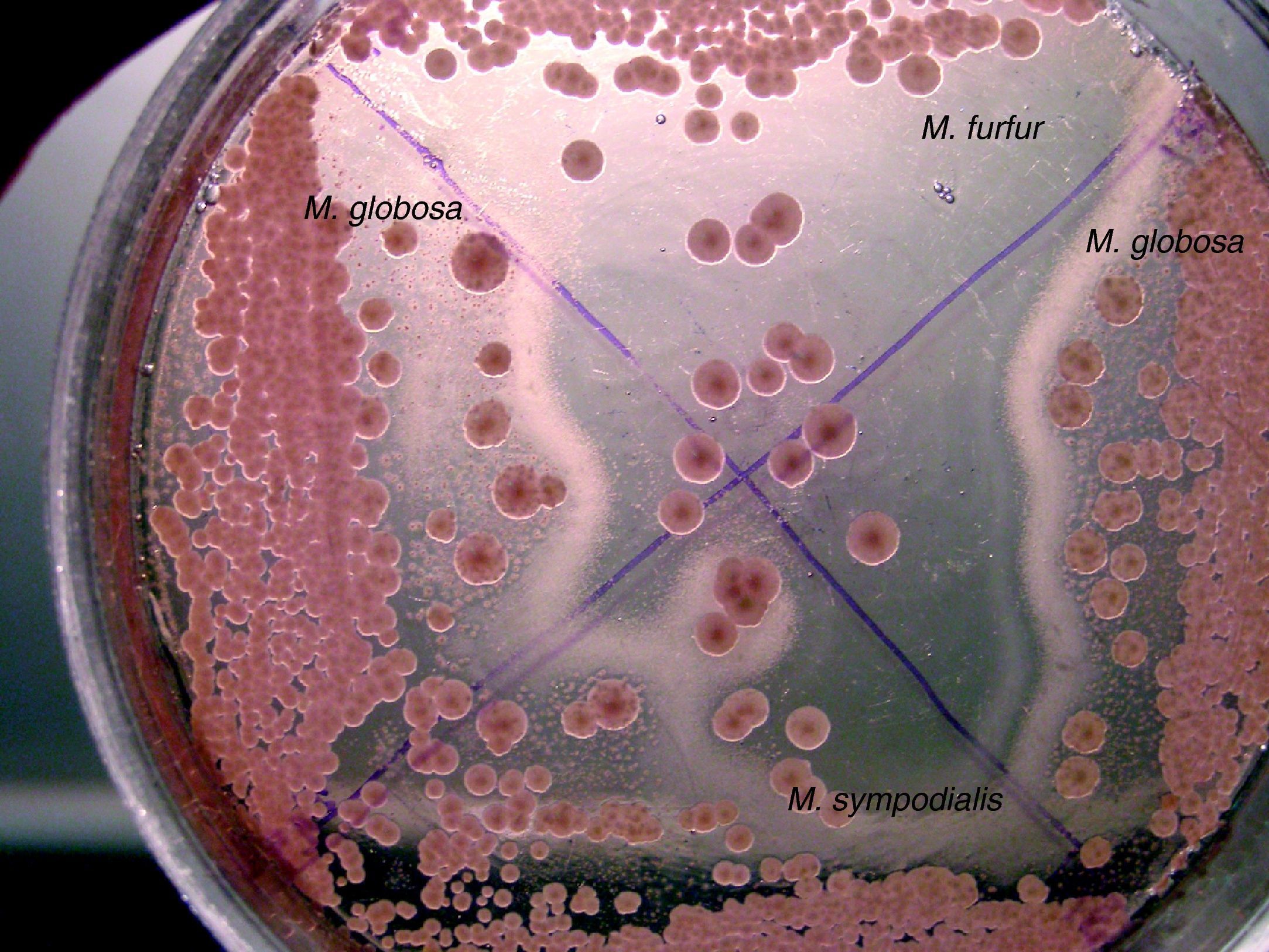
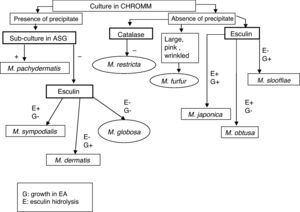
The presence of precipitate and the morphology of the colony – smooth or wrinkled – were checked in CHROMM. Subculture to SGA was used to determine the isolated lipid-dependence. EA evaluated the abilities of specimens to split the esculin showing β-glucosidase activity and the utilization or not of Tween 60. Presence of catalase was evidenced by production of gas bubbles. The assimilation of CrEl was revealed by the production of growth area around this compound. The workflow used to identify species of Malassezia is shown in Fig. 1.
The specimens obtained and identified were conserved in 20% glycerol and 20% skim milk/20% glycerol at −70°C,9 and in dried paper at room temperature.37
ResultsThe 264 patients were referred to the CEREMIC laboratory. The age of patients varied from 5 to 60. Sixty three percent of the patients (n=166) belonged to the range 25–45 years of age.
The direct examination – with two different methods – gave positive samples in 219 cases (83%). Forty five samples (17%) were negative, and this latter group was not included in this study. Among the samples with direct examination showing a positive result, 107 (49%) showed only filaments, and filaments and budding yeasts were observed in 112 samples (51%).
Positive cultures were obtained in 200 samples (91%) and negative in 19 samples (9%).
Identification of clinical isolatesTwo hundred positive cultures were identified by phenotypic analysis. The remaining 19 samples did not yield any isolate in culture and the samples were not enough to repeat the study.
The scheme used for the identification of the isolates proposed by Kaneko et al.,26 showed good results in our laboratory to identify species. All the clinical isolates showed positive catalase reaction.
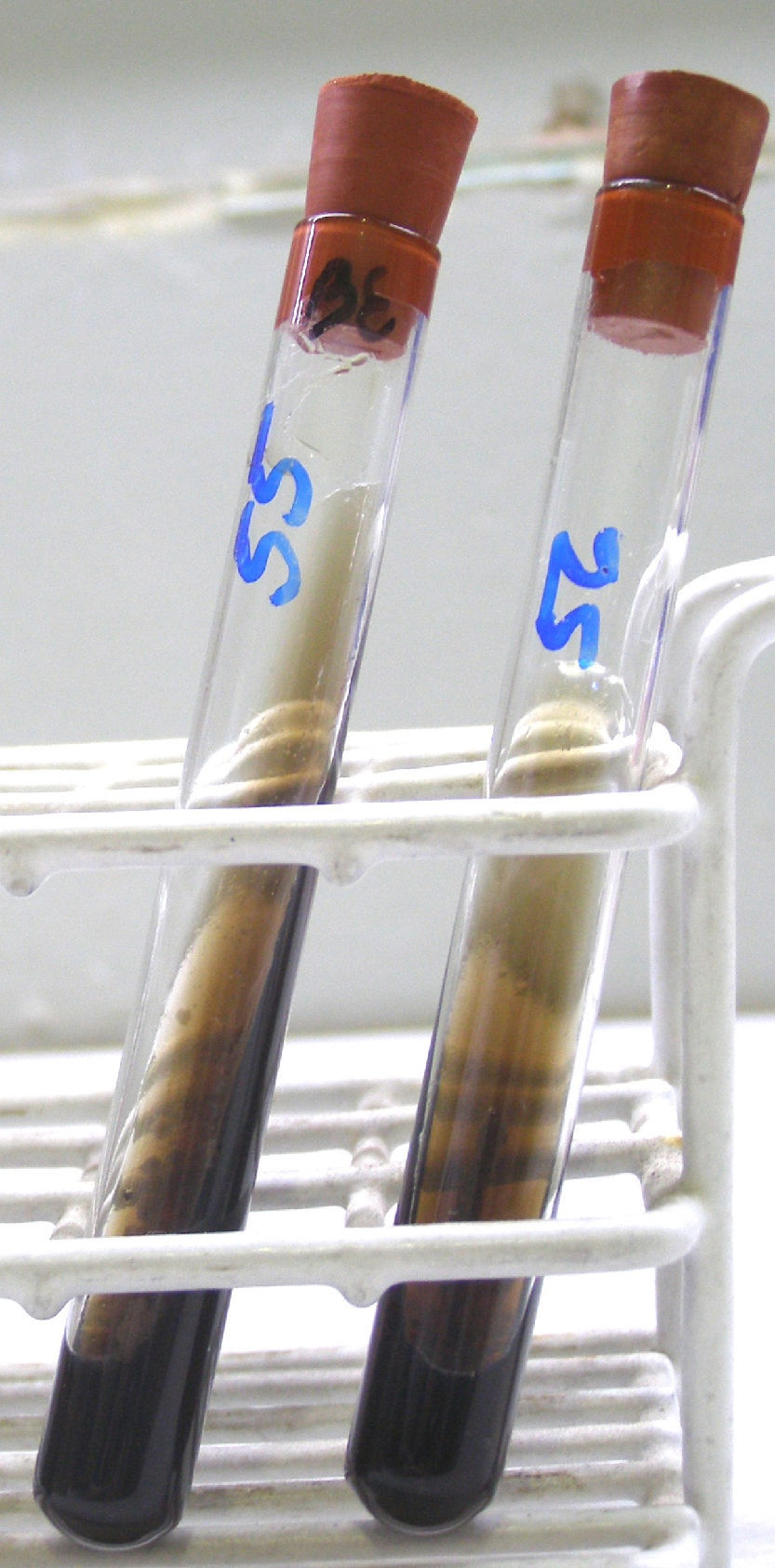
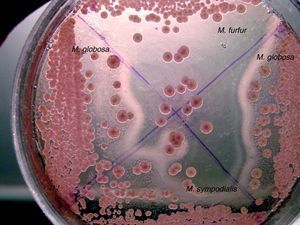
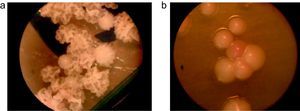
Isolates that showed a precipitates in CHROMM (Fig. 2) and did not grow in SGA were lipid-dependent. In EA, 102 samples (51%) showed production of a black zone due to esculin hydrolysis products and ferrous iron. These tests positive results are compatible with M. sympodialis (Fig. 3). On the other hand, 80 samples (40%) were negative to esculin hydrolisis and were identified as M. globosa.
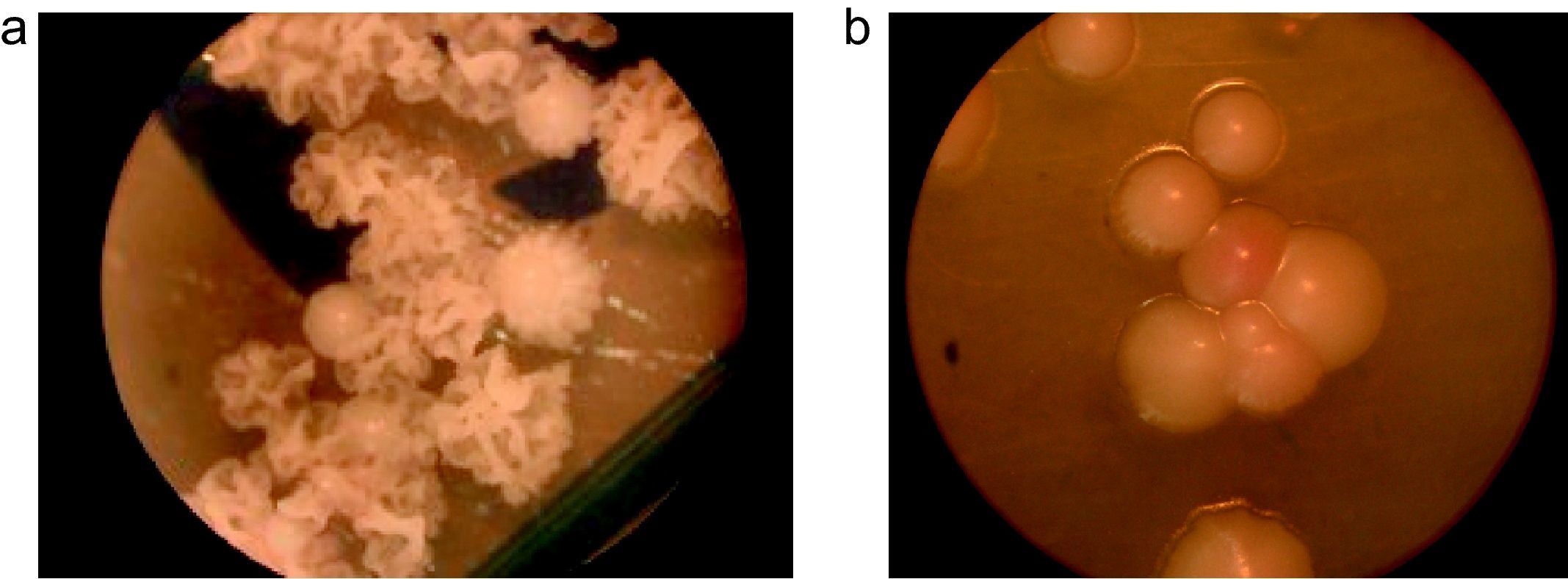
Fourteen strains (7%) out of 18 (9%) that did not produce any precipitate in CHROMM (Fig. 2) were characteristically large, pale pink, wrinkled and compatible to M. furfur (Fig. 4). Two (1%) were EA positive, and were identified as M. obtusa, whereas another 2 EA negative (1%) were identified as M. slooffiae. The tests of growth with CrEl and splitting of esculin (β-Glucosidase) were useful to confirm the identification of M. sympodialis, M. furfur and M. slooffiae.29
The reference strains followed the same scheme of identification and showed the expected results.
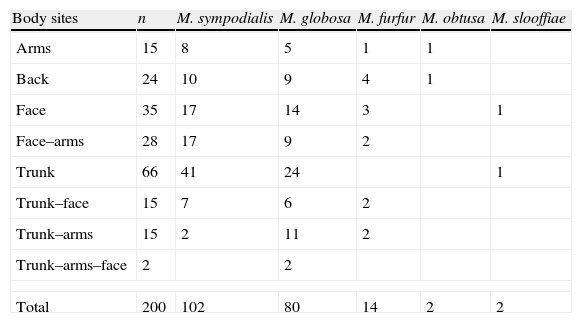
Among the 200 specimens, 59 were recovered from patients with two different body site lesions, and 2 from patients with three different body site lesions. Only one agent was recovered from these patients (Table 2).
Distribution of Malassezia species isolated from specimens studied.
| Body sites | n | M. sympodialis | M. globosa | M. furfur | M. obtusa | M. slooffiae |
| Arms | 15 | 8 | 5 | 1 | 1 | |
| Back | 24 | 10 | 9 | 4 | 1 | |
| Face | 35 | 17 | 14 | 3 | 1 | |
| Face–arms | 28 | 17 | 9 | 2 | ||
| Trunk | 66 | 41 | 24 | 1 | ||
| Trunk–face | 15 | 7 | 6 | 2 | ||
| Trunk–arms | 15 | 2 | 11 | 2 | ||
| Trunk–arms–face | 2 | 2 | ||||
| Total | 200 | 102 | 80 | 14 | 2 | 2 |
The most frequently isolated species in PV lesions from all body sites analysed were M. sympodialis [102/200 (51%)] and M. globosa [80/200 (40%)], followed by M. furfur [14/200 (7%)], M. obtusa [2/200 (1%)], and M. slooffiae [2/200 (1%)]. From those 200 specimens, 192 (96%) PV patients had a single Malassezia species in their lesions and 8 (4%) had 2 species with a distribution as follows: M. sympodialis–M. globosa (n=4); M. sympodialis–M. furfur (n=2) and M. furfur–M. globosa (n=2). M. obtusa and M. slooffiae, known to be relatively uncommon species in general, were obtained from single PV lesion.
DiscussionSimilar to other researches,8,38,21 the highest prevalence of PV in this study was observed in the 25- to 45-year-old group, suggesting that the peak of the infection coincides with ages when the sebum production is in its highest level.
The role of sex in propensity to develop of PV is still unclear. Some studies found that PV is more common in men than in women,7,10 while others indicated that the incidence of these infections is higher in women,20 which may be due to extra attention of women to beauty and the more frequent visits to their dermatologist in comparison to men. However, like many reports,11,19 this study has found no differences in the development of PV between both sexes. (The P-values <0.05 were required to consider statistically significant difference.)
In the present study, the most affected areas of the body were trunk and face, in concordance with the majority of studies worldwide.1,31,40 The back and the arms were the second most commonly affected sites, followed by the neck. Similar to other studies,40 we found no statistical difference in the distribution of Malassezia species on various body sites (P>0.01). This might be related to the frequent presence of patients with extensive PV lesions.13
When possible, the direct examination with Calcofluor white is recommended. This compound has high affinity for the chitin present in the fungal cell wall and in the budding zone. Hence, extra information, such as budding pattern, may be obtained.36
Culture is necessary to distinguish between the Malassezia species by morphological and physiological methods because the detection of a mixed infection would be important when the species have different responses to antifungal agents.13
In our study, the recovery rate of Malassezia species was 91% (200 samples), which showed good concordance with a recent study carried out by Nakabashi et al.,32 and higher than some previous studies.19,40 The difference may be due to the fact that the centre of the PV lesions yields more viable material for culture8,9,11 and avoid the isolation of surrounding commensal species.38,44
In the patients studied, the most frequently isolated species in PV lesions were M. sympodialis (51%) and M. globosa (40%), followed by M. furfur (7%), M. obtusa (1%), and M. slooffiae (1%). These results are comparable with those founded by Canteros et al.5 in Buenos Aires city (central region of Argentina) and Giusiano et al.13 in Chaco city (Argentine northeast). Gupta et al.19,20 in Ontario, Canada, found M. sympodialis as the main agent of PV in temperate climate regions and M. globosa as the predominant agent in tropical regions. However, Rasi et al.38 found M. globosa as the main agent of PV lesions in temperate region of Iran. In contrast, other studies, mainly carried out in areas with tropical or subtropical climates, showed a predominance of M. furfur in PV lesions.2 Makimura et al.27 in Japan, by molecular identification of the species, observed M. furfur and M. sympodialis as the predominant agents of PV, but not M. globosa. Species different from M. globosa, which is mainly observed in Northern countries, might predominate in PV in other climates. Hence, more studies are necessary to either confirm or reject this hypothesis.2 Further, the differences among these studies may be explained by different sampling techniques (scraping/contact plaques), the use of culture and non-culture methods and different culture media (modified Dixon agar/Leeming–Notman agar). There are probably geographical variations, that might influence the species recovered.31
Culture is not necessary for routine diagnosis, but is indispensable to recognize the species involved in the disease.2 The usefulness of the study of morphological characteristics for the differentiation of Malassezia species is rather subjective since it depends, to a large extent, on the observer and the conditions of the test, which can be subject to variation according to the culture medium employed and the temperature at which it is performed. Besides, identification of these species based on a microscopic observation is not able to provide unequivocal results. Thus, a simple, reliable and cost-effective identification method is required in most clinical laboratories.26 Physiological tests, namely culture in CHROMM, catalase test, lipidic dependence and esculin test are useful to achieve a correct identification of clinically important Malassezia species in our region. The employed methodology26 is suitable for the identification of dermatologic significant M. furfur, M. globosa and M. restricta species with a specificity of 100% and M. sympodialis with a specificity of 97.7%. Furthermore, using CHROMM as primary culture allows the detection of mixture of species directly from specimens due to the different characteristic that Malassezia species present in this differential culture medium.
At present, an increase in the isolation of M. globosa is being observed in patients with PV diagnosis (unpublished data) in our city. This is probably due to the climate changes our region is suffering (temperate climate originally) and how these variations modify the ecology of Malassezia genus. Isolation and identification of the different species is necessary to deepen the knowledge of regional epidemiology and ecology of the strains of Malassezia and their relationship with dermatologic disorders. Hence, systematically sampling and recording epidemiological data from hosts residing in various geographical locations and correlating with pathophysiological and environmental information could improve management of Malassezia associated infections and provide biological insights.
Conflict of interestThe authors have no conflict of interest to declare.
Reference strains used in this work were kindly provided by Dr J. Guillot (École Nationale Véterinaire College d’Alfort, Maisons-Alfort, France) and by the Dr. Carlos Malbrán Institute (INEI-Micología, Buenos Aires, Argentina).