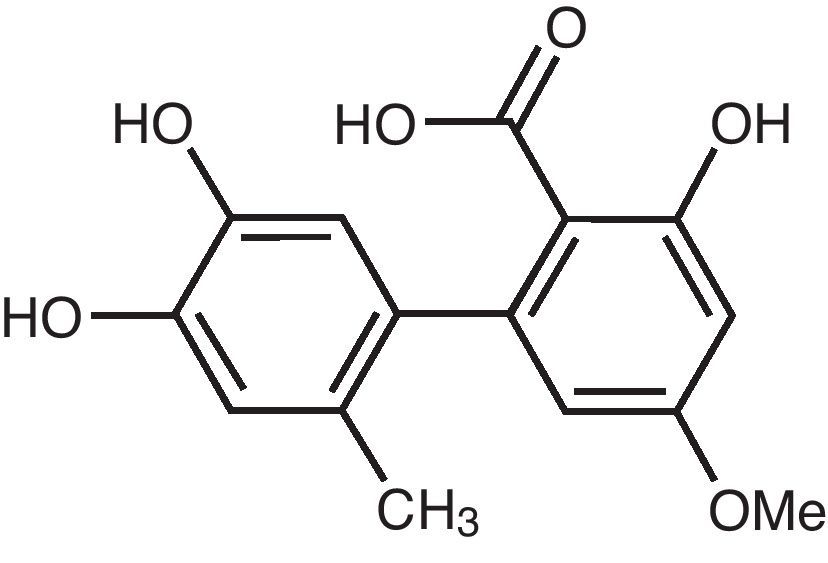
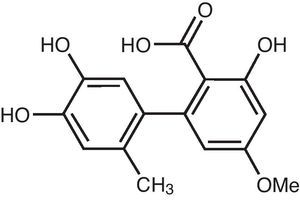
Altenusin is a biphenyl derivative isolated from different species of fungi, which presents several biological activities.
AimsWe report the antifungal activity of the altenusin isolated from the endophytic fungus Alternaria sp., against clinical isolates of Paracoccidioides brasiliensis, and its action on cell walls of P. brasiliensis and the nonpathogenic yeast Schizosaccharomyces pombe.
MethodsIn vitro antifungal activity of altenusin was evaluated using the broth microdilution method against 11 strains of P. brasiliensis and one strain of S. pombe. The effects of the altenusin on the cell wall were estimated using the sorbitol protection assay.
ResultsThe altenusin presented strong activity against P. brasiliensis with MIC values ranging between 1.9 and 31.2μg/ml, and 62.5μg/ml for S. pombe. Our results demonstrated that the MIC values for altenusin were increased for P. brasiliensis Pb18 and for S. pombe when the medium was supplemented with sorbitol. Additionally, S. pombe cells treated with altenusin were more rounded in shape than untreated cells.
ConclusionsAltenusin showed activity against clinical strains of P. brasiliensis at the concentration tested, and this compound probably affects fungal cell walls. These findings suggest that altenusin could act through the inhibition of cell wall synthesis or assembly in P. brasiliensis and S. pombe, and could be considered as a lead compound for the design of new antifungals.
La altenusina es un derivado bifenilo aislado de diferentes especies de hongos, que presenta una diversidad de actividades biológicas.
ObjetivosDescribimos la actividad antifúngica de la altenusina aislada del hongo endofítico Alternaria sp. frente a aislamientos clínicos de Paracoccidioides brasiliensis, y su acción sobre las paredes celulares de P. brasiliensis y la levadura no patógena Schizosaccharomyces pombe.
MétodosSe valoró la actividad antifúngica de la altenusina in vitro usando un método de microdilución en caldo frente a 11 cepas de P. brasiliensis y una cepa de S. pombe. Los efectos de la altenusina sobre la pared celular se estimaron utilizando un análisis de protección con sorbitol.
ResultadosLa altenusina presentó una potente actividad frente a P. brasiliensis con valores de concentración inhibitoria mínima (CIM) que variaron entre 1,9 y 31,2μg/ml, y de 62,5μg/ml para S. pombe. Los resultados del presente estudio demostraron que los valores CIM de la altenusina aumentaron para Pb18 de P. brasiliensis y para S. pombe cuando el medio se suplementó con sorbitol. Además, las células de S. pombe tratadas con altenusina adoptaron una forma más redondeada que las no tratadas.
ConclusionesCon la concentración examinada, la altenusina demostró actividad frente a las cepas clínicas de P. brasiliensis, y es probable que este preparado afecte a las paredes de las células micóticas. Estos hallazgos sugieren que la altenusina podría actuar a través de la inhibición de la síntesis o ensamblado de la pared celular en P. brasiliensis y S. pombe y podría considerarse la molécula inicial para el diseño de nuevos antimicóticos.
Altenusin is a biphenyl derivative isolated from different species of fungi, which presents antioxidant properties and the ability to inhibit several enzymes, such as myosin light chain kinase,13 sphingomyelinase,25 acetylcholinesterase,10 and HIV-1 integrase.19 Altenusin also exhibits broad antimicrobial activity against several drug-resistant pathogens with minimum inhibitory concentration (MIC) values of 31.2 to 125μg/ml.9 Cota et al.2 isolated altenusin from the endophytic fungus Alternaria sp. UFMGCB 55 associated with the plant Trixis vauthieri DC (Asteraceae), and this compound presented the ability to inhibit the enzyme trypanothione reductase (TryR) from Trypanosoma cruzi, an enzyme involved in the protection of Trypanosoma and Leishmania species against oxidative stress.5
The increase in frequency of immunocompromised individuals among the world's human population has resulted in an ever-growing number of serious fungal infections, which will require new antimicrobial therapy.16 The pathogenic fungus Paracoccidioides brasiliensis is the etiologic agent of paracoccidioidomycosis (PCM), a human systemic mycosis for which the portal of entry of the fungus is the respiratory tract via the inhalation of airborne propagules. Although geographically confined, paracoccidioidomycosis constitutes one of the most prevalent deep mycoses in Central and Southern America.7 Antifungals used in cases of PCM are sulfonamides, amphotericin B, or azoles, mainly itraconazole. Extended periods of treatment are necessary and relapses of the disease commonly occur.23 In the present work we report the antifungal activity of altenusin isolated from the endophytic fungus Alternaria sp. UFMGCB 55, against clinical strains of P. brasiliensis, and we also show the action of altenusin on cell walls of P. brasiliensis and the nonpathogenic yeast Schizosaccharomyces pombe.
Materials and methodsIsolation of altenusinThe biphenyl derivative altenusin (Fig. 1) was isolated from ethyl acetate extract of the endophytic fungus Alternaria sp. UFMGCB 55, which was recovered from leaves of the bioactive plant Trixis vauthieri DC (Asteraceae) as described by Cota et al.2 This compound was stored at −20°C at 20mg/ml.
Antifungal activity assaysMaintenance of P. brasiliensis and S. pombe strainsEleven clinical P. brasiliensis strains, of two established cryptic phylogenetic species (S1 and PS2) and one possibly new cryptic species, were used in the biological assays (Table 1). Isolates Pb18, PbB339, Pb14, Pb8 and Pb11 belong to the cryptic species S1, and isolates Pb03, Pb2 and Pb4 belong to the PS2 cryptic phylogenetic species.11 Isolates Pb01, ED01 and Pb1578 are considered representative of a new phylogenetic species “Pb-01-like”.1 The strains of P. brasiliensis were maintained at Departamento de Microbiologia of Universidade Federal de Minas Gerais, Brazil, by weekly transfer in solid YPD medium (yeast extract, peptone and dextrose) at 37°C. The wild type of S. pombe (PN556) was maintained on Sabouraud dextrose agar (Oxoid, Basingstoke, UK).
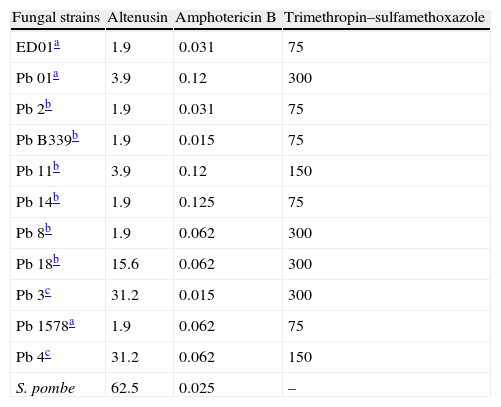
Antifungal activityd of altenusin against 11 strains of Paracoccidioides brasiliensis and one of Schizosaccharomyces pombe.
| Fungal strains | Altenusin | Amphotericin B | Trimethropin–sulfamethoxazole |
| ED01a | 1.9 | 0.031 | 75 |
| Pb 01a | 3.9 | 0.12 | 300 |
| Pb 2b | 1.9 | 0.031 | 75 |
| Pb B339b | 1.9 | 0.015 | 75 |
| Pb 11b | 3.9 | 0.12 | 150 |
| Pb 14b | 1.9 | 0.125 | 75 |
| Pb 8b | 1.9 | 0.062 | 300 |
| Pb 18b | 15.6 | 0.062 | 300 |
| Pb 3c | 31.2 | 0.015 | 300 |
| Pb 1578a | 1.9 | 0.062 | 75 |
| Pb 4c | 31.2 | 0.062 | 150 |
| S. pombe | 62.5 | 0.025 | – |
Paracoccidiodes brasiliensis isolates representative of the phylogenetic species S1 (Pb-18, Pb-B339, Pb-14, Pb-8 and Pb-11) (reported by Matute et al.11 as B17, B18, B22, B25, B21, Table S1).
Paracoccidiodes brasiliensis isolates representative of the phylogenetic species PS2 (Pb-2, Pb-3 and Pb-4) (reported by Matute et al.11 as V2, B26, B23 Table S1).
The bioassays with all clinical strains of P. brasiliensis and S. pombe (PN556) were performed following the CLSI M27-A2 guidelines12,14 with the modifications suggested by Johann et al.8 Amphotericin B (AMB) (Sigma, St Louis, USA) and trimethoprim/sulfamethoxazole (SMT/TMP) were included as positive antifungal controls, being the stock solutions prepared in DMSO and water, respectively. Twofold serial dilutions were prepared exactly as outlined in CLSI document M 27-A2.14
Minimal fungicidal concentrations determinationThe microtiter plate used to determine MIC values was also used to determine MFC values. The in vitro minimal fingicidal concentrations (MFC) of each compound tested was determined by streaking 10μl from each well that showed complete inhibition (100% inhibition or a clear well), from the last positive well (growth similar to that of the growth control well), and from the growth control well onto YPD plates. The MFC was determined as the lowest drug concentration at which counts lower than three colonies were recovered after cultivation on YPD agar for 10 days at 37°C.4,18
Sorbitol protection assaysMIC values were determined using P. brasiliensis strain Pb18 and S. pombe by the standard broth microdilution procedure as described above. Duplicate plates were prepared: one of them contained altenusin plus 0.8M sorbitol as an osmotic support and the other one contained only altenusin. MICs were determined after 14 days for P. brasiliensis and 48h for S. pombe.3
Cell morphology analysisThe model organism for cell morphology analysis was the nonpathogenic yeast S. pombe. S. pombe cell morphology was analyzed by fluorescence microscopy after cell staining with Calcofluor white. The S. pombe cells were grown in YES-yeast extract plus supplements: adenine, leucine, histidine, or uracil (100mg/ml; Sigma) liquid medium to mid log-phase to an A600 of 0.6.21 Images were captured with a Leica DM 4000B fluorescence microscope coupled to a cooled Leica DC 300F camera and IM50 software. For analyses of vacuoles, the cells of S. pombe were stained with CDCFDA (carboxydichlorofluorescein diacetate; Molecular Probes) and observed under fluorescence microscopy.
ResultsPreliminary results demonstrated that altenusin was not active against Candida albicans (>250.0μg/ml) (data not showed), but P. brasiliensis strains were susceptible to altenusin, with MIC values between 1.9 and 31.2μg/ml. P. brasiliensis strains Pb ED01, 2, B339, 11, 14, 8, and 1578 were the most susceptible with MIC values of 1.9μg/ml whereas the strains Pb03 and Pb04 were less susceptible to altenusin with an MIC value of 31.2μg/ml. MIC values found for amphotericin B were between 0.031 and 0.12μg/ml for the P. brasiliensis strains tested, with better activity against the isolates Pb ED01 and Pb2. The drug trimethropin–sulfamethoxazole presented high MIC values, ranging between 75.0 and 300.0μg/ml. For all strains of P. brasiliensis the MFC values obtained for altenusin were equal to the MIC values. In addition, altenusin also presented activity against S. pombe with a MIC value of 62.5μg/ml.
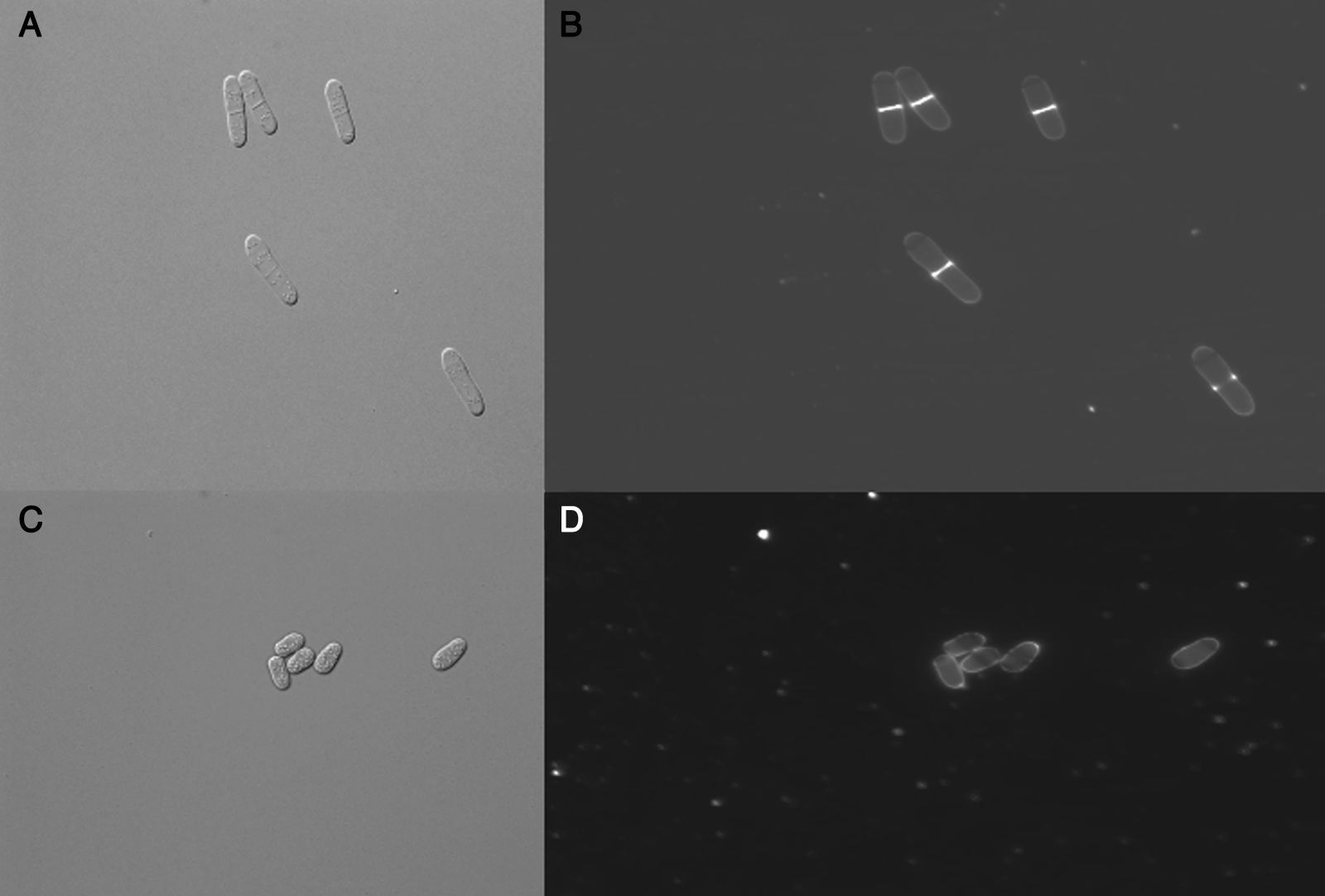
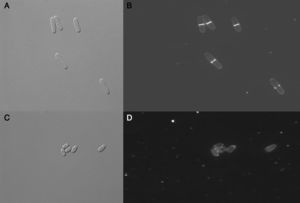
Altenusin modified MIC values to P. brasiliensis and S. pombe after addition of sorbitol to the culture medium. The MIC value against P. brasiliensis (strain Pb18) in culture medium treated with altenusin and supplemented with sorbitol was 62.5μg/ml whereas the MIC was 15.6μg/ml in the same medium without the addition of sorbitol. MIC values obtained against S. pombe were 62.5μg/ml and 125.0μg/ml in absence and presence of sorbitol in the medium, respectively. These results suggested that the antifungal activity of altenusin could affect fungal cell walls. When S. pombe cells stained with calcofluor white were observed in fluorescence microscopy, cells treated with altenusin were more rounded than untreated cells (Fig. 2). Tests to observe whether vacuoles of the cells treated with altenusin were affected by the compound were negative. Differences between control S. pombe cells and cells treated with altenusin were not observed (Fig. 3).
Schizosaccharomyces pombe morphology after treatment with altenusin. A: Differential interphase contrast (DIC) micrographs of S. pombe cells in the absence of altenusin; B: Fluorescence micrographs of S. pombe stained with calcofluor white in the absence of altenusin; C: S. pombe DIC in the presence of altenusin (31.2μg/ml); D: S. pombe stained with calcofluor white in the presence of altenusin (31.2μg/ml).
We assayed the altenusin against fungal isolates of P. brasiliensis of two distinct cryptic phylogenetic species: S1 (Pb18, PbB339, Pb14, Pb8 and Pb11) and PS2 (Pb03, Pb2 and Pb4).11 The cryptic species S1 was more susceptible than the cryptic species S2, with MIC values of 1.9–15.5μg/ml for the first and MIC values of 1.9–31.2μg/ml for the second. Altenusin was also tested against P. brasiliensis isolated Pb01, ED01 and Pb1578, which are considered representative of a new phylogenetic species “Pb-01-like”.1 Teixeira et al.22 recommended the formal description of the “Pb-01-like” (Pb 01, 1578 and ED01) cluster as a new species, Paracoccidioides lutzii.22 The isolates of the P. lutzii group treated with altenusin presented the highest susceptibility, with MIC values ranging between 1.9 and 3.9μg/ml. On the basis of the MIC values for altenusin, isolates of P. brasiliensis were around 50 times more susceptible to this compound than to trimethropin–sulfamethoxazole in vitro assays. Sulfonamides are the first class of drugs available for treating patients with PCM, but long periods of treatment may be required (more than 2 years).24 In addition, the identical MFC and MIC values presented by altenusin may be important as the altenusin could kill the pathogen in situations when infection occurs in sites not easily accessed by host defenses.
According to Frost et al.,6 a distinctive feature of the specific inhibitors of the fungal cell wall is that the antifungal effect is reversed in media containing an osmotic stabilizer such as sorbitol. Cell wall disruptive and osmotic destabilizing agents lead to cell wall rearrangements that enable fungal cells to survive.14 Our results suggest that altenusin could act through the inhibition of cell wall synthesis or assembly in P. brasiliensis and S. pombe cells. Cell morphology analysis was performed with S. pombe, a yeast that is an excellent model organism for the study of cell walls. The major S. pombe glucose polymers of the cell wall are similar to those of P. brasiliensis, with presence of β-d-(1,3) glucan and α-d-glucan.15,17,20 In the present work, the S. pombe cells treated with altenusin in sub-inhibitory concentration were smaller in size and more rounded than control (altenusin absence) cells. It is known that β-d-(1,3) glucan synthase inhibitors produce hallmark changes in the morphologies of yeasts and filamentous fungi.3,17 According to Frost et al.,6 the target in cell walls is unknown when C. albicans presents smaller rounded cells. Altenusin showed interesting in vitro activity against clinical strains of both phylogenetically cryptic species of P. brasiliensis, with low MIC values when compared to trimethropin–sulfamethoxazole. This compound could be considered as a lead compound for the design of new antifungals. Our results suggested that the antifungal activity of altenusin affects fungal cell walls, but new assays will be performed to establish its specific action mechanism.
Conflict of interest statementThe authors declare that they have no conflict of interest.
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo Pesquisa Estado de Minas Gerais (FAPEMIG) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. This work was also supported by FIOCRUZ through a PDTIS grant (RPT-10).