Fusariosis is an emergent opportunistic hyalohyphomycosis produced by fungi belonging to the genus Fusarium. These molds are capable of producing life-threatening diseases in immunocompromised hosts, especially in those suffering from leukemia. It has also been described in immunocompetent patients, where it usually causes non-invasive localized lesions. Fusariosis in immunocompromised individuals has a high morbidity and mortality mainly because of the low sensitivity of these fungi to the antifungal drugs available.
Case reportWe describe here the case of a patient with acute mieloblastic leukemia who developed fusariosis by a species of the Fusarium dimerum species complex. The early diagnosis was made on the basis of microscopic observation of samples from cutaneous lesions, and voriconazole treatment was prescribed. A subsequent complete study of the fungal isolate by culture and molecular methods allowed the identification of F. dimerum, a species rarely described as a human pathogen. The sensitivity of the strain was tested using the Sensititre YeastOne® commercial system, which showed sensitivity to voriconazole and posaconazole, as well as to amphotericin B. The patient died after 7 days at hospital due to an hemodynamic failure.
ConclusionsComplete identification of new isolates of Fusarium and their antifungal susceptibility patterns is of high interest to improve our knowledge about the epidemiology of the disease and how to best manage patients.
La fusariosis es una hialohifomicosis oportunista, emergente, producida por hongos pertenecientes al género Fusarium. Estos hongos pueden provocar enfermedades que amenazan la vida en pacientes inmunodeficientes, en especial en portadores de leucemia. También se ha descrito en individuos inmunocompetentes, en los que induce lesiones localizadas, no invasivas. En pacientes inmunodeficientes, la fusariosis se asocia a una elevada morbimortalidad, sobre todo debido a la falta de sensibilidad de estos hongos a los antimicóticos disponibles.
Caso clínicoDescribimos el caso de una paciente con leucemia mieloblástica aguda que experimentó una fusariosis por una especie del complejo Fusarium dimerum. El diagnóstico precoz se estableció en función de la observación microscópica de muestras de las lesiones cutáneas y se prescribió tratamiento con voriconazol. Más tarde, un estudio completo del aislamiento fúngico por cultivo y métodos moleculares permitió la identificación de F. dimerum, una especie apenas descrita como patógeno en el ser humano. La sensibilidad de la cepa se examinó con el método comercializado Sensititre YeastOne®, que reveló su sensibilidad a voriconazol y posaconazol, al igual que a anfotericina B. La paciente falleció a los 7 días del ingreso debido a una insuficiencia hemodinámica.
ConclusionesLa identificación completa de nuevos aislamientos de Fusarium y su patrón de sensibilidad a los antimicóticos suscita un gran interés para incrementar nuestros conocimientos sobre la epidemiología de la enfermedad y el tratamiento óptimo de los pacientes.
Fusarium spp. are important plant pathogens and may occasionally cause infections in humans. These fungi cause a broad spectrum of infections ranging from superficial to locally invasive and disseminated processes. The clinical form depends largely on the immune status of the host and the portal of entry of the fungus.7 In this document we describe the case of a patient with Acute Mieloblastic Leukemia (AML) who developed fusariosis by a species of the Fusarium dimerum species complex.
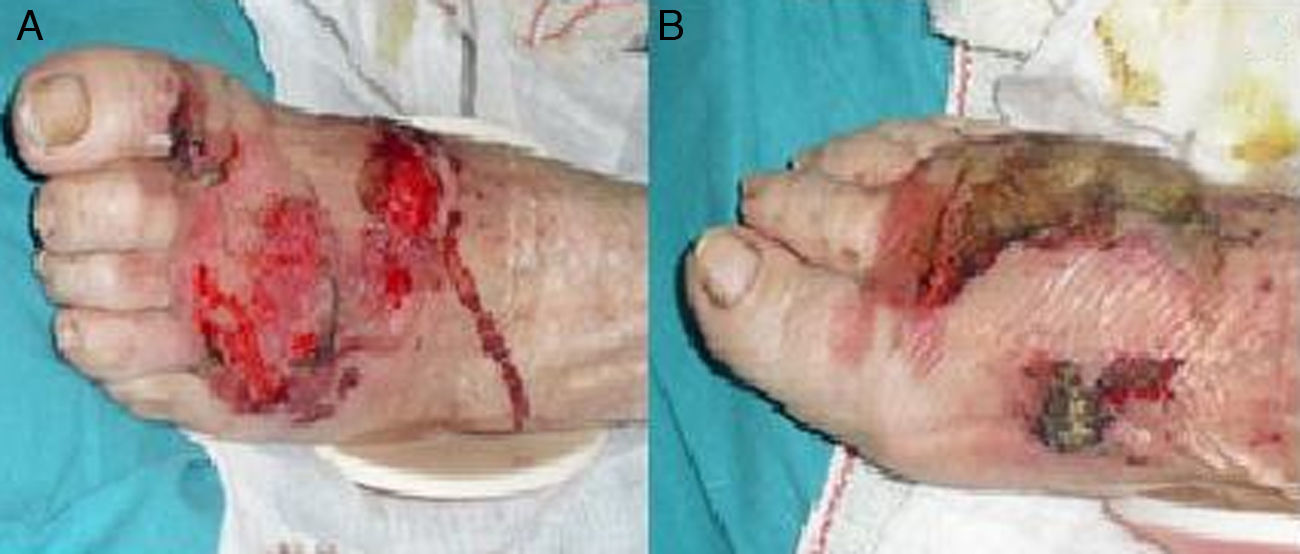
Case reportA 74-year-old woman with a medical history of high blood pressure and dyslipidemia, was admitted to hospital with bilateral edema and pain in both legs and feet. The anterior surface of the feet presented blisters and painful ulcerous lesions with pus discharge and perilesional erythema (Fig. 1). These symptoms had developed over the previous week. At that moment she was being treated with hidroclorotiazida–valsartan 25/160mg/day. The patient had been previously admitted to primary care services and received levofloxacin 500mg/12h and ibuprofen 600mg/8h, but the situation did not improve. She also showed symptoms of cardiac failure: dyspnea, orthopnea and increased abdominal perimeter. The patient did not currently present or remember to have had fever.
Physical examination revealed a bad general status and hemodynamic compromise with BP 65/38mmHg. The cardio-pulmonary examination showed low rhythmic tones and bilateral basal crackles. Laboratory investigations revealed signals of renal failure (creatinine: 8.73mg/dl; glomerular filtration rate: 5ml/min), metabolic acidosis (pH: 7.26, HCO3: 16.2mequiv./L), light thrombocytopenia (122×103/μL), leukocytosis (37×103/μL) and anemia (Hb: 7.6mg/dl; VCM: 115.6fl). Transaminase enzymes and coagulation were normal. After these findings, a subsequent study of bone marrow and peripheral blood resulted in the diagnosis of acute mieloblastic leukemya (AML). Hemodynamic support measures and intravenous treatment with antibiotic were established considering the renal function. Piperaziline–tazobactam 2g/8h and levofloxacin 250mg/48h were prescribed. Two samples of the curettage of the ulcer lesions were taken and sent to the microbiology laboratory. Both samples were studied under optical microscopy and cultured on sheep blood agar, blood-CNA agar, Polivitex agar, McConkey agar and Sabouraud–Cloramfenicol–Gentamicin agar. After Gram staining, microscopic observation of the sample from the right foot showed a filamentous fungus as the only detectable microorganism. The morphology of the abundant conidia suggested Fusarium sp. as the causative agent (Fig. 2). Based on this finding, a first dose of voriconazole 400mg was administered followed by a regimen of 200mg/12h. The direct observation of the left foot sample was not informative.
After two days of incubation at 37°C, the sample from the right foot yielded a pure culture of a filamentous fungus, which was consistent with the previous Gram stain observation. An Enterococcus faecalis was isolated on sheep blood agar from the sample of the left foot and i.v. ampicillin (1g/8h) was prescribed. Additionally, two samples of peripheral blood were cultured and came out negative after 5-day incubation. Seven days after admittance to the hospital and after 5 days of treatment with voriconazole, no favorable response was obtained and the patient died.
Based on the difficulty to identify Fusarium at a species level, the isolate was sent to the Medical Mycology laboratory (Faculty of Medicine) where it was subcultured on Potato Dextrose Agar, Corn Meal Agar and Malt Extract Agar. Macroscopic and microscopic features were exhaustively studied and concluded in the identification of the strain as F. dimerum (Fig. 3). For molecular identification, ITS1-ITS4 and the 5.8S region of the rDNA were amplified with specific primers in a semi-nested PCR.4 The sequence of a fragment of 561 nucleotides was obtained and analyzed by comparison in GenBank and EMBL database. A similarity of 100% with the sequence of Fusarium delphinoides was found. F. delphinoides is one of the species described within the F. dimerum morphotype group.2 Nevertheless, this finding was not enough to assess the identification within the F. dimerum species complex, where other species (e.g. F. nectrioides) could yield the same similarity of the ITS region.9 The sensitivity of this isolate against conventional antifungal drugs was determined by the Sensititre YeastOne® system.6 The isolate showed resistance to all echinocandins (MICs>8μg/ml), 5-fluorocytosine (MIC>64μg/ml), itraconazole (MIC>16μg/ml) and fluconazole (MIC>256μg/ml), and sensitivity to amphotericin B (MIC: 1μg/ml), voriconazole (MIC: 0.5μg/ml) and posaconazole (MIC: 0.5μg/ml).
DiscussionFusariosis in patients with hematological malignancies usually occurs in the shape of disseminated infections where the original site of infection of the fungus is difficult to establish.3 Skin lesions lead to the diagnosis in more than 50% of patients and usually precede the fungaemia in approximately 5 days. In contrast with aspergillosis, where blood cultures are nearly always negative, fusariosis is accompanied by positive blood cultures in 40–50% of the patients.5,8 In our case, F. dimerum was rapidly detected in the lesions of the right foot. The absence of fever and the negative hemocultures first suggested a possible localized infection. Nevertheless, the diagnosis of AML and the deadly progress of the infection were more in accordance with the existence of fungaemia. Even though a rapid detection of the fungus and a subsequent prescription of appropriate treatment were done, the hemodynamic complications were severe and the patient died.
Fusarium has emerged as an important cause of infection in immunocompromised patients and is now considered the second most frequent mold involved in fungal infections.5 Especially, the disease not only is frequent in patients with hematological malignancies but can also occur as localized infections in healthy patients.10 The infection in this case was due to one of the 12 phylogenetic species belonging to the morphototype F. dimerum.2 This fungus had been scarcely described as a causative agent of human fusariosis.2 Its susceptibility to antifungals showed a short spectrum of effective drugs, which is a common feature for Fusarium species.1
The rapid detection of Fusarium spp. by direct microscopy is a very useful tool and allows a rapid prescription of antifungal treatment. However, it is not enough for a correct management of the infection. The diversity of species involved in fusariosis is underestimated because of the frequent lack of identification at a species level. The species distribution varies per geographic region and different species have different drug susceptibility patterns.11 Therefore, complete identification of the fungi and their antifungal susceptibility patterns is of high interest to improve our knowledge about the epidemiology of the disease and how to best manage patients.
Conflicts of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors acknowledge María Abad for the professional review of the English writing of the manuscript.