Maize is considered one of the crops more susceptible to mycotoxins in the world. Two of the mycotoxins commonly associated with maize are fumonisins and ochratoxin A. Aspergillus niger is a known producer of ochratoxin A and is easily found in maize. Recently, however, A. niger has been reported to produce as well fumonisins, mainly fumonisin B2.
AimsThe aim of this study was to isolate A. niger strains from maize samples collected in three Portuguese maize growing regions and to detect the production of both fumonisin B2 and ochratoxin A.
MethodsNinety five maize samples were collected, plated, and all observable Aspergillus section Nigri strains were isolated. Strains were morphologically characterized and mycotoxin production was determined by HPLC-FD.
ResultsIsolations resulted in a total of 270 strains of black Aspergillus from 73 samples (77% of the samples). About 14% of those strains were found to produce ochratoxin A and 39% of the strains were found to produce fumonisin B2.
ConclusionsAn association between the production of these two mycotoxins could not be found and no conclusions could be taken whether the presence of A. niger aggregate strains will increase the risk of maize contamination with fumonisins and more specifically with fumonisin B2.
En todo el mundo se considera que el maíz es uno de los cereales más vulnerables a las micotoxinas. Dos de las asociadas con más frecuencia a este cereal son las fumonisinas y la ocratoxina A. Se sabe que A. niger produce ocratoxina A, que suele estar presente en el maíz. Sin embargo, recientemente, se ha descrito que A. niger puede producir fumonisinas, principalmente fumonisina B2.
ObjetivosEl objetivo del presente estudio fue aislar las cepas de Aspergillus niger a partir de muestras de maíz provenientes de la siega en tres regiones agrícolas portuguesas y detectar la producción de fumonisina B2 y ocratoxina A.
MétodosSe obtuvieron 95 muestras de maíz de la siega en estas regiones, se sembraron en placas y se aislaron todas las cepas de Aspergillus sección Nigri observadas. Se caracterizaron morfológicamente las cepas y se determinó la producción de micotoxinas mediante cromatografía líquida de alto rendimiento con detección de fluorescencia.
ResultadosSe aislaron un total de 270 cepas de Aspergillus sección Nigri de 73 muestras (77% de las muestras). Se observó que alrededor del 14% de las cepas producían ocratoxina A, mientras que aproximadamente el 39% producían fumonisina B2.
ConclusionesNo pudo identificarse una asociación entre la producción de estas dos micotoxinas y no pudimos extraer conclusiones sobre si la presencia de cepas de A. niger aumentará el riesgo de contaminación del maíz por fumonisinas, en especial por fumonisina B2.
Maize (Zea mays L.) constitutes one of the most important crops worldwide, being, however, a good substrate for growth, development and activity of filamentous fungi.13,32 Maize is associated with a large number of fungal species belonging to the genders of Aspergillus, Fusarium, and Penicillium that can cause spoilage and mycotoxin contamination.17,26 Toxin-producing fungi can invade food at pre-harvest period, harvest-time or post-harvest during storage. Colonization and eventual contamination may occur in the same stage or a later one.18 Concerns about mycotoxin occurrence in maize are usually associated to Aspergillus flavus, for its ability to produce aflatoxins, and to Fusarium verticillioides and Fusarium proliferatum, for the production of fumonisins.7 Other fungi associated with this commodity are the ochratoxigenic ones.41 In a recent survey, ochratoxin A (OTA) was detected in 70% of maize bread samples, from the central zone of Portugal. From these, one sample exceeded the European maximum limit established for OTA in cereal products for human consumption (3μgkg−1).8,15 The presence of OTA in maize has also been associated with the contamination of beer,30 belonging the main Aspergillus OTA producer species to the A. niger aggregate.33
A. niger has a great economical and biotechnological interest and is extensively used for production of extracellular enzymes and organic acids such as citric acid.5,37A. niger has even been granted the GRAS (Generally Regarded As Safe) status in certain industrial production processes by the Food and Drug Administration of the US government.35
The discovery of A. niger strains producing OTA by Abarca et al.1 raised concerns not only for their biotechnological safety but also for their food safety risk, due to their common presence in different commodities.10,14,20 OTA is a potent nephrotoxin and has teratogenic, immunosuppressive and carcinogenic properties.11 Cereals and cereal based food and feed are the main contributors to OTA intake in humans and animals, since OTA is stable under normal food processing operation conditions and it is carried-over from raw materials to processed products.24 Recently, however, these concerns may have widened. There are several reports stating that A. niger can also produce fumonisin B2 (FB2) along with OTA.9,19,27 Fumonisins are suspected to cause human and animal toxicoses and are regarded as carcinogenic.12 Of the seven fumonisins currently identified, fumonisins B1 (FB1), B2 (FB2) and B3 (FB3) are the most frequently detected in fungal cultures or in naturally contaminated maize.31 What concerns Aspergillus niger aggregate strains, the main fumonisin produced is the FB2, and reports show that FB2 production by these species is higher while growing in low water activity (aw) culture media,28 when comparing with its production by Fusarium species. Therefore, maize and other commodities with low aw may be more susceptible to FB2 contamination in the presence of A. niger aggregate species.
The aim of this study was to evaluate the FB2 and OTA production by A. niger aggregate strains isolated from maize grains of three Portuguese regions.
Materials and methodsChemicalsOTA standard was supplied by Biopure (Austria). A standard solution of OTA was prepared in the OTA vial purchased from Biopure. The stock solution was made in 4mL toluene:acetic acid (99:1) at 250μgml−1, and stored at −20°C. An intermediate standard solution was prepared at 250ngml−1 by dilution with the same solvent, and stored at −20°C.
Fumonisin B2 standard was supplied by Sigma Chemical Co. (St. Louis, MO, USA) at 5mgml−1. An intermediate standard solution was prepared at 5μgml−1 by dilution in acetonitrile:distilled water (50:50), and stored at −20°C.
All solvents employed were of glass distilled or HPLC grade and all reagents used were of analytical grade.
Sampling planThe survey was carried out in three center-south Portuguese regions, from north to south: Beira Litoral, Ribatejo, and Alto Alentejo. Beira Litoral region (center west) climate is transitional between Atlantic and Mediterranean with annual precipitation of 984mm and the annual average temperatures are very moderate, 14.6°C. In higher areas the annual precipitation can easily exceed 1000mm. Ribatejo (central west) climate is temperate south-Mediterranean. The annual average temperatures are very moderate, 15°C with an annual precipitation of about 500–600mm. The region of Alentejo (center-southeast) has a Mediterranean climate feature of continental, i.e. hot and dry. The average annual temperature exceeds 17°C, and in the summer temperatures above 30°C are recorded throughout the eastern region. Average annual precipitation is less than 600mm.
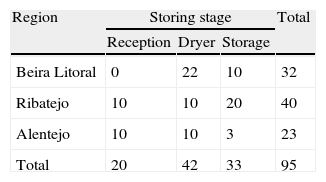
Thirty-two samples were taken in Beira Litoral region, forty samples from the Ribatejo and twenty three from Alto Alentejo, making a total of ninety five samples being taken in three distinct stages of the storage chain. Samples were collected into paper bags to prevent water condensation with the indication of the date, water content and transported to the laboratory within 24hours.
Twenty samples were taken at reception with a water content between 19 and 22%, 42 samples were taken after drying with a water content between 14 and 15% and 33 samples were taken after storage with a water content between 10 and 14%, between the months of November of 2008 and April of 2009 (Table 1).
Isolation of fungiFifteen grains of each maize sample were plated in Petri dishes with MEA10 (containing per liter: malt extract 20g; peptone 1g; glucose 20g; agar 20g; sodium chloride 100g) for 7 days at 25°C. All Aspergillus section Nigri strains were isolated after observing the plates under a stereomicroscope. The strains were purified by transferring them to Malt Extract agar (MEA; containing per liter: malt extract 20g, glucose 20g, peptone 1g, agar 20g) and incubating for 7 days at 25°C. Identification of the isolates was carried out by morphological characterization following the taxonomic keys and guides available for the Aspergillus genus.16,36 The taxonomy of fungi belonging to the section Nigri comprises one of the most confusing and complex due to the subtle differences between the species. In this manner, some biseriate species, such as Aspergillus carbonarius and Aspergillus ibericus can be recognized through the larger conidia size (6–8μm), for instance, and the uniseriate species (Aspergillus aculeatus, Aspergillus japonicus and Aspergillus uvarum), can be easily recognized through the absence of metullae. On the other hand, colonies representative of A. niger aggregate are very difficult to distinguish morphologically. In this study, because molecular analysis was not carried out, all black biseriate aspergilli strains other than A. carbonarius, Aspergillus ellipticus and A. ibericus will be referred to as the A. niger aggregate. Fungal frequency at each region was calculated as the ratio between the number of isolates and the number of samples taken. Fungal incidence was determined as the percentage of samples where the fungus was isolated.
HPLC-FDOTA samples were analysed by HPLC-FD with a Jasco FP-920 fluorescence detector (333nm excitation wavelength; 460nm emission wavelength). Chromatographic separations were performed on a reverse phase C18 column YMC-Pack ODS-AQ (250mm×4.6mm ID, 5μm) fitted with a precolumn with the same stationary phase. The mobile phase was water:acetonitrile:methanol (99:99:2, v/v) pumped at 0.8ml min−1. The injection volume was 30μl.
FB2 samples were analysed by HPLC-FD with a Jasco FP-920 fluorescence detector (460nm excitation wavelength; 500nm emission wavelength). Chromatographic separations were performed on a reverse phase C18 column YMC-Pack ODS-AQ (250mm×4.6mm ID, 5μm), fitted with a precolumn with the same stationary phase. The mobile phase was acetonitrile: water:acetic acid (60:40:1, v/v) pumped at 1.0mlmin−1. The injection volume was 30μl.
Mycotoxin productionOTA productionStrains were tested for OTA production in Yeast Extract Sucrose (YES) agar medium (containing per liter: yeast extract 20g, sucrose 150g, agar 15g). Strains were inoculated on 6cm diameter plates and incubated at 25°C for 7days in the dark. Extraction methodology consisted in removing 3 agar plugs (mean 3 agar plug weight of 0.424g) from one colony and placing into a 4ml vial where 1ml of methanol was added. After 60minutes, the extract was filtered through 0.45μm filters, evaporated and further dissolved in 1ml of mobile phase as described by Bragulat et al.6
Samples were taken as positive for each of the toxins when a peak was obtained at a retention time similar to each standard, with a height five times higher than the baseline noise. Limit of detection (LOD) was of 0.7ngml−1.
OTA production values were converted to μgkg−1 using the mean agar plugs value.
Fumonisin B2 productionAll strains were tested for fumonisin B2 production in Czapek Yeast Autolysate (CYA) agar (containing per liter: sucrose 30g, Difco yeast extract 5g, K2HPO4 1g, NaNO3 3g, KCl 0.5g, MgSO4·7H2O 0.5g, FeSO4·7H2O 0.01g, ZnSO4·7H2O 0.01g, CuSO4·5H2O 0.005g, agar 20g). Strains were inoculated on 6cm diameter plates and incubated at 25°C for 7days in the dark. Extraction and derivatization methodology as described in Abrunhosa et al.2 was employed: 5 agar plugs (mean 5 agar plug weight of 0.707g) were removed from one colony, and placed into a 4ml vial, where 1ml of methanol:water (75:25) was added. After sonication for 50minutes, the extract was filtered through 0.45μm filters, evaporated and further derivatized with 200μl of methanol, 200μl of borate buffer, 100μl of sodium cyanide and 100μl of NDA.
Samples were taken as positive for each of the toxins when a peak was obtained at a retention time similar to each standard, with a height five times higher than the baseline noise. LOD was of 75ngml−1.
FB2 production values were converted to μgkg−1 using the mean agar plugs value.
Association between OTA and FB2 productionTo estimate the possible association between OTA and FB2 production, a 2×2 contingency table test was used.4 A low association between variables is indicated when Phi is close to 0, and a strong association is indicated when Phi is close to 1. Statistical analyses were performed using SPSS Statistics version 19.0.
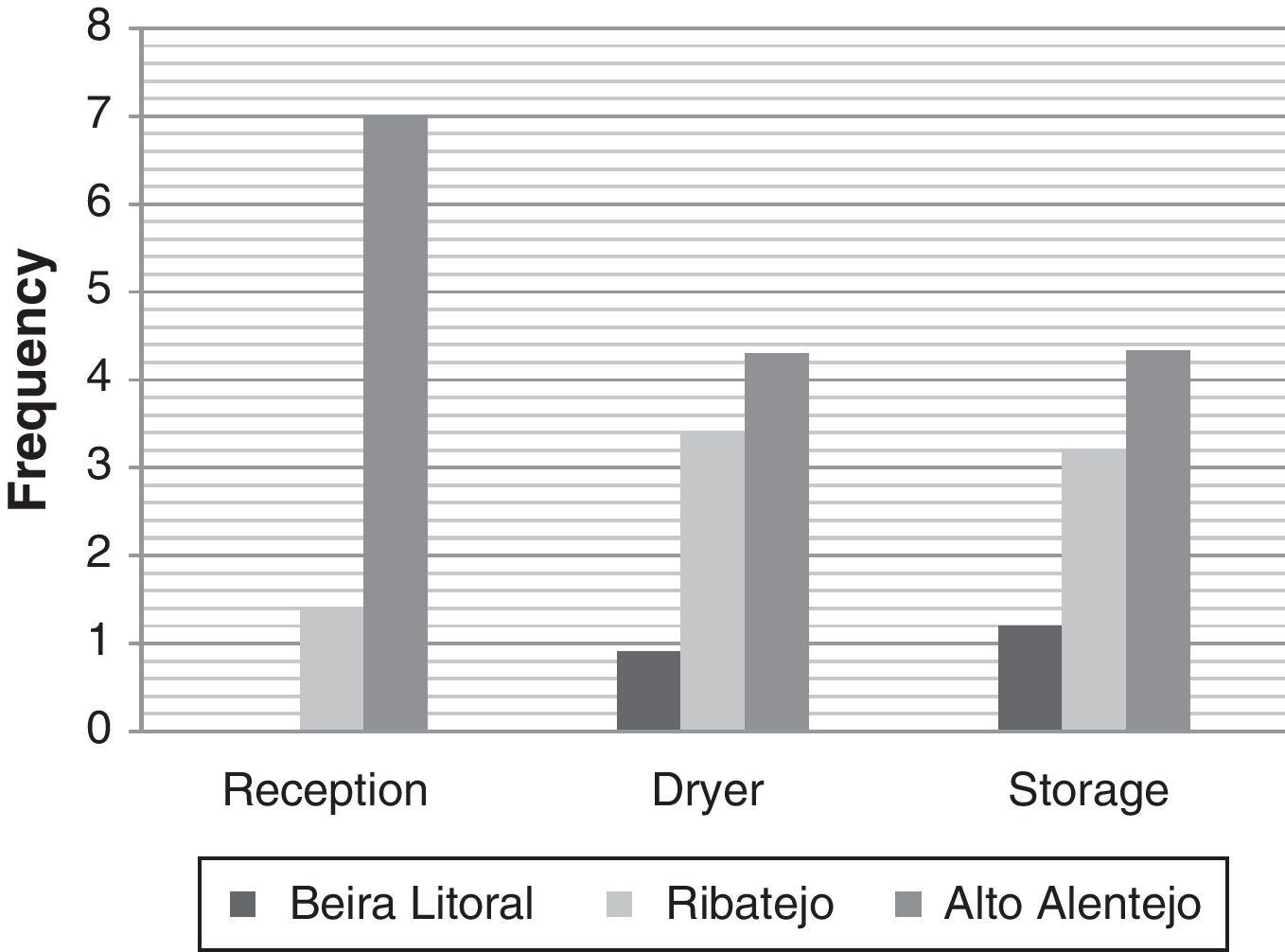
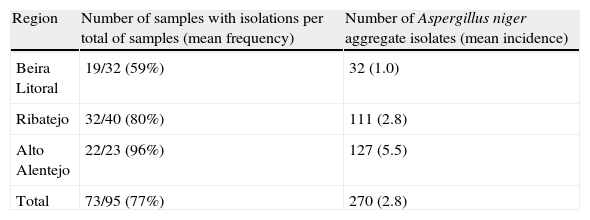
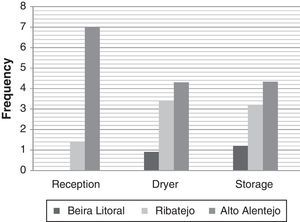
ResultsIsolation of black AspergillusFrom the ninety five samples of maize grains obtained in the three Portuguese regions, Aspergillus section Nigri isolates were found in seventy three samples (77%). From these, two hundred and seventy strains were isolated (Table 2). Morphological analysis revealed that all were bisseriate and within the A. niger aggregate; uniseriate species and other bisseriate species, such as A. carbonarius or A. ibericus, were not isolated. The incidence of Aspergillus section Nigri differed among regions being present in 59%, 80% and 96% of the samples from Beira Litoral, Ribatejo and Alto Alentejo, respectively. Fungal frequency followed the same trend, being higher in Alto Alentejo, in all stages, than in the other regions. Beira Litoral is the region that exhibits the lowest frequency (Fig. 1).
Number of isolates and samples from three Portuguese regions.
| Region | Number of samples with isolations per total of samples (mean frequency) | Number of Aspergillus niger aggregate isolates (mean incidence) |
| Beira Litoral | 19/32 (59%) | 32 (1.0) |
| Ribatejo | 32/40 (80%) | 111 (2.8) |
| Alto Alentejo | 22/23 (96%) | 127 (5.5) |
| Total | 73/95 (77%) | 270 (2.8) |
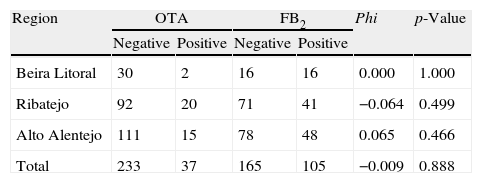
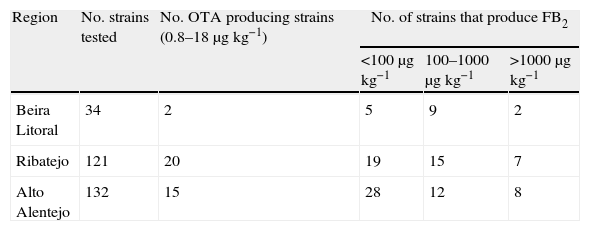
In Aspergillus section Nigri strains isolated from maize, OTA and FB2 were produced by 14% and 39% of the isolates, respectively (Table 3). Thirty-seven OTA producer strains and 105 FB2 producer strains were isolated. Beira Litoral exhibits the lowest incidence of ochratoxigenic strains (6%) and the highest incidence of FB2 strains (50%); Ribatejo has the highest incidence of ochratoxigenic strains (18%) and the smallest incidence of FB2 producers (34%); while Alto Alentejo has a mean incidence of OTA (12%) and FB2 (38%) producing strains.
Mycotoxin production of Aspergillus niger aggregate isolated in three Portuguese regions.
| Region | OTA | FB2 | Phi | p-Value | ||
| Negative | Positive | Negative | Positive | |||
| Beira Litoral | 30 | 2 | 16 | 16 | 0.000 | 1.000 |
| Ribatejo | 92 | 20 | 71 | 41 | −0.064 | 0.499 |
| Alto Alentejo | 111 | 15 | 78 | 48 | 0.065 | 0.466 |
| Total | 233 | 37 | 165 | 105 | −0.009 | 0.888 |
To evaluate if there is an association between the production of OTA and FB2, the Phi association value was calculated (Table 3). For the overall results the Phi – association value is fair (Phi<0.1), and not significant (p>0.05), meaning that their production is independent from each other.
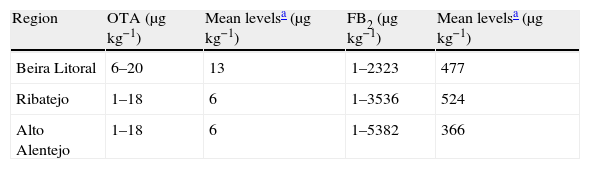
As expected the OTA production by these strains is relatively low in all regions (Table 4), with production values ranging between 1μgkg−1 and 20μgkg−1 (0.4ngml−1 and 8.5ngml−1). The FB2 production is much higher and ranged between 1μgkg−1 and 5382μgkg−1. Regarding OTA, even though Beira Litoral exhibits the lowest amount of producing isolates, the production mean for OTA is the highest when comparing with Ribatejo and Alto Alentejo. The same does not apply to FB2, being Ribatejo the region with the highest production mean. The region with higher amount of isolates, Alto Alentejo, is the one with lower production means.
FB2 production varied between strains where almost 50% produce below 100μgkg−1, 34% produce between 100 and 1000μgkg−1, and 16% produce at levels above 1000μgkg−1 (Table 5).
Number of A. niger aggregate strains producing OTA and FB2 and its distribution in three Portuguese regions.
| Region | No. strains tested | No. OTA producing strains (0.8–18μgkg−1) | No. of strains that produce FB2 | ||
| <100μgkg−1 | 100–1000μgkg−1 | >1000μgkg−1 | |||
| Beira Litoral | 34 | 2 | 5 | 9 | 2 |
| Ribatejo | 121 | 20 | 19 | 15 | 7 |
| Alto Alentejo | 132 | 15 | 28 | 12 | 8 |
Morphological analysis revealed that all isolated strains were bisseriate and within the Aspergillus niger aggregate. Uniseriate species were not found in these maize samples, even though it had been previously isolated from Nigerian and Argentinean maize.1,23 Also in other study, with Portuguese wine grapes, uniseriate strains were rarely isolated (2 isolates in 770 isolated strains),40 and only in a grape growing area located northern from these maize growing areas.
Aspergillus section Nigri species are common soil inhabitants contaminating ripening crops in Mediterranean, tropical and subtropical regions21 being more resistant to higher solar exposure and higher temperatures. Therefore, the higher incidence of isolates in Ribatejo and in Alto Alentejo (Table 2) may be explained by the fact that both these regions have Mediterranean climates, very hot and dry during summer time, frequently achieving temperatures around 40°C. Beira Litoral, on the other hand, has Atlantic influences with more moderate temperatures and higher precipitation, being more common other fungal species. The black aspergilla presence in Portuguese regions with Mediterranean climate had already been assessed in a survey of ochratoxigenic fungi in Portuguese wine grapes39,40 where these species have been isolated from 22% of grape samples. Although the sampling sites were not the same, it was also possible to observe a higher incidence in Alto Alentejo (72–100%) than in Ribatejo (18–48%).39 In these studies, temperature and relative humidity were found to determine the relative incidence of Aspergillus section Nigri in grapes.
Mycotoxin production by fungiThe production of OTA within Aspergillus section Nigri varies according to different authors: from 3% to 28%, being extreme value up to 80% reported in the literature, as reviewed by Palumbo et al.34 In the previous Portuguese study concerning the production of OTA from strains isolated from grapes, percentages of OTA producing strains of A. niger aggregate were lower (4%).40 However, OTA producing strains were not equally distributed among all regions, being the incidence in Southern regions higher.38 Magnoli et al. 23 reported 6% of ochratoxigenic A. niger strains in previously disinfected Argentinean maize kernels, which lies within the value obtained from Portuguese grapes and maize.
The number of fumonigenic A. niger aggregate strains is slightly higher (36%) in comparison with similar studies in grapes where 23% and 29% of Aspergillus niger aggregate strains produced FB2.3,42 However, the values are much lower than in other studies in grapes and coffee beans 19,27,29 where 60–70% of the isolates were found to produce FB2 although in these cases a very low number of strains were tested.
It is clear the A. niger species can produce both OTA and FB2; however, it was not clear if there is an association between the productions of these two mycotoxins. Although statistically not significant, the distribution of OTA and FB2 producing strains among regions seems to indicate that there is no connection between the production of both these toxins by the same fungus.
OTA production values (0.4μgl−1 to 8.5μgl−1) are lower than the ones reported by Magnoli et al. 24 which ranged between 2μgl−1 and 24.5μgl−1. The FB2 production is much higher and ranged between 1μgkg−1 and 5382μgkg−1, being lower, however, than the ones obtained by Mogensen et al. 27 that ranged between 229μgkg−1 and 6476μgkg−1 and by Susca et al. 42 that ranged between 100μgkg−1 and 293,000μgkg−1. The requirements for FB2 production by Aspergillus and Fusarium strains are not the same. Fusarium spp. are predominantly considered as field fungi and except under extreme conditions, the concentrations of fumonisins produced by Fusarium spp. do not increase during grain storage.25 However, Aspergillus have the ability to grow and produce mycotoxins at lower water availability conditions.22 Although the optimum temperature and aw differ, the levels produced under optimum condition are of the same order of magnitude.28
The fact that Aspergillus section Nigri fungi are associated to commodities not previously associated with Fusarium and, consequently, with fumonisins, the production of FB2 by Aspergillus section Nigri strains may extend the concern about fumonisins to a wider range of commodities.
In conclusion, Aspergillus section Nigri strains isolated from maize can produce both OTA and FB2 but, being maize a well-known natural substrate for Fusarium species and their fumonisins, it is still unclear if the presence of black aspergilla will increase the risk of maize contamination with fumonisins.
Conflict of interestThe authors declare no conflict of interest.
Célia Soares and Thalita Calado were supported by grants from Fundação para a Ciência e Tecnologia (references SFRH/BD/37264/2007 and SFRH/BD/79364/2011, respectively).
The authors are grateful for the support and cooperation of the National Producers Association of Maize and Sorghum (ANPROMIS – Associação Nacional de Produtores de Milho e de Sorgo).