Trichosporon asahii is a yeast-like fungus that has recently gained importance as a cause of opportunistic systemic infections. The pathogenicity and virulence factors of T. asahii remain largely unknown. Because of the association between invasive infections and the use of catheters and related devices, the ability of the microorganism to adhere and form biofilms may play an important role in the pathogenicity during a trichosporonosis.
AimsThe aim of this study is to identify an association between biofilm formation by T. asahii isolates and their genotype and/or clinical source.
MethodsThe biofilm production of 49 T. asahii strains isolated from Mexican patients was measured using the crystal violet stain method, and a comparison made with different adhesion phase incubation times. Antifungal susceptibility testing was performed using a modified CLSI protocol coupled with the quantification of the viable cells with the XTT reduction method.
ResultsAll the T. asahii isolates assayed were able to produce biofilm in vitro, with an intraspecific variability being observed. Overall, increased biofilm production was found when extending the adhesion phase incubation time from 2 to 4h. No association could be established between the biofilm-producing phenotype and either the genotype or clinical source. Higher antifungal resistance to amphotericin B and fluconazole was linked to increased biofilm production by T. asahii.
ConclusionsAll clinical isolates tested were able to produce biofilm. No association could be established between biofilm formation and genotype or clinical source.
Trichosporon asahii es un hongo levaduriforme de gran importancia en los últimos años por causar infecciones sistémicas oportunistas. Existe escasa información sobre su patogenicidad y factores de virulencia. Debido a la asociación entre las infecciones invasivas y el uso de catéteres y otros dispositivos médicos, la capacidad de este microorganismo para adherirse y formar biopelículas puede incrementar su patogenicidad durante la tricosporonosis.
ObjetivosEn el presente trabajo se estudia la asociación entre la formación de biopelícula por diferentes aislamientos de T. asahii y su genotipo y/o lugar de aislamiento.
MétodosSe cuantificó la producción de biopelícula en 49 aislamientos de T. asahii aislados de pacientes mexicanos mediante el método de tinción con cristal violeta, y se compararon diferentes tiempos de incubación para la fase de adhesión. Además se realizaron pruebas de sensibilidad a los antifúngicos mediante el protocolo del CLSI, con modificaciones, acoplado a la cuantificación de células viables con el método de reducción del XTT.
ResultadosTodos los aislamientos de T. asahii analizados produjeron biopelícula; se observó variabilidad intraespecifica en dicha producción. En general se observó un incremento en la producción de biopelícula al aumentar el tiempo de incubación de la fase de adhesión de 2 a 4h. No se logró establecer una asociación entre la producción de biopelícula y el genotipo u origen clínico de los diferentes aislamientos de T. asahii. El incremento en la resistencia a la anfotericina B y el fluconazol resultó proporcional al incremento en la producción de biopelícula.
ConclusionesTodos los aislamientos clínicos evaluados fueron capaces de producir biopelícula. No se logró establecer una asociación entre la formación de biopelícula y el genotipo u origen clínico del aislamiento.
Trichosporon genus comprises yeast-like organisms commonly colonizing the gastrointestinal and respiratory tract, as well as human skin. Although the genus is generally associated with the superficial infection white piedra, it has recently acquired importance as the cause of opportunistic systemic infections since the first reported case in 1970 of brain invasive trichosporonosis.36
Trichosporon asahii is the most prevalent species in cases of trichosporonosis.22,30 This pathogen is considered an important cause of fungal disseminated infections by non-Candida species, particularly in patients with malignant hematological diseases.26,35 Trichosporonosis caused by T. asahii has a mortality rate of 50–80% in immunocompromised patients.30 Neutropenia is the main risk factor. A correlation has also been established between trichosporonosis and patients undergoing invasive clinical procedures, such as the placement of catheters and prosthetic materials.14,20,26,30
The pathogenicity and virulence factors of T. asahii remain unknown. There are reports on the production of esterases,1,9 hemolysins,32 phospholipases, proteases and DNAses4; however, the role of these lytic compounds during the course of an infection by T. asahii is not clear.
Some microorganisms have the ability to produce an extracellular matrix formed by polysaccharides, proteins and extracellular DNA that allows the conglomeration of cells to adhere to organic or inorganic surfaces.16 These structures, called biofilms, facilitate the colonization, growth and proliferation of the microorganism, and provide it with antibiotic and environmental stress resistance. It is well known that 95% of the microorganism load can be found within these structures.11 Because of the association between invasive infections and the use of catheters and related devices, the ability of the microorganism to adhere and form biofilms may have an important role in the pathogenicity during a trichosporonosis. Di Bonaventura et al. evaluated the kinetics of in vitro biofilm production for the type strain T. asahii ATCC 201110. They observed that T. asahii cells could adhere to polystyrene surfaces after only 30min of incubation. At 72h, cells presented yeast-like and filamentous structures embedded in an extracellular matrix, forming a mature biofilm 25–40μm thick. The formation of biofilm was associated with an increase in resistance to antifungals, mainly voriconazole, in comparison to planktonic cells.12 Other groups have reported variable biofilm production by different T. asahii strains 2,9,17,32as well as by other non-T. asahii Trichosporon clinical isolates.10,17,18
The existing reports on in vitro production assays suggest differences in the capability of biofilm production by various T. asahii strains. To date, there are no studies on the biofilm production of T. asahii strains isolated in Mexico. Here we quantified the biofilm production and antifungal resistance of 49 clinical isolates of T. asahii from Mexican patients, and looked for a possible association between biofilm formation and isolate genotype or clinical source. This is the largest biofilm production study of T. asahii reported so far.
Material and methodsStrainsWe studied 49 isolates of T. asahii obtained from different patients between 2003 and 2015 at the Microbiology Reference Center of the School of Medicine, Universidad Autónoma de Nuevo León, Monterrey, Mexico. The strains had been previously identified with the API 20C AUX system (bioMérieux, France) and the urease test on Christensen's agar (positive result), and were later genotyped by intergenic spacer 1 (IGS1) sequence analysis as previously reported.31 All isolates were stored in sterile water at room temperature.
Biofilm production and quantificationBiofilm production was performed with modifications to the method described by Di Bonaventura.12 All T. asahii strains were subcultured in Sabouraud glucose agar (SGA) at 30°C for 24h, transferred to yeast extract broth (YEPD; 1% yeast extract, 2% peptone, 2% glucose) and incubated overnight at 30°C and 150rpm. Cells were collected by centrifugation, washed twice with sterile phosphate buffer (PBS) and re-suspended in RPMI 1640 with l-glutamine buffered to pH 7.0 with 0.165M morpholinepropanesulfonic acid (RPMI 1640-MOPS; Hardy Diagnostics, Santa Maria, CA). Inoculum was spectrophotometrically adjusted to 1×107cfu/ml at 590nm, and 100μl were transferred to pre-sterilized 96-well polystyrene flat bottom plates (Corning Incorporated, Corning, NY). Plates were incubated for 1, 2, 4, 6, and 8h at 37°C, after which the medium was poured off and wells were washed 2 times with 150μl of PBS to remove non-adhered cells. One hundred microlitres of RPMI 1640-MOPS were added to each well and plates were incubated at 37°C for 72h, substituting the medium every 24h. At the end of the 72h period, plates were washed twice with PBS.
Quantification of biofilm was carried out as described by Melo et al.24 Following the PBS wash, wells were left to dry for 1h at 37°C. Wells were then dyed with 110μl of an aqueous solution of 0.4% crystal violet (CV), and left at room temperature for 45min. The CV solution was poured off, and the wells were washed thrice with 200μl of sterile distilled water. To dissolve the dye, 200μl of 95% ethanol were added to each well, being the plates at room temperature for 45min. One hundred microlitres of this solution were transferred to a clean plate and quantified by spectrophotometry at 595nm. Experiments were performed twice. Candida albicans ATCC 90028 was used as biofilm producer control.
Biofilm production was categorized following an arbitrarily established phenotype classification based on the quartiles of absorbance readings obtained at the 4 h-adhesion phase. Thus, isolates were sorted as weak producers (A595≤0.531), intermediate producers (A595=0.532–0.763), strong producers (A595=0.764–0.999), and very strong producers (A595≥1.0).
Antifungal susceptibility testAmphotericin B (AMB; Apothecon, Princeton, NJ) and fluconazole (FLC; Pfizer, Inc., Amboise, France) were prepared in stock solutions and diluted using RPMI-1640 MOPS.
Susceptibility testing for planktonic cells was performed according to the M27-A3 broth microdilution method published by the Clinical and Laboratory Standards Institute (CLSI).7 96-well round-bottom plates were inoculated for a final concentration of 0.5×103 to 2.5×103cfu/ml in each well. Plates were incubated at 37°C for 48h. Candida krusei ATCC 6258 and Candida parapsilosis 22019 were used as quality control strains. The minimum inhibitory concentrations (MIC) were determined according to the CLSI guidelines. Readings were done after 48h for both antifungals. The MIC for AMB corresponded to the visually lowest concentration with 100% inhibition of growth. The MIC for FLC was the concentration in which the growth inhibition observed was ≥50% when compared to the growth in the positive control well (without antifungal).
Antifungal susceptibility testing for biofilms was carried out using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) colorimetric method. Following a 4h adhesion phase, biofilms were washed twice with PBS and challenged with the antifungal agents for 48h at 37°C. XTT (Sigma, St. Louis, MO) was prepared in RPMI 1640-MOPS at a concentration of 0.5mg/ml with 0.4mM menadione, and 100μl of the XTT-menadione solution were added to each well. Plates were then incubated in the dark at 37°C for 2h. Finally, viable cells were quantified spectrophotometrically at 490nm. Antifungal-free cells were included as controls. The MIC of each isolate to AMB was determined by a ≥90% decrease of metabolic activity in comparison with the control without the antifungal. The MIC for FLC was determined by a 50% decrease of the metabolic activity in relation to the positive control. Experiments were performed twice.
Statistical analysisNot Normal distribution of the data was confirmed with the Shapiro–Wilk Normality test (p=0.01). Associations between biofilm production, genotypes and clinical sources were analyzed with a χ2 test. Comparison of biofilm production among different experimental groups was performed with the Kruskal–Wallis test. Shifts in biofilm producing phenotype were analyzed by comparing proportions using the χ2 test. All statistical analyses were performed in GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA). A p-value<0.05 was considered significant.
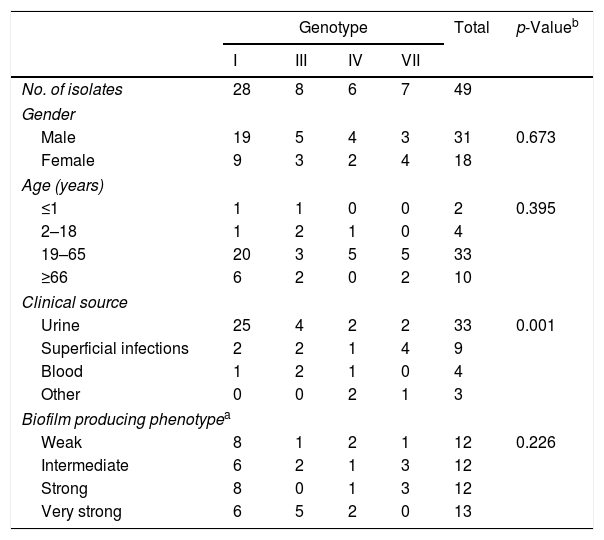
ResultsThe demographic information and source of the 49 isolates are shown in Table 1. Genotype distribution of T. asahii isolates was as follows: 57.14% genotype I, 16.33% genotype III, 12.24% genotype IV, and 14.29% genotype VII. Isolates were obtained from both male (63.27%) and female patients (36.73%). Most of the patients were adults (67.35%), with a distribution of 60.61% for genotype I, 15.15% for each genotype IV and VII, and 9.09% for genotype III. The second age group with the most cases was the senior group (20.41%), followed by children and teenagers (8.16%), and infants and newborns (4.08%). T. asahii isolated from urine represented most of the analyzed strains (67.35%); 25 of 49 belonged to genotype I, followed by 4 isolates of genotype III, and 2 isolates for each genotype IV and VII. Other isolates were obtained from superficial infections (18.37%), blood (8.16%), and other sources (6.12%). We found a significant association between genotype I and isolates obtained from urine (p<0.001, r=0.6). No association between genotypes and other clinical sources could be established (p ≥ 0.3). This finding may be due to the small sample size.
Demographic information and biofilm producing phenotype of the 49 T. asahii clinical isolates included in this study.
| Genotype | Total | p-Valueb | ||||
|---|---|---|---|---|---|---|
| I | III | IV | VII | |||
| No. of isolates | 28 | 8 | 6 | 7 | 49 | |
| Gender | ||||||
| Male | 19 | 5 | 4 | 3 | 31 | 0.673 |
| Female | 9 | 3 | 2 | 4 | 18 | |
| Age (years) | ||||||
| ≤1 | 1 | 1 | 0 | 0 | 2 | 0.395 |
| 2–18 | 1 | 2 | 1 | 0 | 4 | |
| 19–65 | 20 | 3 | 5 | 5 | 33 | |
| ≥66 | 6 | 2 | 0 | 2 | 10 | |
| Clinical source | ||||||
| Urine | 25 | 4 | 2 | 2 | 33 | 0.001 |
| Superficial infections | 2 | 2 | 1 | 4 | 9 | |
| Blood | 1 | 2 | 1 | 0 | 4 | |
| Other | 0 | 0 | 2 | 1 | 3 | |
| Biofilm producing phenotypea | ||||||
| Weak | 8 | 1 | 2 | 1 | 12 | 0.226 |
| Intermediate | 6 | 2 | 1 | 3 | 12 | |
| Strong | 8 | 0 | 1 | 3 | 12 | |
| Very strong | 6 | 5 | 2 | 0 | 13 | |
Biofilms were produced for each of the 49 strains of T. asahii, assaying adhesion phases of 2, 4, 6, and 8h. The median of absorbance obtained for those phase times were A595=0.295, 0.764, 0.764, and 0.803, respectively. We observed a significant increase of biofilm production with adhesion phases of 4h or more (p<0.0001). Furthermore, broader absorbance ranges for adhesion phases of 4, 6, and 8h suggest a high intraspecific variability in biofilm production by T. asahii (data not shown).
Table 1 shows the phenotypes of the 49 isolates concerning the biofilm production. Overall, isolates were evenly distributed among the different phenotypes: 12 isolates each for weak, intermediate, and strong producers, and 13 isolates for very strong biofilm producers. We did not observe any association between the biofilm production phenotype and either the genotype (p=0.226, r=0.44) or the clinical source (p=0.23, r=0.439).
Any increase or decrease in biofilm production was carefully studied when comparing the 4 and 8h adhesion times for each isolate. In the same way, any difference leading to change in the phenotype classification was also registered. Furthermore, we analyzed if any classification shift was associated to a particular genotype or clinical source. Out of the 49 isolates, 34.7% increased their biofilm production, 10.2% of the isolates diminished their production, and 55.1% remained the same (data not shown). We found a significant association between phenotype change and genotypes I (p=0.036) and VII (p=0.022), and between phenotype change and isolates from superficial sites (p=0.01); overall, increased adhesion phase incubation time had a significant effect in the biofilm production of T. asahii strains (p=0.003).
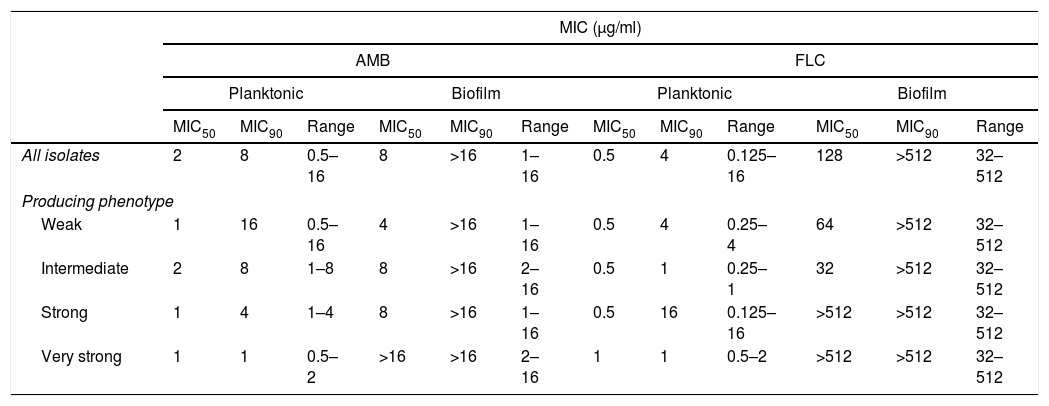
The MIC readings of in vitro susceptibility tests for planktonic cells and biofilm were performed after 48h of incubation with the antifungal agents. The MIC50, MIC90 and ranges of the 49 isolates against both AMB and FLC are summarized in Table 2. When testing planktonic cells, 52.94% of the strains had MICs of ≥2μg/ml for AMB, but only 1 strain showed resistance to FLC. When challenging biofilms, all strains showed an increased resistance to the antifungals tested, since generally both MIC50 and MIC90 were greater than the concentration needed for inhibiting planktonic cells. Overall, antifungal resistance increased ≥2log2 times for AMB, and ≥8log2times for FLC. Furthermore, a trend of increased antifungal resistance with increased biofilm production was observed, particularly in the MIC50 readings. For AMB, weak and intermediate biofilm producers increased resistance ≥1 log2 times, while strong and very strong producers increased resistance ≥3log2 times. Diminished FLC efficiency is even clearer, with a resistance increase of 6–7log2 for weak and intermediate producers, and ≥10log2 for strong and very strong biofilm producers. We were not able to correlate antifungal susceptibility profiles with genotype or clinical source for any of the antifungals.
In vitro antifungal susceptibility of planktonic cells and biofilm of T. asahii.
| MIC (μg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB | FLC | |||||||||||
| Planktonic | Biofilm | Planktonic | Biofilm | |||||||||
| MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | |
| All isolates | 2 | 8 | 0.5–16 | 8 | >16 | 1–16 | 0.5 | 4 | 0.125–16 | 128 | >512 | 32–512 |
| Producing phenotype | ||||||||||||
| Weak | 1 | 16 | 0.5–16 | 4 | >16 | 1–16 | 0.5 | 4 | 0.25–4 | 64 | >512 | 32–512 |
| Intermediate | 2 | 8 | 1–8 | 8 | >16 | 2–16 | 0.5 | 1 | 0.25–1 | 32 | >512 | 32–512 |
| Strong | 1 | 4 | 1–4 | 8 | >16 | 1–16 | 0.5 | 16 | 0.125–16 | >512 | >512 | 32–512 |
| Very strong | 1 | 1 | 0.5–2 | >16 | >16 | 2–16 | 1 | 1 | 0.5–2 | >512 | >512 | 32–512 |
MIC: minimum inhibitory concentration; MIC50: MIC needed to inhibit the growth of 50% of the isolates; MIC90: MIC needed to inhibit the growth of 90% of the isolates.
Biofilm production has been associated to high mortality in systemic infections by Candida species.33,34 Although the biofilm producing capabilities of T. asahii are well documented, in vitro differences suggest a variability in this production that may be related to the isolation site, adhesion phase time, or intrinsic characteristics of the particular strains, such as genotype. We show that all the isolates tested were able to produce biofilms that were not susceptible to amphotericin B or fluconazole, and that resistance was directly proportional to increased biofilm production by T. asahii.
As well as in other reports,3,8,17,30 most of the isolates included in this study were obtained from urine samples (33 out of 49). Regarding their identification, IGS1 sequence analysis demonstrated the presence of 4 different genotypes in this subset of isolates from Mexican patients and most of these belonged to genotype I (28 out of 49), as has been reported in Brazil,3,6 Spain,28 Turkey,19 Taiwan,37 and Thailand.23
Biofilm production by T. asahii has been assayed using different support materials,27 growth phase incubation time,17 temperatures and pH values.13 One group compared different adhesion phases of 60, 90, and 120min, and reported that incubation times of 90min offered the most reproducible results.17 In our study, we did not observe relevant absorbance readings for CV assays when testing 1 and 2h adhesion phase times, as all isolates were A595≤0.472, or weak producers. We decided to broaden our analysis to include longer incubation times in that phase. To our knowledge, this is the first work that reports the effect of a prolonged adhesion phase on the formation of biofilm by this fungus. This acquires relevance as we did observe a significantly higher biofilm production when increasing adhesion phase incubation time from 2 to 4h. These results may be reflected in increased exposure times of T. asahii to adequate surfaces in the clinical setting, and translate into elevated virulence or persistence of infection.
Although all strains tested produced biofilm, only 26.53% were able to produce robust biofilms comparable to those produced by the C. albicans strain included in this study as a control. This contrasts with the works by Dağ and Cerikçioğlu and Araújo de Almeida et al., whose strains were classified as non-producers to moderate producers of biofilm.2,9 Our results also differ from those reported by Iturrieta-González et al., in which 24 of 36 T. asahii isolates had CV absorbance readings of ≥1.0. They, however, measured biofilm formation at 48h of incubation post-adhesion phase, whereas we used 72h biofilms as described in our methods. They discuss that when comparing the results at both times, 72h biofilms showed lower absorbance values than the 48h biofilms, which they interpreted as biofilm detachment from the wells.17 Di Bonaventura et al. and Sun et al. reported biofilm formation by the strains included in their analyses; however, both groups used the XTT reduction method to quantify the biofilms.12,32 The XTT method generally yields lower absorbance readings than those obtained with the CV method, impeding an adequate comparison of the results. Although the use of CV staining as a reliable method for biofilm quantification in bacteria and yeasts has been previously demonstrated,5,24,25 biofilm analysis with regard to T. asahii has seen the application of different methods or modifications in various protocols. A standardized biofilm quantification method is needed to appropriately compare and contrast results by different research groups.
It remains unclear whether biofilm formation is associated to either genotype or site of isolation. Sun et al. analyzed T. asahii genotypes I, III, and IV, and found a significantly higher biofilm production by genotype III and IV strains when compared to the biofilms produced by isolates of genotype I.32 This contrasts with the results by Iturrieta-González et al., who found no difference in biofilm production among the 4 genotypes analyzed (I, III, IV and V). The same group reported an association between a higher biofilm production and those Trichosporon strains isolated from superficial infections; however, this finding refers to all Trichosporon species analyzed, and does not specify this behavior for the T. asahii strains.17 We did not observe a correlation between biofilm formation and either the genotype or clinical source. However, the isolates from superficial infections increased their biofilm production when increasing the adhesion phase incubation time.
Susceptibility testing showed that planktonic cells had a variable response to AMB, similar to what has been previously described.6,19,21,23 All but one strain were susceptible to FLC. Although triazole antifungal agents efficiency has made them the treatment of choice against T. asahii infections, in vitro MICs of ≥8μg/ml have been reported for this species.15,19,23,29 When testing against biofilms, we found that all strains increased their resistance against both antifungals. Overall, the efficiency decrease for AMB was at least 4-fold, and 256-fold for FLC. We also observed a trend of increased antifungal resistance associated to stronger biofilm producers. Similar results were obtained by Sun et al., in which the strongest biofilm producers had higher MICs for AMB and FLC (>1024μg/ml) compared to the MICs of the weakest producers (128–512μg/ml).32 There is, however, a contradictory report in which no correlation between antifungal resistance and biofilm quantification could be established.17
In the present study we confirmed the in vitro formation of biofilm by clinical isolates of T. asahii, and demonstrated intraspecific variability in their production. Overall, we showed an increased biofilm production when extending the adhesion phase incubation time from 2 to 4h. Besides, we report increased antifungal resistance to amphotericin B and fluconazole directly proportional to increased biofilm production. Although all clinical isolates tested were able to produce biofilm, the involvement of this complex structure in the pathogenicity of the microorganism remains unknown. Further studies are needed to understand the precise role of biofilm formation as a virulence factor in T. asahii infections.
FundingThis work was supported by the Programa de Apoyo a la Investigación Científica y Tecnológica (PAICyT) [CS-656-11].
Conflict of interestThe authors have no conflict of interest to declare. The authors alone are responsible for the content and the writing of the paper.
We thank Dr. Sergio Lozano of the “Dr. José E. González” University Hospital (Monterrey, Mexico) for his review of the manuscript prior to submission.