One of the main problems for the preservation of genetics resources of Agaricus subrufescens is to maintain the viability of the strains because the mycelium is very sensitive to cooling and therefore it ages rapidly.
AimsEvaluate the viability of A. subrufescens strains stored as cultures on sorghum grain (spawn) at different temperatures.
MethodsEighteen strains of A. subrufescens and three strains of Agaricus bisporus were studied. Spawn's viability was evaluated under the following conditions: (1) control at 25°C (C), (2) cooling to 4°C (R) and (3) freezing in liquid nitrogen at −196°C (LN). Samples were recovered from week 4 every 2 weeks until week 12 and week 24 in C and R, whereas in LN samples were recovered at 4, 12 and 24 weeks. Viability was evaluated in 50 seeds, by strain and condition, recovering the mycelium in Petri dishes with potato dextrose agar medium (PDA). Mycelium growth was also evaluated on PDA after 14 days of recovery.
ResultsMost strains showed 100% viability and they were recovered usually in 1 day. In LN the viability ranged between 84 and 100% depending on the strain, but in some cases recovery took more than 10 days. Mycelial growth decreased gradually over time and although the results show significant differences between treatments C and R, the decline is associated with ageing of the mycelium rather than the treatment itself.
ConclusionsCulture on sorghum grain and storage at low temperature is an interesting way to preserve genetic resources of A. subrufescens.
Uno de los principales problemas para la preservación de los recursos genéticos de Agaricus subrufescens es mantener la viabilidad de sus cepas ya que el micelio es sensible al frío y, en consecuencia, envejece rápidamente.
ObjetivosEvaluar la viabilidad de cepas de A. subrufescens y cepas de Agaricus bisporus en cultivos en semillas de sorgo a diferentes temperaturas.
MétodosSe estudiaron 18 cepas de A. subrufescens y 3 cepas de A. bisporus. Se evaluó la viabilidad de A. subrufescens en las condiciones siguientes: (1) control a 25°C (C), (2) enfriamiento hasta 4°C (E) y (3) congelación en nitrógeno líquido a −196°C (NL). Las muestras se recuperaron a las 4, 6, 8, 10, 12 y 24 semanas en C y E, mientras que en NL se recuperaron a las 4, 12 y 24 semanas. La viabilidad se evaluó en 50 semillas, por cepa y condición, en placas de Petri con medio de agar patata dextrosa (APD). También se evaluó el crecimiento de los micelios en APD tras obtención a los 14 días.
ResultadosLa mayoría de cepas mostraron un 100% de viabilidad, y en general, se obtuvieron en 24h. En la condición NL la viabilidad varió entre el 84 y el 100%, pero en algunos casos su obtención requirió > 10 días. El crecimiento de los micelios se redujo gradualmente con el tiempo y, aunque los resultados indicaron diferencias significativas entre los tratamientos C y E, este declive se asocia al envejecimiento del micelio más que al propio tratamiento.
ConclusionesEl cultivo en semillas de sorgo y el almacenamiento a bajas temperaturas es un medio eficaz para preservar los recursos genéticos de A. subrufescens.
Agaricus subrufescens Peck, also named Agaricus blazei Murrill sensu Heinemann, Agaricus rufotegulis Nauta or Agaricus brasiliensis Wasser, M. Didukh, Amazonas & Stamets11 is a commercially cultivated mushroom appreciated by its medicinal and therapeutic properties, of note its antitumor, immunomodulatory, antioxidant, antimutagenic, antiviral, and antibacterial capacities, and its effects on autoimmune diseases, such as lupus and arthritis.1,2,4,6,7,13,15,17,19,28,33,34 This species is popularly known as cogumelo do sol, cogumelo piedade, cogumelo de deus, almond mushroom, almond Portobello, king Agaricus, Himematsutake or Kawariharatake, among others8,32 Commercially is often found with the initials ABM, abbreviation of Agaricus blazei mushroom. For taxonomy and synonymy of this taxon we followed Kerrigan,11 Arrilaga & Parra,3 Ludwig21 and Cappelli5 and the fact that the homonyme A. subrufescens Ellis & Everh is posterior as this has been corrected in Index Fungorum (http://www.indexfungorum.org).
One of the main attractions of the cultivation of A. subrufescens is its ability to grow at relatively high temperatures, making this species an ideal candidate for cultivation in the tropics and subtropics. Brazilian and Japanese authors have investigated the genetic polymorphism among cultivated strains, mainly by using RAPD markers, and they showed a high genetic homogeneity.16 In each country, the strains currently cultivated probably derived from a single or a very few sporophores, because the growers select the better strains. In addition to the cultivated strains with their low genetic diversity presented above, the collection of genetic resources for this organism is enriching with few North American,11 European,20 and more recently Asiatic isolates (Callac, personal communication). Due to the new interest for this mushroom with the growing industry of A. subrufescens, which has consolidated in recent years, works are in progress for increasing the number of specimens in collections and studying their diversity. Breeding programs leading to hybrid production are in progress.12 However both mushroom growers, breeders and biologists studying the biodiversity of this species have problems linked to the management of the strains because A. subrufescens is the only species of the genus Agaricus known suffering damage when exposed for prolonged periods at temperatures of 4°C or lower.11 Growers have difficulties for maintaining spawn under refrigeration for long time periods without loss of mycelium viability and breeders and biologists have to find other preservation methods of the genetic resources than conventional storage of mycelium at low temperature. Mushroom spawn is made of mycelium grown under sterile conditions on cereal grains. Most commercial growers acquire spawn and they store it, usually under refrigeration, until it is used for compost inoculation. The use of spawn as an alternative support for preservation of Agaricus genetic resources has been proposed by several authors after San Antonio and Hwang31 and Mata and Pérez-Merlo.23
The present work evaluated mycelium viability of both cultivars and wild strains from different origins of A. subrufescens using spawn on sorghum grain during storage at 4°C and in liquid nitrogen (−196°C). The aims were to check if the use of mycelium developed on cereal grains could be an efficient way for storage and preservation of A. subrufescens strains as previously shown for the white button mushroom, A. bisporus,23 to enlarge the ways of preservation in addition of the recent proposition by Colauto et al.7 of cryoconservation at −80°C on rice grain and the low-cost preservation by immersion proposed by Maia et al.,22 and to observe the phenotypic variability between strains representative of the diversity in the today germplasm of A. subrufescens.
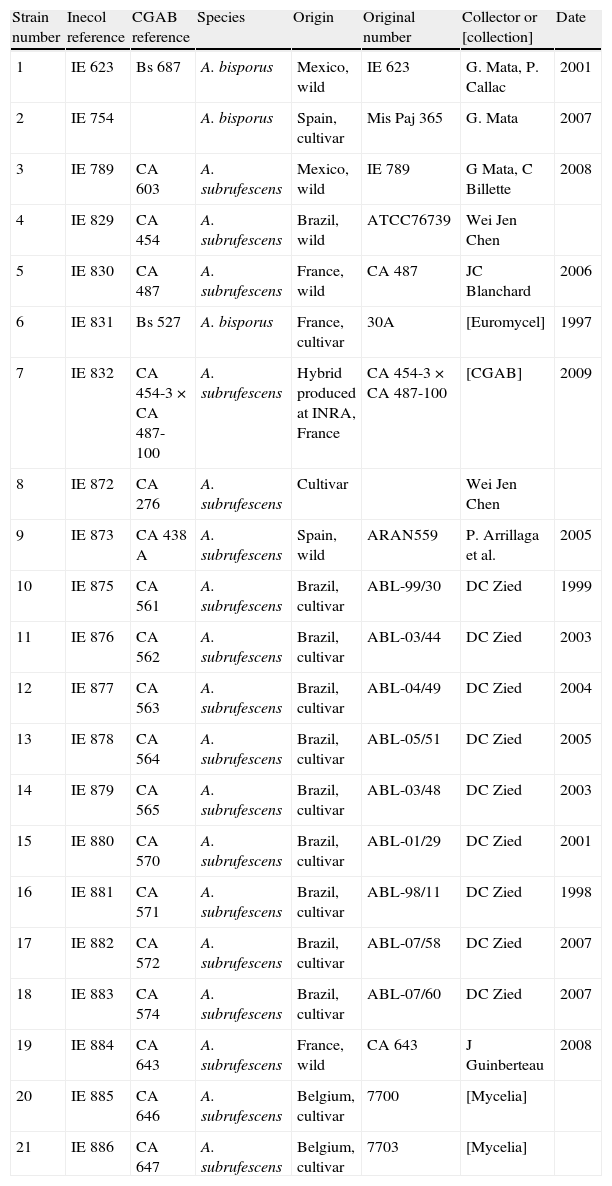
Materials and methodsStudied strains and spawn preparationEighteen strains of A. subrufescens and 3 strains of A. bisporus from different origins were studied. Table 1 shows the strains with their registration numbers in the strain collection at INRA in Bordeaux, France (CGAB) and the Institute of Ecology AC in Mexico (IE). The strains were maintained in culture medium of potato dextrose agar (PDA).
Studied strains with their original registration number and their geographical origin.
| Strain number | Inecol reference | CGAB reference | Species | Origin | Original number | Collector or [collection] | Date |
| 1 | IE 623 | Bs 687 | A. bisporus | Mexico, wild | IE 623 | G. Mata, P. Callac | 2001 |
| 2 | IE 754 | A. bisporus | Spain, cultivar | Mis Paj 365 | G. Mata | 2007 | |
| 3 | IE 789 | CA 603 | A. subrufescens | Mexico, wild | IE 789 | G Mata, C Billette | 2008 |
| 4 | IE 829 | CA 454 | A. subrufescens | Brazil, wild | ATCC76739 | Wei Jen Chen | |
| 5 | IE 830 | CA 487 | A. subrufescens | France, wild | CA 487 | JC Blanchard | 2006 |
| 6 | IE 831 | Bs 527 | A. bisporus | France, cultivar | 30A | [Euromycel] | 1997 |
| 7 | IE 832 | CA 454-3×CA 487-100 | A. subrufescens | Hybrid produced at INRA, France | CA 454-3×CA 487-100 | [CGAB] | 2009 |
| 8 | IE 872 | CA 276 | A. subrufescens | Cultivar | Wei Jen Chen | ||
| 9 | IE 873 | CA 438 A | A. subrufescens | Spain, wild | ARAN559 | P. Arrillaga et al. | 2005 |
| 10 | IE 875 | CA 561 | A. subrufescens | Brazil, cultivar | ABL-99/30 | DC Zied | 1999 |
| 11 | IE 876 | CA 562 | A. subrufescens | Brazil, cultivar | ABL-03/44 | DC Zied | 2003 |
| 12 | IE 877 | CA 563 | A. subrufescens | Brazil, cultivar | ABL-04/49 | DC Zied | 2004 |
| 13 | IE 878 | CA 564 | A. subrufescens | Brazil, cultivar | ABL-05/51 | DC Zied | 2005 |
| 14 | IE 879 | CA 565 | A. subrufescens | Brazil, cultivar | ABL-03/48 | DC Zied | 2003 |
| 15 | IE 880 | CA 570 | A. subrufescens | Brazil, cultivar | ABL-01/29 | DC Zied | 2001 |
| 16 | IE 881 | CA 571 | A. subrufescens | Brazil, cultivar | ABL-98/11 | DC Zied | 1998 |
| 17 | IE 882 | CA 572 | A. subrufescens | Brazil, cultivar | ABL-07/58 | DC Zied | 2007 |
| 18 | IE 883 | CA 574 | A. subrufescens | Brazil, cultivar | ABL-07/60 | DC Zied | 2007 |
| 19 | IE 884 | CA 643 | A. subrufescens | France, wild | CA 643 | J Guinberteau | 2008 |
| 20 | IE 885 | CA 646 | A. subrufescens | Belgium, cultivar | 7700 | [Mycelia] | |
| 21 | IE 886 | CA 647 | A. subrufescens | Belgium, cultivar | 7703 | [Mycelia] |
The strains were cultured for 7 days in Petri dishes with PDA. Spawn was prepared according to the method of Guzman et al.9 in sorghum seeds (Sorghum vulgare Pers.) 65% hydrated and sterilized at 121°C for 1h. The seeds, placed in Petri dishes were inoculated with a disc (±0.5cm diameter) of agar with mycelium precultures of each strain and incubated in the dark for 2 weeks at 25°C to allow the grains were completely covered by mycelium.
Preservation treatmentsAfter the 2-week incubation of spawn, Petri dishes were placed with spawn of each strain under refrigeration (R) at 4°C. The samples were recovered every 2 weeks from week 4 until week 12 and at week 24. Control samples (C) were maintained at 25°C and recovered in the same way that the treatment R.
For freezing samples the method proposed by Mata and Perez Merlo23 was used. Fully incubated sorghum seeds were placed in sterile polycarbonate (Nalgene) vials (25 seeds per vial) each vial containing 1.5ml of sterile cryoprotectant solution prepared with 10% glycerol (v/v). The seeds remained in contact with the cryoprotective solution for 1h and then samples were placed in polycarbonate boxes and transferred directly into the container of liquid nitrogen (−196°C) (LN). Samples were thawed at 4, 12 and 24 weeks, in polycarbonate boxes dipping in water at 30°C for 10min.26 Once thawed, the vials were cleaned for 1min in an alcohol solution (70%, v/v), then seeds were removed from vials and placed in Petri dishes with PDA.
Viability and vitality of the samplesTo evaluate the effect of cooling on mycelial viability, after treatments (C, R, LN) seeds were placed in Petri dishes containing PDA and incubated at 25°C. After treatments the percentage of sample recovery was evaluated through daily observations of the seeds. A sample was considered recovered when mycelial growth was noted by observing the seeds with a stereoscopic microscope. The delay for recovering was also recorded. For each treatment and week of incubation recovery was evaluated with 50 spawn seeds.
Moreover, the mycelial growth was evaluated by placing a spawn seed in a Petri dish with PDA and recording the diameter of the mycelium on 2 perpendicular axes.25 Ten samples were prepared per treatment and strain. Petri dishes with spawn seeds were incubated in darkness at 25°C for 14 days and the mycelial diameters were measured on two perpendicular axes.
Statistical analysis of dataData recorded for mycelial diameter were analysed using ANOVA followed by Tukey's multiple-range test (p=95%) to identify statistical differences in the average diameter of the mycelia obtained with different treatments.
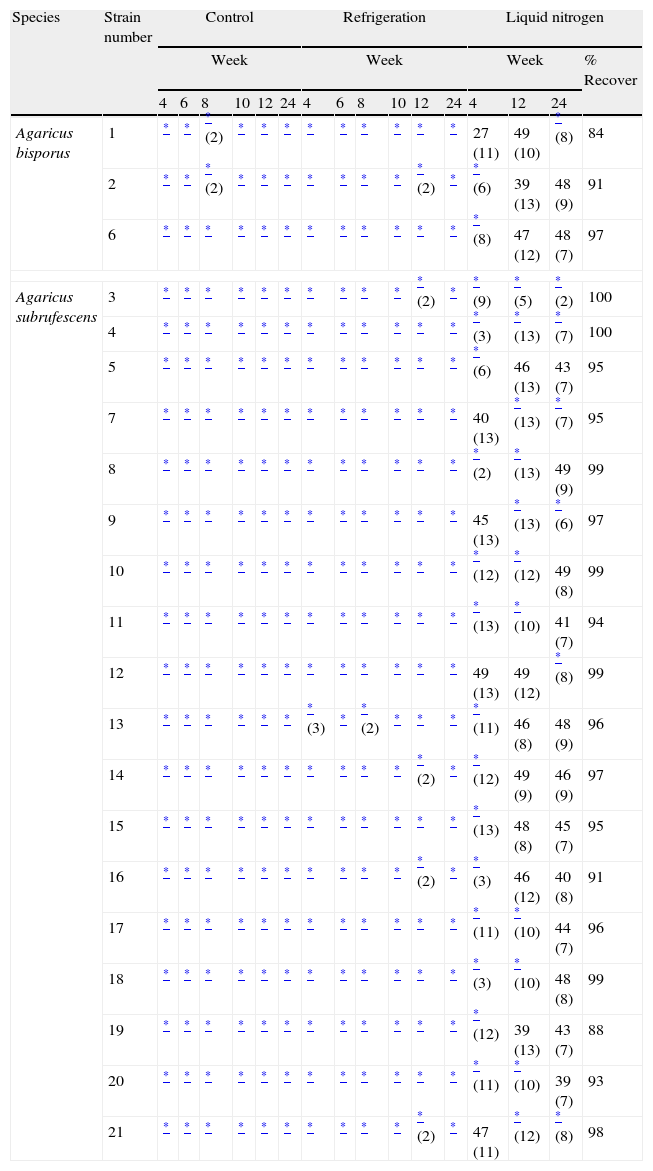
ResultsRecovery rates of the samples varied between the treatments and between strains in some treatments. They were 100% in controls (C) and treatment at 4°C (R). No sectoring was observed during the fungal colony extension. Most samples were recovered in one day after being placed in the culture medium. However, in some sampling times the delay for total recovery of the samples was between 2 and 3 days. In controls only two strains required two days to reach full recovery, they both were A. bisporus. In the treatment R, one A. bisporus strain out of three and five A. subrufescens strains out of 18 had recovery delays higher than one day, and one of them was recovered completely only on the third day (Table 2). Samples stored in liquid nitrogen showed recovery rates between 84 and 100% depending on the strain and the sampling date. It is noteworthy that the lower rate was for the Mexican strain of A. bisporus after four weeks of storage and required between 8 and 11 days for total or partial recovery, while some other strains required up to 13 days (Table 2). In all strains and conditions tested, the recovered mycelia showed normal appearance in color and texture, with no apparent alteration.
Number of samples recovered in treatments C, R and LN and percentage of recover.
| Species | Strain number | Control | Refrigeration | Liquid nitrogen | |||||||||||||
| Week | Week | Week | % Recover | ||||||||||||||
| 4 | 6 | 8 | 10 | 12 | 24 | 4 | 6 | 8 | 10 | 12 | 24 | 4 | 12 | 24 | |||
| Agaricus bisporus | 1 | * | * | * (2) | * | * | * | * | * | * | * | * | * | 27 (11) | 49 (10) | * (8) | 84 |
| 2 | * | * | * (2) | * | * | * | * | * | * | * | * (2) | * | * (6) | 39 (13) | 48 (9) | 91 | |
| 6 | * | * | * | * | * | * | * | * | * | * | * | * | * (8) | 47 (12) | 48 (7) | 97 | |
| Agaricus subrufescens | 3 | * | * | * | * | * | * | * | * | * | * | * (2) | * | * (9) | * (5) | * (2) | 100 |
| 4 | * | * | * | * | * | * | * | * | * | * | * | * | * (3) | * (13) | * (7) | 100 | |
| 5 | * | * | * | * | * | * | * | * | * | * | * | * | * (6) | 46 (13) | 43 (7) | 95 | |
| 7 | * | * | * | * | * | * | * | * | * | * | * | * | 40 (13) | * (13) | * (7) | 95 | |
| 8 | * | * | * | * | * | * | * | * | * | * | * | * | * (2) | * (13) | 49 (9) | 99 | |
| 9 | * | * | * | * | * | * | * | * | * | * | * | * | 45 (13) | * (13) | * (6) | 97 | |
| 10 | * | * | * | * | * | * | * | * | * | * | * | * | * (12) | * (12) | 49 (8) | 99 | |
| 11 | * | * | * | * | * | * | * | * | * | * | * | * | * (13) | * (10) | 41 (7) | 94 | |
| 12 | * | * | * | * | * | * | * | * | * | * | * | * | 49 (13) | 49 (12) | * (8) | 99 | |
| 13 | * | * | * | * | * | * | * (3) | * | * (2) | * | * | * | * (11) | 46 (8) | 48 (9) | 96 | |
| 14 | * | * | * | * | * | * | * | * | * | * | * (2) | * | * (12) | 49 (9) | 46 (9) | 97 | |
| 15 | * | * | * | * | * | * | * | * | * | * | * | * | * (13) | 48 (8) | 45 (7) | 95 | |
| 16 | * | * | * | * | * | * | * | * | * | * | * (2) | * | * (3) | 46 (12) | 40 (8) | 91 | |
| 17 | * | * | * | * | * | * | * | * | * | * | * | * | * (11) | * (10) | 44 (7) | 96 | |
| 18 | * | * | * | * | * | * | * | * | * | * | * | * | * (3) | * (10) | 48 (8) | 99 | |
| 19 | * | * | * | * | * | * | * | * | * | * | * | * | * (12) | 39 (13) | 43 (7) | 88 | |
| 20 | * | * | * | * | * | * | * | * | * | * | * | * | * (11) | * (10) | 39 (7) | 93 | |
| 21 | * | * | * | * | * | * | * | * | * | * | * (2) | * | 47 (11) | * (12) | * (8) | 98 | |
Numbers in parentheses are days for recovery.
Without number in parentheses means that all samples were recovered in 1 day.
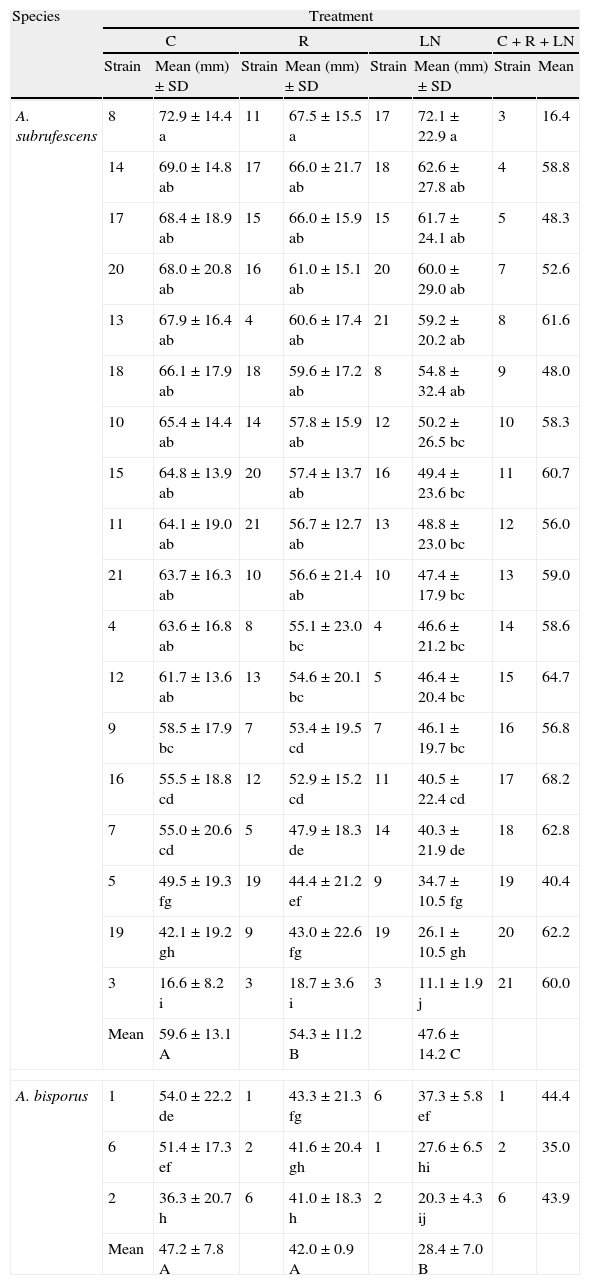
The average of mycelial diameter for all the A. subrufescens strains after incubation for 14 days was statistically different between the 3 treatments when the recovering times were grouped. Mean values for LN treatment reached 80% of the control value, and 88% of that of R treatment (Table 3). However, for half of the strains the differences between controls and refrigerated mycelia were not significant (Table 4). For the other half, higher diameters were recorded in the control treatment than in R. The two Brazilian cultivars 14 and 16 were the only A. subrufescens strains with a significant lower mycelial diameter in LN than in R. There was no significant correlation between the recovery rates and the mycelial growth ability which appeared to be a characteristic of strains. The strain with the slowest growth was strain 3 (wild Mexican isolate) with a mean diameter of 16.4mm, and the fastest growing strain was strain 17 (one of the more recent Brazilian cultivar) with 68.2mm (Table 3). It is noteworthy that the wild strains of A. subrufescens had the significantly lowest mycelial diameters after 14 days of incubation in each treatment.
Average of mycelial diameter in mm and standard deviations in treatments combining all the recovery times.
| Species | Treatment | |||||||
| C | R | LN | C+R+LN | |||||
| Strain | Mean (mm)±SD | Strain | Mean (mm)±SD | Strain | Mean (mm)±SD | Strain | Mean | |
| A. subrufescens | 8 | 72.9±14.4 a | 11 | 67.5±15.5 a | 17 | 72.1±22.9 a | 3 | 16.4 |
| 14 | 69.0±14.8 ab | 17 | 66.0±21.7 ab | 18 | 62.6±27.8 ab | 4 | 58.8 | |
| 17 | 68.4±18.9 ab | 15 | 66.0±15.9 ab | 15 | 61.7±24.1 ab | 5 | 48.3 | |
| 20 | 68.0±20.8 ab | 16 | 61.0±15.1 ab | 20 | 60.0±29.0 ab | 7 | 52.6 | |
| 13 | 67.9±16.4 ab | 4 | 60.6±17.4 ab | 21 | 59.2±20.2 ab | 8 | 61.6 | |
| 18 | 66.1±17.9 ab | 18 | 59.6±17.2 ab | 8 | 54.8±32.4 ab | 9 | 48.0 | |
| 10 | 65.4±14.4 ab | 14 | 57.8±15.9 ab | 12 | 50.2±26.5 bc | 10 | 58.3 | |
| 15 | 64.8±13.9 ab | 20 | 57.4±13.7 ab | 16 | 49.4±23.6 bc | 11 | 60.7 | |
| 11 | 64.1±19.0 ab | 21 | 56.7±12.7 ab | 13 | 48.8±23.0 bc | 12 | 56.0 | |
| 21 | 63.7±16.3 ab | 10 | 56.6±21.4 ab | 10 | 47.4±17.9 bc | 13 | 59.0 | |
| 4 | 63.6±16.8 ab | 8 | 55.1±23.0 bc | 4 | 46.6±21.2 bc | 14 | 58.6 | |
| 12 | 61.7±13.6 ab | 13 | 54.6±20.1 bc | 5 | 46.4±20.4 bc | 15 | 64.7 | |
| 9 | 58.5±17.9 bc | 7 | 53.4±19.5 cd | 7 | 46.1±19.7 bc | 16 | 56.8 | |
| 16 | 55.5±18.8 cd | 12 | 52.9±15.2 cd | 11 | 40.5±22.4 cd | 17 | 68.2 | |
| 7 | 55.0±20.6 cd | 5 | 47.9±18.3 de | 14 | 40.3±21.9 de | 18 | 62.8 | |
| 5 | 49.5±19.3 fg | 19 | 44.4±21.2 ef | 9 | 34.7±10.5 fg | 19 | 40.4 | |
| 19 | 42.1±19.2 gh | 9 | 43.0±22.6 fg | 19 | 26.1±10.5 gh | 20 | 62.2 | |
| 3 | 16.6±8.2 i | 3 | 18.7±3.6 i | 3 | 11.1±1.9 j | 21 | 60.0 | |
| Mean | 59.6±13.1 A | 54.3±11.2 B | 47.6±14.2 C | |||||
| A. bisporus | 1 | 54.0±22.2 de | 1 | 43.3±21.3 fg | 6 | 37.3±5.8 ef | 1 | 44.4 |
| 6 | 51.4±17.3 ef | 2 | 41.6±20.4 gh | 1 | 27.6±6.5 hi | 2 | 35.0 | |
| 2 | 36.3±20.7 h | 6 | 41.0±18.3 h | 2 | 20.3±4.3 ij | 6 | 43.9 | |
| Mean | 47.2±7.8 A | 42.0±0.9 A | 28.4±7.0 B | |||||
Different letters in the same column indicate significant differences between strains in mycelial diameters using Tukey's multiple range test (p=0.05%). Different capital letters on a line indicate significant differences in mycelial diameters between the treatments for each species.
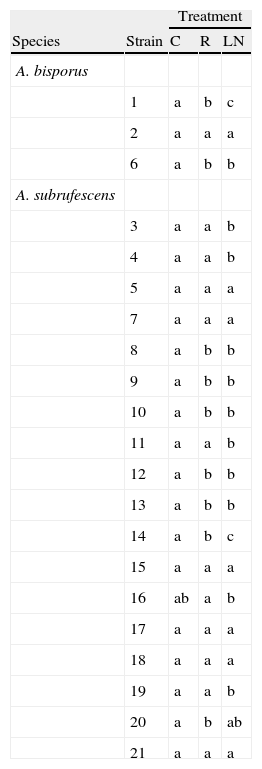
Significant differences by each strain in the tested treatments.
| Treatment | ||||
| Species | Strain | C | R | LN |
| A. bisporus | ||||
| 1 | a | b | c | |
| 2 | a | a | a | |
| 6 | a | b | b | |
| A. subrufescens | ||||
| 3 | a | a | b | |
| 4 | a | a | b | |
| 5 | a | a | a | |
| 7 | a | a | a | |
| 8 | a | b | b | |
| 9 | a | b | b | |
| 10 | a | b | b | |
| 11 | a | a | b | |
| 12 | a | b | b | |
| 13 | a | b | b | |
| 14 | a | b | c | |
| 15 | a | a | a | |
| 16 | ab | a | b | |
| 17 | a | a | a | |
| 18 | a | a | a | |
| 19 | a | a | b | |
| 20 | a | b | ab | |
| 21 | a | a | a | |
Different letters in the rows indicate significant differences in mycelial diameters using Tukey's multiple range test (p=0.05%).
For A. bisporus, the three strains had different reactions to the treatments (Tables 3 and 4). Whereas the Mexican wild strain was significantly gradually affected by the low temperatures, the observed differences in the cultivars were not or slightly significant. The mycelial diameters after 14 days of incubation were in the same range than that of the wild A. subrufescens strains.
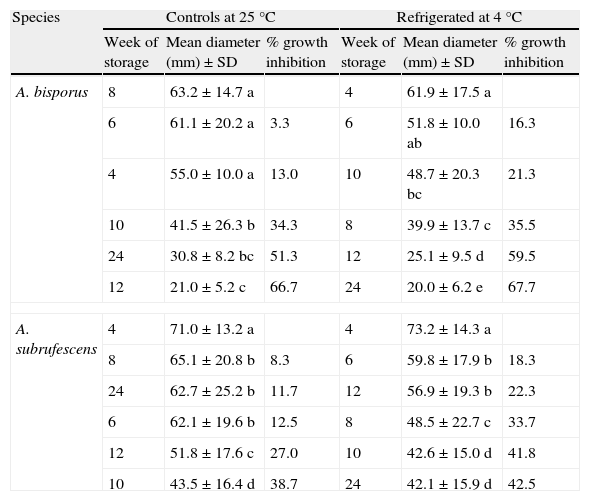
The highest growth ability was observed in the first's weeks of storage in treatments C and R, thereafter with the passage of time, mycelial diameters decreased gradually reaching significant lower values in both treatments. A. bisporus strains showed no significant difference in mycelial growth between weeks 4 and 8 in C treatment, while in R treatment it did not differ between weeks 4 and 6. The growth inhibition at week 10 in treatment C was 34.3%, while in treatment R was 35.5% at week 8 (Table 5). Moreover strains of A. subrufescens showed their greatest mycelial growth at week 4 in both C and R treatments. The observed growth from week 6 was significantly different and growth inhibitions were obtained up to 38.7% at week 10 of treatment C and 42.5% at week 24 of treatment R (Table 5).
Mycelial diameter average and standard deviation in all samples of treatments C and R in different weeks.
| Species | Controls at 25°C | Refrigerated at 4°C | ||||
| Week of storage | Mean diameter (mm)±SD | % growth inhibition | Week of storage | Mean diameter (mm)±SD | % growth inhibition | |
| A. bisporus | 8 | 63.2±14.7 a | 4 | 61.9±17.5 a | ||
| 6 | 61.1±20.2 a | 3.3 | 6 | 51.8±10.0 ab | 16.3 | |
| 4 | 55.0±10.0 a | 13.0 | 10 | 48.7±20.3 bc | 21.3 | |
| 10 | 41.5±26.3 b | 34.3 | 8 | 39.9±13.7 c | 35.5 | |
| 24 | 30.8±8.2 bc | 51.3 | 12 | 25.1±9.5 d | 59.5 | |
| 12 | 21.0±5.2 c | 66.7 | 24 | 20.0±6.2 e | 67.7 | |
| A. subrufescens | 4 | 71.0±13.2 a | 4 | 73.2±14.3 a | ||
| 8 | 65.1±20.8 b | 8.3 | 6 | 59.8±17.9 b | 18.3 | |
| 24 | 62.7±25.2 b | 11.7 | 12 | 56.9±19.3 b | 22.3 | |
| 6 | 62.1±19.6 b | 12.5 | 8 | 48.5±22.7 c | 33.7 | |
| 12 | 51.8±17.6 c | 27.0 | 10 | 42.6±15.0 d | 41.8 | |
| 10 | 43.5±16.4 d | 38.7 | 24 | 42.1±15.9 d | 42.5 | |
Different letters in the column by species indicate significant differences in mycelial diameters using Tukey's multiple range test (p=0.05%).
For the preservation of genetic resources and breeding programs, the longevity of the strains is an important question. Whereas the mycelial cultures of A. subrufescens in liquid or agar media are known to suffer damage when exposed for prolonged periods at temperatures of 4°C or lower,11 storage of mycelium on sorghum grain at 4°C did not affect the resilience of mycelia. The final recovery observed during 24 weeks of storage was always 100% and mostly in a single day, even if some strains showed a slightly greater recovery time. These results suggest that mycelia can survive at sub-lethal temperature, provided that they are embedded within and protected by the sorghum seeds in the spawn. Using paddy rice grains and 5 strains, Maia et al.22 reported no mycelial recovery after storage for 3 months at 4°C, whereas at room temperature good mycelial recovery was obtained until 6 months but the ability decreased after. The higher longevity obtained here with sorghum grain might be due to differences in physical attributes of the seeds that allow the mycelia to survive. The round shape and the low size of sorghum might contribute to a higher physical protection of the mycelium. With A. bisporus on wheat straw, Mata and Rodriguez-Estrada24 observed that mycelia only penetrated the external layer of the seed. After cryopreservation without cryoprotectant, mycelia were always recovered from seed hila, or from fissures on the seed surface, supporting the idea that seeds offer protection to mycelia. Sorghum seed hilum is an easy gate for mycelial penetration and the protective effect of the hull of the individual rice kernels might be less efficient than the sorghum pericarp. Sorghum seeds had previously been used for cryopreservation of other mushrooms.23
For improving the efficiency of damage protection during the cryoconservation in liquid nitrogen, spawn grains were immersed in 10% glycerol. As above such a treatment was reported to be inefficient with paddy rice by Maia et al.,22 whereas recovery percentages ranged from 88 to 100% for the 18 A. subrufescens strains studied. Fast freezing from ambient temperature to −196°C was used. Such a freezing process was inefficient using rice whereas a slow freezing from 8 to −80°C allowed to efficiently cryopreserving 5 A. subrufescens strains at −80°C for one year with 100% recovery.7 One can expect to improve the recovery efficiency after storage in liquid nitrogen by using a slow freezing process with the protecting sorghum grains. However, consistently with the present observations, the mycelial growth of cryopreserved cultivar strains of A. subrufescens (−80°C) was recovered after nine days of incubation in Colauto et al.7 The delayed recovery observed in liquid nitrogen treatment could be linked to the damage suffered by the mycelium during the thawing process of the spawn and the difficulty to clean the samples from the remains of glycerol. This delay at the beginning of mycelial growth was observed when spawn was frozen in liquid nitrogen using cryoprotectant solutions.23,24 Therefore, the differences between growth in LN treatment and treatments C and R could be linked directly to the delayed onset of mycelial growth rather than negative effects caused by the treatment itself.
The behavior of the 18 strains of A. subrufescens was similar to that of three A. bisporus strains that are less susceptible to low temperatures, either at 4°C or at −196°C. The recovery percentages and the delays observed after storage in liquid nitrogen with A. bisporus were in the same range than for A. subrufescens, whereas germplasm of this species is preserved efficiently in liquid nitrogen (on spawn or agar media) for many years in different laboratories, since cryogenic preservation for the maintenance of A. bisporus was proposed by San Antonio and Hwang.30 Despite their initial susceptibility difference to freezing, both species can be cryopreserved efficiently as spawn on sorghum grain, showing the interest of this preservation method.
Our chief interest is the longevity of the strains, but effects imposed by cold stress cannot be ignored. Despite the good results on the recovery of strains, the mycelial growth measured after incubation for 14 days showed a significant decrease with increasing storage time. This was observed for all the strains both with storage at 4°C and at 25°C. This phenomenon could be linked to an aging process of the mycelium independent of the temperature in a tested range. Changes over time are frequently observed in A. bisporus and are expressed as sectors of different growth rates and aerial mycelium density in cultures on agar media.18 The observed changes in mycelial growth could be linked to the instability of the strains and frequently are considered as irreversible degeneration of the strains. Due to the large standard deviation observed, it is expected that during the recovering process, one can select intentionally or not, the variant keeping their original vitality and productivity.
However, even with the delayed recovery in the liquid nitrogen treatment, high recoveries of all the strains were obtained and that is encouraging to protect the genetic material of A. subrufescens through freezing spawn on sorghum grain. Several studies10,14,31 have shown an efficient recovery of A. bisporus spawn without affecting their productivity. Similarly spawn has been used successfully for the conservation of Pleurotus strains,27Lentinula edodes and Lentinula boryana,26 and Volvariella volvacea.29 These results are encouraging and suggest that conservation of A. subrufescens genetic resources on sorghum grain for long periods of time could be a viable option even if experiments will be necessary for testing longer freezing times in order to evaluate the possible effects of freezing on the productivity of the strains.
The results obtained in this work suggest that spawn of A. subrufescens remains viable even under refrigeration, however, diminishes their ability to grow in relatively short periods of time. This has important implications for the management of spawn on commercial terms as the storage periods cannot be too long. The spawn should be used in an appropriate physiological time, this is particularly important when using cultivation substrates without a good selectivity because rapid growth of the mycelium to avoid competition with antagonistic organisms is required.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors are grateful to Rosalía Pérez and Carlos Ortega of Instituto de Ecología A.C. for their technical support. This work is part of research funded by a bilateral cooperation between Mexico (CONACYT project 115790) and France (ANR-09-BLAN-0391-01) in the “AgaSub” project: “Biology of the gourmet and medicinal mushroom Agaricus subrufescens, development of its cultivation and of new products of therapeutic interest or for diseases prevention”.