Although in the last decade the management of invasive fungal infections has improved, a number of controversies persist regarding the management of complicated intra-abdominal infection and surgical extended length-of-stay (LOS) patients in intensive care unit (ICU).
AimsTo identify the essential clinical knowledge and elaborate a set of recommendations, with a high level of consensus, necessary for the management of postsurgical patients with complicated intra-abdominal infection and surgical patients with ICU extended stay.
MethodsA Spanish prospective questionnaire, which measures consensus through the Delphi technique, was anonymously answered and e-mailed by 30 multidisciplinary national experts, all of them specialists in fungal invasive infections from six scientific national societies; these experts were intensivists, anesthesiologists, microbiologists, pharmacologists and specialists in infectious diseases. They answered 11 questions drafted by the coordination group after conducting a thorough review of the literature published in the last few years. For a category to be selected, the level of agreement among the experts in each should be equal to or greater than 70%. In a second round, 73 specialists attended a face-to-face meeting which was held after extracting recommendations from the chosen topics and in which they validated the pre-selected recommendations and derived algorithm.
ResultsAfter the second Delphi round, the following 11 recommendations with high degree of consensus were validated. For “surgical patients” seven recommendations were validated: (1) risk factors for invasive candidiasis (IC), (2) usefulness of blood culture and direct examination of abdominal fluid to start empirical treatment; (3) PCR for treatment discontinuation; (4) start antifungal treatment in patients with anastomotic leaks; (5) usefulness of Candida score (CS) but not (6) the Dupont score for initiating antifungal therapy in the event of anastomotic leakage or tertiary peritonitis, and (7) the administration of echinocandins as first line treatment in this special population. For “surgical ICU extended LOS patients” four recommendations were validated: (1) risk factors for IC, (2) presence of multi-colonization by Candida as a required variable of the CS, (3) starting antifungal treatment with CS≥4, and (4) to perform non-culture-based microbiological techniques in stable septic patients without evident focus.
ConclusionsThe diagnosis and management of IC in ICU surgical patients requires the application of a broad range of knowledge and skills that we summarize in our recommendations. These recommendations, based on the DELPHI methodology, may help to identify potential patients, standardize their global management and improve their outcomes.
Aunque en la última década se ha observado un mejor control de la infección fúngica invasiva, todavía existen numerosas controversias en el manejo del paciente posquirúrgico con infección intraabdominal complicada y del paciente quirúrgico de larga estancia en UCI.
ObjetivosIdentificar los principales conocimientos clínicos necesarios y elaborar recomendaciones con un alto nivel de consenso para el tratamiento del paciente posquirúrgico con infección intraabdominal complicada y del paciente quirúrgico de larga estancia en UCI.
MétodosSe realizó un cuestionario español prospectivo que mide el grado de consenso mediante la técnica Delphi. Dicho cuestionario fue realizado de forma anónima y por correo electrónico por 30 expertos multidisciplinarios nacionales, especialistas en infecciones fúngicas invasivas, de 6 sociedades científicas nacionales. Los expertos incluían intensivistas, anestesistas, microbiólogos, farmacólogos y especialistas en enfermedades infecciosas que respondieron a 11 preguntas preparadas por el grupo de coordinación, preguntas que fueron confeccionadas tras una revisión exhaustiva de la literatura de los últimos años. El grado de acuerdo alcanzado entre los expertos en cada una de las categorías debía ser igual o superior al 70% para redactar una recomendación. En un segundo término, después de extraer las recomendaciones de los temas seleccionados, se celebró una reunión presencial con 73 especialistas y se les solicitó la validación de las recomendaciones preseleccionadas y de los algoritmos derivados de estas.
ResultadosConcluida la segunda ronda se validaron 11 recomendaciones con un elevado grado de consenso. Para los pacientes con infección intraabdominal complicada se validaron 7 recomendaciones: 1) factores de riesgo para la candidiasis invasiva; 2) utilidad del hemocultivo y del examen directo del líquido abdominal para iniciar tratamiento empírico; 3) PCR para la discontinuación del tratamiento; 4) inicio de tratamiento antifúngico en pacientes con dehiscencia de sutura anastomótica; 5) utilidad del Candida Score; 6) no utilidad de la escala de Dupont para el inicio de tratamiento antifúngico en caso de dehiscencia de sutura anastomótica o peritonitis terciaria, y 7) administración de equinocandinas como primera opción de tratamiento para esta población específica. Para los pacientes quirúrgicos de larga estancia en la UCI se validaron 4 recomendaciones: 1) factores de riesgo para candidiasis invasiva; 2) presencia de multicolonización por Candida como variable requerida del Candida Score; 3) inicio de tratamiento antifúngico si Candida Score≥4, y 4) determinación de técnicas microbiológicas no basadas en el cultivo en el paciente estable con sepsis sin foco evidente.
ConclusionesEl diagnóstico y abordaje de la candidiasis invasiva en los pacientes quirúrgicos en UCI requiere de la aplicación del amplio conocimiento y habilidades establecidas en nuestras recomendaciones. Estas recomendaciones, basadas en la metodología Delphi, pueden ayudar a identificar a los potenciales pacientes, estandarizar su manejo en conjunto y mejorar sus resultados clínicos.
The approach to invasive fungal infection (IFI) is still nowadays a relevant challenge in Intensive Care Units (ICUs).37 The reason for this is an increasing incidence of IFI in the ICUs in the last years,4 as well as, in comparison with the rest of the hospital services, a higher complexity of its diagnosis and treatment in critically-ill patients.42
The most frequent IFI in ICUs is invasive candidiasis (IC), especially candidemia and Candida peritonitis, and implies a mortality factor independent from the mortality rate in critically-ill patients.2,38 ICU and surgery wards are the services with the highest incidence of candidemia, summing up to two-thirds of every episode detected in hospital centers.23
The gastrointestinal tract is, together with the skin, the most frequent entrance for Candida infection.7 Multi-colonization is considered an essential factor for the IC pathogenesis.40 Several studies have identified different risk factors for the development of nosocomial or community-acquired IC34,39 in patients undergoing surgery. In the same way, enough evidence has been gathered to establish a universal empirical therapy for postsurgical patients, which is the case of high-risk patients with secondary peritonitis acquired in the community.33
Microbiological diagnostic techniques,22 not based on cultures and Candida Score (CS)19 may be useful for IFI diagnoses and, therefore, for the decision to prescribe antifungal treatment for postsurgical patients and ICU extended length-of-stay (LOS) patients.
Our primary goal is to analyze the current situation regarding the management of IC in postsurgical and surgical ICU extended LOS patients to obtain a set of therapeutic recommendations for different scenarios through the DELPHI methodology.
Material and methodsA panel (coordinator group) including 7 specialists from six scientific societies was formed – Spanish Society of Mycology (AEM), as the promoter; Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC); Spanish Society of Anesthesiology and Reanimation (SEDAR); Spanish Society of Intensive, Critical Care and Coronary Units (SEMICYUC); Spanish Society of Chemotherapy (SEQ); Spanish Society of Hospital Pharmacies (SEFH) – all with extensive experience in the treatment of critically-ill patients. They were responsible for developing a questionnaire after conducting a thorough review of the existing literature, as was done in the two previous editions of this project.43,44
An “expert panel” was made up of 30 specialists from different geographic locations in our country from six scientific societies involved in this research. The criteria followed for their inclusion in the panel were their experience in the investigation of IFI, as well as their expertise in the management of postsurgical patients and surgical extended LOS patients in ICUs. They were requested to answer a questionnaire drafted by the seven coordinators responsible for this research, as was done in the two previous editions of this project.43,44
In a second round, and after the elaboration of the resulting recommendations by the coordinators’ group, a second analysis was requested in a face-to-face meeting, in which 73 specialists from all around the country who care for postsurgical and surgical ICU extended LOS patients, validated the pre-selected recommendations and the derived algorithm through a voting procedure.
The Delphi methodology was used for the development of this study to optimize the consultation process of the 30 panel members. More specifically, thanks to the Delphi methodology, we were able to learn the groups’ opinions; not only that of a given individual, but the opinion of each of the experts in different areas of information suggested by the coordinators. The level of consensus required to make a recommendation was equal or higher than 70% (21 out of 30) in Top 4 (score of 7 or more points) by all of the experts consulted in each question. A total of 10 questions, all posed by the coordinators (Annex 1) were evaluated by the experts using a metric scale.
A total of 11 questions, prepared by the coordinators (Annex 1) were distributed in two sections: postsurgical patient section, 7 questions (developed by E.M and G.A); and surgical ICU extended LOS patients, 4 questions (developed by A.R and J.P).
The study methodology was based on the development of only one phase aimed to discover the level of consensus of the different questions. To meet this goal, on May 19th and 26th, 2014, 30 specialists (Annex 2) participating in the research anonymously answered the online questionnaire that included 11 questions. The coordinators responsible for the systematic research of the literature for the development of the questions, did not answer the questionnaire.
Thereafter, as mentioned above, recommendations and an algorithm were produced and validated by 73 experts in a face-to-face meeting held on September 25th, 2014 (Annex 3).
ResultsPatients with complicated intra-abdominal infection1. Agreement regarding the variables considered risk factors to initiate early empirical antifungal treatment in patients with community-acquired peritonitis, located in the upper GI tract (above the angle of Treitz).
Answers provided by the coordinators: Recent or current immunosuppressive therapy, pancreatic neoplasm, severe sepsis or septic shock, previous antibiotic treatment (5 days), upper GI neoplasm, and chronic PPT-antiH2 treatment.
Rationale. Published literature on community-acquired peritonitis caused in the upper GI tract have not determined a precise distinction of risk factors to initiate early/empirical antifungal therapy.6,27,35 In fact, to date there is still no evidence to support the use of empirical antifungal therapy in patients with secondary community-acquired peritonitis, including high-risk patients.15,31,33 This is why the recommendation deriving from the experts’ opinions have not determined a precise distinction of risk factors to initiate early/empirical antifungal therapy.6,27,35 Currently, there is not enough evidence to support empirical antifungal therapy in patients with secondary community-acquired peritonitis.
The panel members agreed to consider the administration of empirical/early antifungal therapy in this population in every situation proposed by the coordinators. Specifically, on a scale of 0–10 points in which 10 stands for the highest level of agreement, 24 out of the 28 specialists (85.7%) granted 7 or more points to pancreatic neoplasm and recent or current immunosuppressive therapy; 23 experts (82.1%) agreed in the case of upper gastrointestinal neoplasm, previous antibiotic therapy during 5 or more days and severe sepsis or septic shock; also 21 specialists (75%) agreed in the event of chronic treatment with proton pump inhibitors (PPTs) or histamine-2 receptor antagonists (antiH2). There was a high level of agreement for every variable (Top 4≥70%).
2. Agreement on the microbiological diagnostic techniques based on their positive predictive value and their possible usefulness to initiate empirical therapy in patients with intra-abdominal candidiasis.
Answers provided by the coordinators: blood culture, direct examination of abdominal fluid, (1→3)-β-D-glucan, and PCR.
Rationale. The armamentarium available for IC diagnosis includes direct detection and culture and indirect detection in which surrogate markers and polymerase-chain-reaction (PCR) assay are used. The sensitivity of blood cultures is far from ideal with a sensitivity of 21–71% according to autopsy studies.9 Published studies confirm that the polymerase chain reaction and the (1→3)-β-D-glucan quantification test are useful for the diagnosis of IC.29 The meta-analysis published by Karageorgopoulos et al., which is based on 16 studies, proves that the (1→3)-β-D-glucan test presents a sensitivity of 76.8% for the diagnosis of probable and proven infections caused by Candida.17 The study by Tissot et al., which includes critically-ill surgical patients with a high risk, confirms that the (1→3)-β-D-glucan test is superior to the colonization index and to the CS for the diagnosis of intra-abdominal candidiasis.24,36 The study published by Fortún et al., which was developed including patients suffering from deep tissue IC, established the positive predictive value (PPV) of a multiplex real-time PCR in 92.8%,14 a rate significantly superior to (1→3)-β-D-glucan.
The great majority of the experts (82.1%) agreed to point out the usefulness of the positive predictive value of blood cultures and the direct examination of abdominal fluids when empirical/antifungal treatment is administered in patients with intra-abdominal candidiasis. Specifically, on a scale of 0–10 in which 10 stands for the highest level of agreement, 23 out of 28 specialists granted 7 or more points to both microbiological diagnostic techniques in this scenario. Thus, a consensual agreement was established (Top 4≥70%).
In contrast, no consensus was reached regarding the validation of (1→3)-β-D-glucan, nor that of the PCR, which were only granted 7 or more points by 19 specialists (67.9%) and 18 specialists (64.3%), respectively out of the 28 experts.
3. Agreement on the microbiological diagnostic techniques based on their negative predictive value (NPV) and their possible usefulness to discontinue empirical/early antifungal therapy in patients with intra-abdominal candidiasis.
Answers provided by the coordinators: blood culture, direct examination of the abdominal fluid, (1→3)-β-D-glucan, and PCR.
Rationale. In the Tissot et al. study, the NPV of the (1→3)-β-D-glucan test in patients with gastrointestinal recurring perforation was higher than the NPV of the CS and also higher than the NPV of the colonization index in surgical critically-ill patients with a high-risk for the diagnosis of intra-abdominal candidiasis.36 In the study by Fortún et al., which included patients with deep tissue IC, the NPV of a multiplex real-time PCR was set in 98.7%, which was superior to (1→3)-β-D-glucan (94%).14 Tests showing a high NPV are useful for the physician to decide whether or not to discontinue antifungal therapy.29
The great majority of the panel (81.5%) agreed on validating the usefulness of the PCR negative predictive value for the discontinuation of empirical/early treatment in this population. Specifically, on a scale of 0–10 points in which 10 stands for the highest level of agreement, 22 out of 27 specialists granted 7 or more points to the convenience of the microbiological diagnostic technique in this situation, therefore a consensual agreement was reached (Top 4≥70%).
In contrast, the NPV of (1→3)-β-D-glucan, as well as the direct examination of abdominal fluid and the blood culture, were respectively thought to be useful by 18 (66.7%), 17 (63%) and 14 (51.9%) out of a total of 27 specialists, thus no consensus was achieved for any of the referred techniques (Top 4<70%).
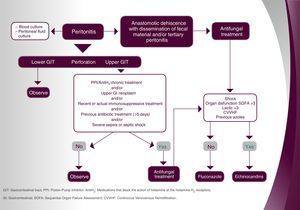
4. Agreement on the systematic initiation of empirical/early antifungal treatment in patients with anastomotic leakage (independent of the CS).
Rationale. Surgery, and especially abdominal surgery, is considered a risk factor in the CS, a scale for the detection of patients with risk of IC according to different risk factors.19 In this context, even though the management of patients with anastomotic leakage is not included in the CS, a number of scientific evidences prove that postsurgical peritonitis is associated to IC, particularly in patients with recurring gastrointestinal perforation.8,15,27
Most of the experienced consultants (75%) agreed on the need to initiate empirical/early antifungal treatment in every patient with anastomotic leakage, regardless of the presence of IC. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 21 out of the 28 participants granted 7 or more points to the need for the systematic administration of a treatment in this situation. Thus, a high consensus was reached (Top 4≥70%).
5. Agreement on the usefulness of the CS to decide on an empirical/early antifungal treatment in patients with anastomotic leaks, and/or tertiary peritonitis.
Rationale. The CS is not a specific scale for IC in patients with post-operative peritonitis. In fact, in most patients with suture dehiscence, the rate of Candida colonization is unknown. Nevertheless, surgery, and very specially, abdominal surgery, is considered a risk factor for IC in the CS.19 Thus, the CS may be useful to help the physician decide whether or not to start empirical/early antifungal treatment in patients with anastomotic leakage and/or tertiary peritonitis.8,15,26
The vast majority of the board members (82%) agreed to consider the use of the CS to determine empirical/early antifungal therapy useful in this population. Specifically, and using a 0–10 point scale where 10 represents the highest degree of agreement, 23 out of the 28 participants awarded 7 or more points to the usefulness of the CS in this situation, establishing a consensus of agreement (Top 4≥70).
6. Agreement on the usefulness of specific scores, such as Dupont's, to decide on the empirical/early antifungal treatment in patients with intra-abdominal sepsis.
Rationale. Nowadays, clinicians still do not possess the tools which enable them to determine if the presence of Candida in a multi-bacterial culture of the intra-abdominal fluid must be considered significant. In this context, the use of specific scales, such as the one developed by Dupont et al., could be helpful for the clinician to decide whether or not to initiate an antifungal treatment while waiting for fungal results.12 Nevertheless, these scales need to be validated by large multi-centric studies.13,26
Only 14 out of the 28 board members (50%) considered that specific scores for intra-abdominal candidiasis are useful to decide on administering an empirical/early antifungal therapy, thus consensus was not reached (Top 4<70%).
7. Agreement on the prescription of the following antifungal drugs as first-line empirical/early treatment in unstable patients with intra-abdominal infection and/or with serum lactate >3mmol/l, previous azoles intake and/or continuous renal replacement therapy.
Answers provided by the coordinators: fluconazole, amphotericin lipid B complex, liposomal amphotericin B, anidulafungin, caspofungin, and micafungin.
Rationale. The clinical practical guide for IC diagnosis and treatment in non-neutropenic adult patients, which was published by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2012, recommends echinocandin therapy as the first-line antifungal treatment, with an AI level of evidence.10 However, the ESCMID guide does not include a specific recommendation for intra-abdominal candidiasis. The consensus document by Bassetti et al. recommends the administration of echinocandins or liposomal amphotericin B as a first-line treatment for intra-abdominal candidiasis, with an AI level of evidence.5
The great majority of experts agreed to administer an echinocandin (micafungin, anidulafungin, caspofungin) as a first-line treatment in this population. Specifically, on a scale of 0–10 in which 10 stands for the highest level of agreement, 26 out of the 28 specialists (92.9%) granted 7 or more points to the administration of micafungin in this situation, an assessment granted by (89.3%) in the case of anidulafungin and caspofungin.
In contrast, although all specialists stated not to administer fluconazole as a first-line antifungal treatment in this situation, a consensus was not reached (Top 4<70%) on the convenience to use liposomal amphotericin B (17 answers with 7 or more points; Top 4: 60.7%) or amphotericin lipid B complex (8; 28.6%) in this patient population.
Surgical patients with ICU extended stay1. Agreement on the variables considered as risk factors for IC in abdominal surgical patients.
Answers provided by the coordinators: parenteral nutrition, patients with extended burns, high severity (APACHE II≥25 points), upper abdominal surgery (above the angle of Treitz), broad-spectrum antibiotic intake for more than 72h, extended hospitalization (>10 days in ICU or hospital), presence of solid neoplasm, and lower abdominal surgery.
Rationale. IC in critically-ill patients is relatively frequent (17%). Due to the lack of specific signs/symptoms of infection, the complexity of the patients and the insidious presentation of the clinical manifestations, it is difficult to diagnose.15 Several studies published in scientific literature identified a wide variety of IC risk factors in non-immunocompromised patients,28,41 even though their role has not yet been clearly determined.11
The variables that reached consensus (Top 4≥70%) were: Parenteral nutrition (26 answers in Top 4, 92.9% of all answers); high level of severity (APACHE II>25 points) (24; 85.7%); extended hospitalization >10 days (ICU or hospital) (24; 85.7%); receiving broad spectrum antibiotics for more than 72h (24; 85.7%); upper abdominal surgery (above the angle of Treitz) (24; 85.7%); and the presence of solid neoplasm (22; 78.6%).
The great majority of specialists (96.4%) agreed on considering parenteral nutrition an IC risk factor in patients with abdominal surgery. Specifically, on a scale of 0–10 points in which 10 stands for the highest level of agreement, 24 out of 28 participants granted 7 or more points, thus a consensual agreement was reached (Top 4≥70%). Consensus was also reached when determining other IC risk factors in this population: patients with extended burns (26 answers with 7 or more points; Top 4: 92.9%), high severity, (25; 89.3%), extended hospitalization (24; 85.7%), treatment with a broad-spectrum antibiotic for more than 72h (24; 85.7%), upper abdominal surgery (24; 85.7%), and the presence of solid neoplasm (22; 78.6%). Contrarily, a consensus (Top 4<70%) was not reached on defining lower abdominal surgery (22; 78.6%) as a risk factor in this population.
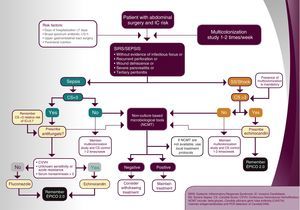
2. Agreement on the need to consider the presence of multi-colonization as a mandatory variable of the CS to prescribe an early antifungal treatment.
Rationale. Validated scales for the detection of patients with risk of suffering IC include a wider variety of risk factors.16 Although there are several authors who think that multi-colonization by Candida is not a significant variable for the detection of IC, a number of studies have proven that it is a very relevant clinical condition in this group of patients.19,20,43
Most of the panel members (75%) agreed on considering the presence of multi-colonization necessary to prescribe an antifungal treatment in this situation. Specifically, on a scale of 0–10, in which 10 stands for the highest level of agreement, 21 out of 28 participants granted 7 or more points to this regard, so a consensual agreement was established (Top 4≥70%).
3. Agreement on initiating fungal treatment based on the following results of the CS in stable (no shock) post-operative abdominal surgery patients, IC risk factors and sepsis with no evident focus.
Answers provided by the coordinators: CS=3, CS=4, and CS=5.
Rationale. Several studies have proven the usefulness of the CS, as a system of risk classification validated for suspicion of IC or candidemia. It assists in making a decision on the prescription of an early treatment in patients hospitalized in the ICU with specific risk factors.1,19 The study by Leon et al. revealed an IC incidence of 13.8% with CS>3, whereas with CS<3 the incidence rate was only 2.3%.20 This incidence progressively increases to 16.8% (RR=7.3), with CS=4 and up to 23.6% (RR=10.3), with CS=5.21
The great majority of the consulted experts agreed on the need to initiate fungal treatment in this population when the CS punctuation is higher than 3. Specifically, on a scale of 0–10 points in which 10 stands for the highest level of agreement, 25 out of 28 specialists (89.3%) granted 7 or more points to the need to administer the treatment when the CS score equals 5, an assessment sustained by 24 experts (85.7%) in the case of CS=4. In contrast, only 8 panel members (28.6%) agreed to administer the treatment to patients with CS=3, thus no consensus was reached (Top 4<70%).
4. Agreement on the performance of the following actions in stable (no shock) post-operative abdominal surgery patients, IC risk factors and sepsis with no evident focus, CS=3 and high clinical suspicion.
Answers provided by the coordinators: determining non-culture-based microbiological techniques to diagnose IC, and initiating antifungal treatment in the event of a positive non-culture-based microbiological method.
Rationale. The mortality rate due to IC continues substantially high.15 Although the empirical and early administration of an antifungal treatment is associated with better survival, a great number of patients still receive unnecessary treatment, leading to potential toxicity and cost increase.18 The detection of antibodies or antigens-related to the components of the fungal wall- or of nucleic acids might improve early diagnosis and guide the treatment.3,17,25,30,32
Most experts agreed not only on the need to initiate antifungal treatment in the event of a positive result in any non-culture-based microbiological technique (26 out of 28 answers received 7 or more points; Top 4 92.2%), but also to carry out non-culture-based microbiological techniques to diagnose IC (24; 85.7%). Thus, a high consensual agreement on both statements was established (Top 4≥70%).
Recommendations and algorithmsOnce the results achieved with the Delphi methodology applied to the management of complicated intra-abdominal infection and surgical patients with ICU extended stay were obtained, the recommendations exhibited were extracted as conclusions. They are based on the questions which reached a consensus equal or higher than 70%. These recommendations and the derived algorithm (Figs. 1 and 2) were validated thereafter during a face-to-face meeting with the hospital experts.
Conflict of interestThis consensus has been sponsored by MSD Laboratories, Spain.
Rafael Zaragoza Crespo
Intensive Care Department, Dr. Peset University Hospital. Valencia, Spain
Ricard Ferrer Roca
Intensive Care Department, Vall d¿Hebron University Hospital. Barcelona, Spain
Alejandro Hugo Rodríguez
Intensive Care Department, Joan XXIII University Hospital. Tarragona, Spain
Emilio Maseda Garrido
Department of Anesthesiology and Surgical Critical Care, La Paz University Hospital. Madrid, Spain
Pedro Llinares Mondéjar
Infectious Diseases Department, A Coruña University Complex. A Coruña, Spain
Santiago Grau Cerrato
Pharmacy Department, Hospital del Mar. Barcelona, Spain
José María Aguado García
Infectious Diseases Department, 12 de Octubre University Hospital. Madrid, Spain
Gerardo Aguilar Aguilar
Department of Anesthesiology and Surgical Critical Care, Valencia Clinical University Hospital. Valencia, Spain
Benito Almirante Gragera
Infectious Diseases Department, Vall d’Hebron University Hospital. Barcelona, Spain
Francisco Álvarez Lerma
Intensive Care Department, Hospital del Mar. Barcelona, Spain
César Aragón González
Intensive Care Department, Carlos Haya University Hospital. Málaga, Spain
María Izaskun Azcárate Egaña
Intensive Care Department, Donostia University Hospital. Donostia, Spain
Marcio Borges Sa
Sepsis Unit Coordinator, Son Llàtzer Hospital. Palma de Mallorca, Spain
Mercedes Bouzada Rodríguez
Anaesthesia, Resuscitation and Pain Therapy Department, University Hospital Clinic of Santiago. Santiago de Compostela, Spain
Juan Carlos del Pozo Laderas
Intensive Care Department, Reina Sofía University Hospital. Córdoba, Spain
Carmen Fariñas Álvarez
Intensive Care Department, Marqués de Valdecilla University Hospital. Santander, Spain
Jesús Fortún Abete
Infectious Diseases Department, Ramón y Cajal University Hospital. Madrid, Spain
Beatriz Galván Guijo
Intensive Care Department, La Paz University Hospital. Madrid, Spain
José Garnacho Montero
Intensive Care Department, Virgen del Rocío University Hospital. Sevilla, Spain
José Ignacio Gómez Herreras
Department of Anesthesiology and Surgical Critical Care, Valladolid Clinical University Hospital. Valladolid, Spain
Rafael Huarte Lacunza
Pharmacy Department, Miguel Servet University Hospital. Zaragoza, Spain
Cristóbal León Gil
Intensive Care Department, Valme University Hospital. Sevilla, Spain
Rafael León López
Intensive Care Department, Reina Sofía University Hospital. Córdoba, Spain
Patricia Muñoz García
Microbiology and Infectious Diseases Department, Gregorio Marañón University Hospital. Madrid, Spain
Jordi Nicolás Picó
Pharmacy Department, Son Llàtzer Hospital. Palma de Mallorca, Spain
Pedro Olaechea Astigarraga
Intensive Care Department, Galdakao Usansolo Hospital. Vizcaya, Spain
Javier Pemán García
Microbiology Unit, La Fe University and Polythecnic Hospital. Valencia, Spain
María Luisa Pérez del Molino Bernal
Microbiology and Parasitology Unit, Santiago de Compostela University Hospital Complex. Santiago de Compostela, Spain
Leonor Periañez Párraga
Pharmacy Department, Son Espases University Hospital. Palma de Mallorca, Spain
Guillermo Quindós Andrés
Microbiology Unit, Faculty of Medicine and Dentistry, Basque Country University. Vizcaya, Spain
Jesús Rico Feijoo
Department of Anesthesiology and Surgical Critical Care, Río Hortega University Hospital. Valladolid, Spain
María Rodríguez Mayo
Microbiology Unit, A Coruña University Hospital Complex. A Coruña, Spain
Eva Romá Sánchez
Pharmacy Department, La Fe University and Polythecnic Hospital. Valencia, Spain
Isabel Ruiz Camps
Infectious Diseases Department, Vall d’Hebron University Hospital. Barcelona, Spain
Miguel Salavert Lleti
Infectious Diseases Department, La Fe University and Polytechnic Hospital. Valencia, Spain
Juan Carlos Valía Vera
Department of Anesthesiology and Surgical Critical Care, General University Hospital Consortium. Valencia, Spain
César Aldecoa Álvarez-Santullano
Department of Anesthesiology and Surgical Critical Care, Río Hortega University Hospital. Valladolid, Spain
Rosa Ana Álvarez Fernández
Department of Anesthesiology and Surgical Critical Care, Asturias Central University Hospital. Asturias, Spain
Rocío Armero Ibáñez
Department of Anesthesiology and Surgical Critical Care, Doctor Peset University Hospital. Valencia, Spain
Fernando Armestar Rodríguez
Intensive Care Department, Germans Trias i Pujol University Hospital. Badalona, Barcelona, Spain
Miguel Ángel Arribas Santamaría
Intensive Care Department, Arnau de Vilanova Hospital. Valencia, Spain
José Ignacio Ayestarán Rota
Intensive Care Department, Son Espases University Hospital. Palma de Mallorca, Spain
María Ángeles Ballesteros Sanz
Intensive Care Department, Marqués de Valdecilla University Hospital. Santander, Spain
María José Bartolomé Pacheco
Department of Anesthesiology and Surgical Critical Care, Marqués de Valdecilla University Hospital. Santander, Spain
Unai Bengoetxea Uriarte
Department of Anesthesiology and Surgical Critical Care, Basurto Hospital. Bilbao, Vizcaya, Spain
Eva Benveniste Pérez
Intensive Care Department, Germans Trias i Pujol University Hospital. Badalona, Barcelona, Spain
José Blanquer Olivas
Intensive Care Department, Valencia Clinical University Hospital. Valencia, Spain
Felipe Bobillo del Amo
Intensive Care Department, San Carlos Clinical University Hospital. Madrid, Spain
Ángel Caballero Sáez
Intensive Care Department, San Millán Hospital Complex- San Pedro Hospital. Logroño, La Rioja, Spain
Andrés Carrillo Alcaraz
Intensive Care Department, Morales Meseguer University General Hospital. Murcia, Spain
José Castaño Pérez
Intensive Care Department, Virgen de las Nieves University Hospital. Granada, Spain
Pedro Castro Rebollo
Intensive Care Department, Clínic i Provincial of Barcelona Hospital. Barcelona, Spain
Milagros Cid Manzano
Department of Anesthesiology and Surgical Critical Care, Complex of Ourense University Hospital. Ourense, Spain
Belén Civantos Martín
Intensive Care Department, La Paz University Hospital. Madrid, Spain
María Victoria de la Torre Prados
Intensive Care Department, Virgen de la Victoria University Hospital. Málaga, Spain
David Domínguez García
Department of Anesthesiology and Surgical Critical Care, Nuestra Señora de la Candelaria University Hospital. Santa Cruz de Tenerife, Spain
Juan Ramón Fernández Villanueva
Intensive Care Department, Complex of Santiago Compostela University Hospital. A Coruña, Spain
Rafael García Hernández
Department of Anesthesiology and Surgical Critical Care, Puerta del Mar University Hospital. Cádiz, Spain
Rafael Franco Llorente
Department of Anesthesiology and Surgical Critical Care, Virgen de las Nieves University Hospital. Granada, Spain
Luis Gajate Martín
Department of Anesthesiology and Surgical Critical Care, Ramón y Cajal University Hospital. Madrid, Spain
Emilio García Prieto
Intensive Care Department, Asturias Central University Hospital. Oviedo, Asturias, Spain
Pau Garro Martínez
Intensive Care Department, Granollers General Hospital. Barcelona, Spain
Carolina Giménez-Esparza Vic
Intensive Care Department, Vega Baja Hospital. Orihuela, Alicante, Spain
Ricardo Gimeno Costa
Intensive Care Department, La Fe University and Polytechnic Hospital. Valencia, Spain
Francisco Javier González de Molina Ortiz
Intensive Care Department, Mutua de Terrassa University Hospital. Barcelona, Spain
Marta Gurpegui Puente
Intensive Care Department, Miguel Servet University Hospital. Zaragoza, Spain
María José Gutiérrez Fernández
Intensive Care Department, San Agustín Hospital. Avilés, Asturias, Spain
Joaquín Lobo Palanco
Intensive Care Department, Navarra Hospital Complex. Pamplona, Navarra, Spain
Mauro Loinaz Bordonabe
Intensive Care Department, Navarra Hospital Complex. Pamplona, Navarra, Spain
Esther María López Ramos
Intensive Care Department, Príncipe de Asturias University Hospital. Alcalá de Henares, Madrid, Spain
María Pilar Luque Gómez
Intensive Care Department, Lozano Blesa Clinic University Hospital. Zaragoza, Spain
Juan Francisco Machado Casas
Intensive Care Department, Jaén Hospital Complex. Jaén, Spain
José Miguel Marcos Vidal
Department of Anesthesiology and Surgical Critical Care, Virgen Blanca Hopital Complex. León, Spain
Fernando Maroto Monserrat
Intensive Care Department, San Juan de Dios del Aljarafe Hospital. Bormujos, Sevilla, Spain
Juan Carlos Martínez Cejudo
Intensive Care Department, Infanta Elena University Hospital. Huelva, Spain
María del Carmen Martínez Ramagge
Intensive Care Department, La Línea Hospital (AGSCampo of Gibraltar). La Línea de la Concepción, Cádiz, Spain
Ignacio Moreno Puigdollers
Department of Anesthesiology and Surgical Critical Care, La Fe University and Polytechnic Hospital. Valencia, Spain
Lorena Mouríz Fernández
Department of Anesthesiology and Surgical Critical Care, Lucus Augusti University Hospital. Lugo, Spain
Luis Alberto López Olaondo
Department of Anesthesiology and Surgical Critical Care, Navarra University Clinic. Pamplona, Navarra, Spain
Sergio Ossa Echeverri
Intensive Care Department, Burgos University Hospital. Burgos, Spain
Juan Carlos Pardo Talavera
Intensive Care Department, Reina Sofía General University Hospital. Murcia, Spain
Inés María Parejo Cabezas
Department of Anesthesiology and Surgical Critical Care, San Pedro de Alcántara Hospital. Cáceres, Spain
Jorge Pereira Tamayo
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Vigo, Pontevedra, Spain
Miguel Ángel Pereira Loureiro
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Vigo, Pontevedra, Spain
Ana Pérez Carbonell
Department of Anesthesiology and Surgical Critical Care, Elche General University Hospital. Alicante, Spain
Marcos Pérez Carrasco
Intensive Care Department, Vall d’Hebron University Hospital. Barcelona, Spain
Demetrio Pérez Civantos
Intensive Care Department, Infanta Cristina University Hospital. Badajoz, Spain
María José Pérez-Pedrero Sánchez-Belmonte
Intensive Care Department, Virgen de la Salud Hospital. Toledo, Spain
David Pestaña Lagunas
Department of Anesthesiology and Surgical Critical Care, Ramón y Cajal University Hospital. Madrid, Spain
Pedro Picatto Hernández
Department of Anesthesiology and Surgical Critical Care, Asturias Central University Hospital. Oviedo, Asturias, Spain
Rosa Poyo-Guerrero Lahoz
Intensive Care Department, Son Llàtzer Hospital. Palma de Mallorca, Spain
Luis Quecedo Gutiérrez
Department of Anesthesiology and Surgical Critical Care, La Princesa University Hospital. Madrid, Spain
Roberto Reig Valero
Intensive Care Department, Castellón General Hospital. Castellón, Spain
Manuel Rodríguez Carvajal
Intensive Care Department, Juan Ramón Jiménez Hospital. Huelva, Spain
Enrique Samsó Sabé
Department of Anesthesiology and Surgical Critical Care, Hospital del Mar. Barcelona, Spain
Catalina Sánchez Ramírez
Intensive Care Department, Doctor Negrín of Gran Canaria University Hospital. Las Palmas de Gran Canaria, Spain
Margarita Sánchez Castilla
Department of Anesthesiology and Surgical Critical Care, Puerta de Hierro-Majadahonda University Hospital. Madrid, Spain
Susana Sancho Chinesta
Intensive Care Department, Doctor Peset University Hospital. Valencia, Spain
Juan Carlos Sotillo Díaz
Intensive Care Department, Gregorio Marañón General University Hospital. Madrid, Spain
José Manuel Soto Blanco
Intensive Care Department, San Cecilio University Hospital. Granada, Spain
Luis Suárez Gonzalo
Department of Anesthesiology and Surgical Critical Care, La Paz University Hospital. Madrid, Spain
Teresa Tabuyo Bello
Intensive Care Department, A Coruña University Hospital. A Coruña, Spain
Eduardo Tamayo Gómez
Department of Anesthesiology and Surgical Critical Care, Valladolid Clinic University Hospital. Valladolid, Spain
Luis Mariano Tamayo Lomas
Intensive Care Department, Río Hortega University Hospital. Valladolid, Spain
Gonzalo Tamayo Medel
Department of Anesthesiology and Surgical Critical Care, Cruces University Hospital. Bilbao, Vizcaya, Spain
Vicente Torres Pedrós
Department of Anesthesiology and Surgical Critical Care, Son Espases University Hospital. Palma de Mallorca, Spain
Montserrat Vallverdú Vidal
Intensive Care Department, Arnau de Vilanova University Hospital. Lleida, Spain
Marina Varela Durán
Department of Anesthesiology and Surgical Critical Care, Pontevedra University Hospital Complex. Pontevedra, Spain
Paula Vera Artazcoz
Intensive Care Department, Santa Creu i Sant Pau Hospital. Barcelona, Spain
María Elena Vilas Otero
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Pontevedra, Spain