The domestic cat is the most susceptible host to Sporothrix infection, developing severe clinical forms. Few effective antifungal agents are available for treating feline sporotrichosis, and cases of treatment failure are common. Treatment success depends on cat health status, therapy-related factors, as well as social/economic issues, but it is mainly contingent upon the host–fungus interaction. The owner's adherence is critical and should be reinforced throughout the treatment to increase the chances of a successful outcome. The antifungal agents described for feline sporotrichosis are most often used in monotherapy regimens. Due to cases in which the treatment with itraconazole failed, the use of antifungal agents in combination should be considered to achieve synergy. The combination of itraconazole and potassium iodide represents an important option for the treatment of naïve cats presenting multiple cutaneous lesions, nasal mucosal lesions and/or respiratory signs, as well as for refractory cases. However, the therapeutic options for unsuccessfully treated cases are scarce. Therefore new options are needed, even more taking into account that there are many in vitro potential molecules not available for use in cats yet. More studies are necessary to correlate in vitro antifungal susceptibility tests results and the outcome of cats treated due to sporotrichosis. This review will briefly discuss both the antifungal drugs and treatment protocols used in cats with sporotrichosis, as well as the determinants of treatment failure.
El gato doméstico es el huésped más susceptible a la infección por Sporothrix, llegando a desarrollar formas clínicas graves. Hay pocos agentes antimicóticos efectivos disponibles para tratar la esporotricosis felina, y los casos de fracaso terapéutico son habituales. El éxito del tratamiento depende del estado de salud del gato, los factores relacionados con la terapia y los problemas sociales/económicos, pero se asocia principalmente con la interacción huésped-hongo. El cumplimiento del tratamiento por parte del propietario es fundamental y debe reforzarse durante todo el proceso para aumentar las posibilidades de éxito. Los agentes antimicóticos descritos para la esporotricosis felina se usan con mayor frecuencia en monoterapia. Debido a los casos de fallo terapéutico en el tratamiento con itraconazol se debe considerar el uso combinado de agentes antifúngicos en sinergia. La combinación de itraconazol y yoduro de potasio es una buena opción en el caso de gatos no tratados previamente y con múltiples lesiones cutáneas, en la mucosa nasal y/o con signos respiratorios, así como para casos refractarios. Sin embargo, las opciones terapéuticas para la mayoría de los casos que fracasan son escasas. Por tanto, son necesarias nuevas opciones terapéuticas, más aún cuando existen muchas moléculas potenciales in vitro, no disponibles de momento para su uso en gatos. Son necesarios más estudios que correlacionen los resultados de las pruebas de sensibilidad in vitro a los antifúngicos con aquellos del tratamiento de gatos con esporotricosis. Esta revisión discutirá brevemente los fármacos antimicóticos y los protocolos terapéuticos utilizados para tratar gatos con esporotricosis, así como los factores determinantes del fracaso del tratamiento.
Sporothrix brasiliensis and Sporothrix schenckii are the main causative agents of feline sporotrichosis.23,49,79Sporothrixglobosa,54Sporothrixhumicola59 and Sporothrixpallida92 are scarcely reported. Cases of feline sporotrichosis have been reported in the United States, Mexico, Argentina, Paraguay, Malaysia, Spain, Germany, Australia, Japan, Thailand, United Kingdom and Brazil.26,27,29,43,59 In this last country, there is a predominance of S. brasiliensis, and epizootics have been reported since 1998.45 On the other hand, S. schenckii has been described as the main causal agent in some Asian countries.49
Feline sporotrichosis ranges from a single lesion to multiple skin lesions and fatal disseminated systemic forms.84Sporothrix brasiliensis is the most virulent species in terms of mortality in a murine model,24 and infections are frequently associated with atypical and severe clinical manifestations in humans,2 with increasing virulence over time.35 Remarkably, the fatal disseminated form of the disease in cats has not been reported outside Brazil, where non-S. brasiliensis species are the major agents.48,49 The cat is considered the most susceptible animal species to Sporothrix infection, leading to severe clinical forms and more difficult treatment in many cases.64,88 The treatment of cats with sporotrichosis is an important measure of disease control as it induces a quick reduction of the fungal burden.64 However, it requires a long period of daily care, and cats do not always respond well to the treatment, leading to therapeutic failure.42,66,78,86
Few effective antifungal agents are available, and scarce clinical studies on cats with sporotrichosis have been conducted. Itraconazole (ITZ) as monotherapy or associated with potassium iodide (KI) is the most common therapeutic regimen. Ketoconazole (KTZ), sodium iodide (NaI), fluconazole (FLZ), amphotericin B deoxycholate (AMB), terbinafine (TRB), posaconazole (POS), local heat therapy, cryosurgery and surgical removal of the lesions have also been described for treating this disease in cats.44 Some progress has recently been made in searching for new antifungal therapies for sporotrichosis. Potential candidates that showed in vitro activity against Sporothrix species include terpinen-4-ol and farnesol, TCAN26 (a structural analogue of miltefosine), H3 (a 24-sterol methyltransferase inhibitor), clotrimazole, β-dihydrofuran naphthoquinone isomers (naphthoquinone), coumarin derivatives, extracts from members of the Piperaceae family (Piper abutiloides), marjoram (Origanum majorana L.), rosemary (Rosmarinus officinalis L.),80 pentathiepin compounds,5 buparvaquone,10 curcumin50 and derivatives from the acylhydrazones.8 Many of these compounds show synergism with ITZ and significant antifungal activity against ITZ-resistant isolates,80 making them candidates for further studies in cats. However, the path taken from the in vitro findings, then the in vivo drug tests to check effectiveness and safety, and the approval of the final product at the end is a long one. Although miltefosine was long considered a candidate in the drug repositioning strategy,9,25 it was not effective in a study that included cats with sporotrichosis.85
This review will briefly discuss about the current antifungal drugs, the treatment protocols used in cats with sporotrichosis, and the determinant factors in treatment failure.
Therapeutic optionsPotassium iodide (20% or as a saturated solution) was the first treatment described for feline sporotrichosis.33 However, few successful treatment outcomes were reported in isolated cases15,36 or series of cases.28,34 The occurrence of dose-related adverse reactions (anorexia, vomiting, lethargy and fever) was common (10–20mg/kg every 12–24h).3,20,21,28,33,68 When KI concentration was lower (2.5–20mg/kg every 24h), the clinical cure rate was 47.9%, and clinical adverse reactions were observed in 52% of the cases.75 Despite the adverse reactions when used in monotherapy, KI remains being an option for feline sporotrichosis when talking about oral capsules with a lower dose than that established in the literature and combining with ITZ, a combination therapy that was proposed.76,78
Sodium iodide was also used for the treatment of cats with sporotrichosis, with adverse reactions similar to those of KI.14,16,20,65,69,71,84 Cases of clinical cure were reported in a single case14 and a series of cases.16,84 However, in these case series, the percentage of clinical cure ranged from 21.4 to 23%, and the occurrence of gastrointestinal adverse reactions ranged from 35.7 to 40%.16,84
Licensed in 1959, AMB (polyene antifungal antibiotic) became the mainstay for the treatment of invasive fungal diseases,58 including the disseminated forms of human sporotrichosis.55 However, its use in cats with sporotrichosis was associated with a lack of clinical response and adverse effects, especially nephrotoxicity.28,56,69 The imidazole KTZ became available in 1979, and the results of recovery and failure in feline sporotrichosis were published thereafter, with doses of KTZ varying from 5 to 27.7mg/day.14,21,28,48,60,65,68,73,84 KTZ is less effective than ITZ (28.6%, 38.3%, respectively), and it is linked to higher occurrence of adverse reactions (42.1%, 30.9%, respectively), which include gastrointestinal alterations (nausea, anorexia, vomiting)73 and hepatotoxicity.39 The first reported case of feline sporotrichosis treated with ITZ occurred one year after its introduction in 1992.71 This drug became the first-line treatment for sporotrichosis in cats due to its effectiveness and improved safety profile compared with AMB and KTZ.20,48,57,64,73,81,84,88 The dosage established for treating cats with sporotrichosis (50–100mg/day) resulted in variable clinical cure rates (38–77%).64,73,88 In comparison to KTZ, the use of ITZ implies lower occurrence of gastrointestinal and liver adverse reactions (30.9%).39,73 Dosage adjustments are not necessary in cases with renal impairment, but the dose must be lowered if administered with hepatic disease or dysfunction.38
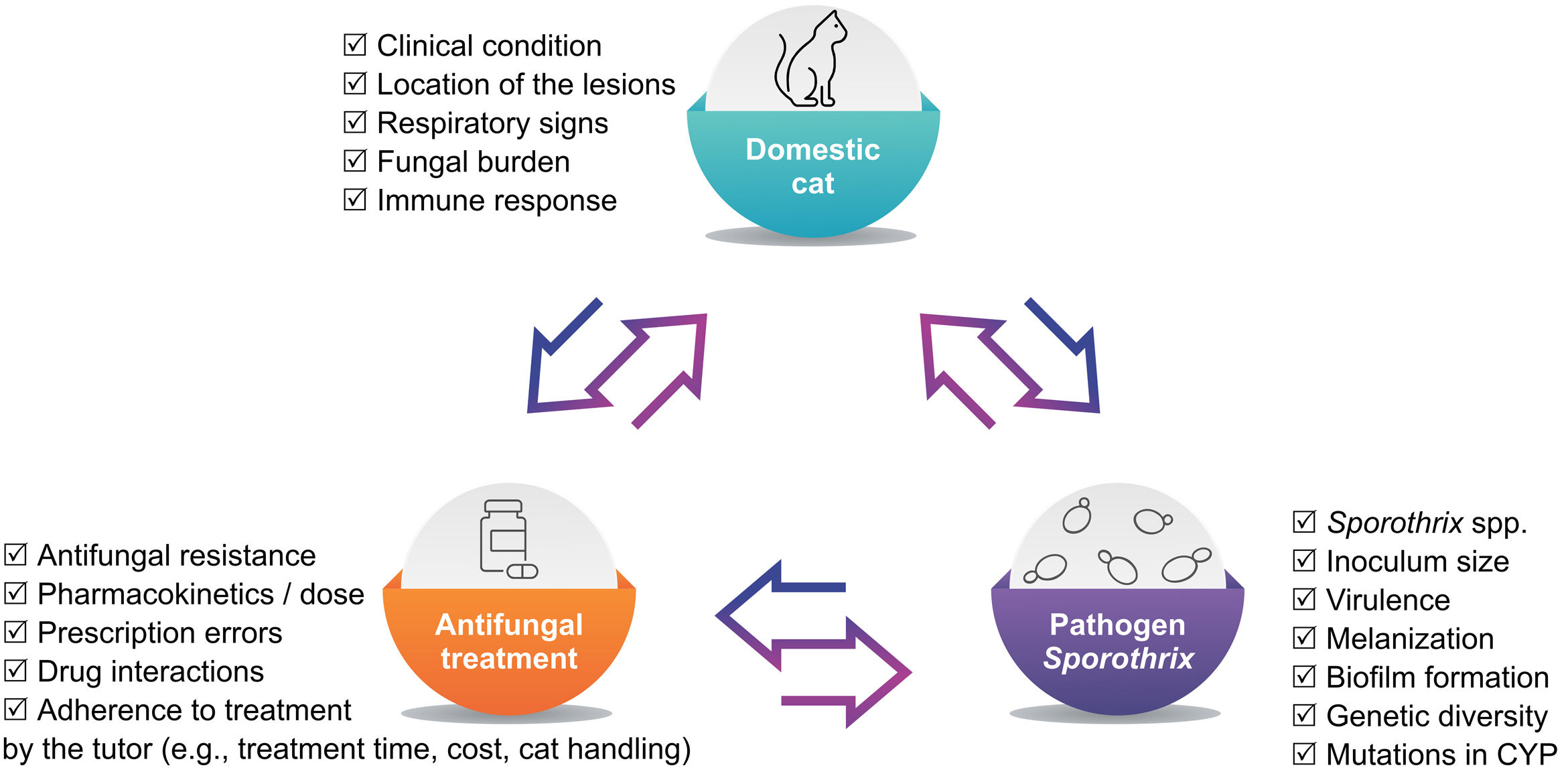
The association of ITZ (50–100mg/day) and KI (2.5–20mg/kg/day) is the most studied combination so far in the treatment of sporotrichosis in cats.64,76,78 This option increases the likelihood of recovery in cats with sporotrichosis that did to respond to ITZ monotherapy,78 as well as for naïve cats, particularly those presenting multiple cutaneous lesions, nasal mucosal lesions and/or respiratory signs64,76 (Fig. 1). In cats with sporotrichosis refractory to ITZ or ITZ+KI, subcutaneous or intralesional AMB combined with ITZ is another therapeutic option.40–42
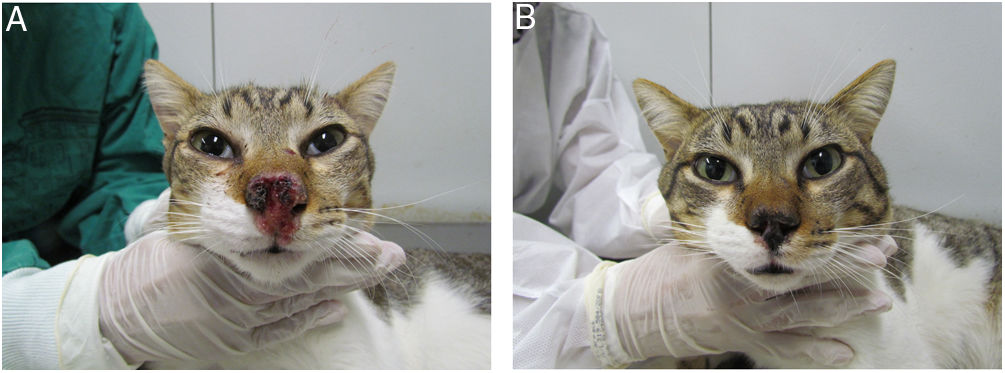
Cat with sporotrichosis successfully treated with itraconazole and potassium iodide at the Laboratory of Clinical Research on Dermatozoonoses in Domestic Animals at INI/Fiocruz, Rio de Janeiro, Brazil (2021). (A) Cat with sporotrichosis presenting epiphora, ulcerated skin lesion and crusts on the nasal planum, philtrum and above the upper lip before receiving the antifungal treatment. (B) The clinical cure was achieved after 28 weeks of itraconazole (100mg/24h) and potassium iodide (5mg/kg/24h) therapy.
It is worth noting that in the 1990s, the lipid-based formulations of AMB reached the market. These drugs have lower toxicity, can be offered at higher doses, and may effectively treat resistant or AMB-unresponsive mycoses. However, the cost may be prohibitive for its use in animals.37 Intravenous administration of liposomal amphotericin B (L-AMB) (1mg/kg) and oral ITZ (100mg/day) were successfully used in a cat presenting skin and mucosal lesions on the nasal region refractory to ITZ 100mg/day, AMB administered both subcutaneously (0.5mg/kg) and intralesional (1mg/kg).42 However, when intravenous L-AMB associated with oral ITZ (100mg/day) was used in three cats with poor general health, multiple lesions and respiratory signs, one cat died due to the progression of the disease, and the others did not respond to the treatment.72
Fluconazole, a broad-spectrum triazole whose use was approved in early 1990, is recommended for systemic antifungal infections, like cryptococcosis, involving difficult-to-reach tissues (central nervous system).39 In a single study, FLZ (10mg/day) was used in monotherapy to treat one cat with sporotrichosis that had cutaneous lesions and respiratory signs. Due to the recurrence of the respiratory signs, this drug was prescribed again shortly after the first treatment had end, and the clinical cure was further achieved.20 Thus, there is no therapeutic or cost advantage that justifies the use of FLZ over ITZ for sporotrichosis infections.
The use of terbinafine, an allylamine antifungal agent, primarily recommended for dermatophytosis, was approved in 1990.46 Terbinafine (30mg/day) as monotherapy and in combination with itraconazole (5–10mg/kg/day) was used in the treatment of feline sporotrichosis in one study, with no information on the clinical cure rate.84 The effectiveness of this drug has been confirmed in humans31,32 and in two dogs94 with sporotrichosis, but not in cats so far.
Posaconazole is a high-cost drug and an analog of ITZ, and was approved in 2006.83 The use of POS was reported in a cat from Australia that presented with a non-ulcerated nodule on its nasal bridge due to an infection of a member whithin the S. pallida complex. After the ITZ-induced hepatic injury, therapy was changed to POS (40mg/day), which eventually resulted in regression of residual infected tissues and clinical resolution.92
When considering cryosurgery or administration of intralesional AMB, some factors should be considered, such as the location of the residual skin lesions and the requirement of anesthetic procedures.40,41,87
Different treatment protocols used in feline sporotrichosis are described in Table 1.
Feline sporotrichosis treatment protocols described since 1956.
| Year | Authors | Origin | Treatment |
|---|---|---|---|
| 1956 | Freitas et al.33 | Brazil | KI |
| 1965 | Freitas et al.34 | Brazil | KI |
| 1973 | Anderson et al.3 | USA | KI |
| 1983 | Burke et al.14 | USA | KTZ+NaI |
| 1983 | Nusbaum et al.69 | USA | NaI |
| 1986 | Dunstan et al.28 | USA | 20% KI, SSKI, KTZ |
| 1986 | Mackay et al.56 | AUS | 20% KI |
| 1989 | Gonzalez Cabo et al.36 | Spain | 20% KI |
| 1991 | Caravalho et al.15 | USA | KI |
| 1993 | Peaston71 | USA | NaI, ITZ |
| 1993 | Marques et al.60 | Brazil | KTZ |
| 1996 | Davies & Troy21 | USA | KI, KTZ |
| 1996 | Nakamura et al.65 | Japan | NaI, KTZ |
| 2001 | Nobre et al.68 | Brazil | KTZ, KI, ITZ |
| 2004 | Schubach et al.84 | Brazil | NaI, KTZ, TRB, ITZ, ITZ+FLZ, ITZ+TRB |
| 2009 | Crothers et al.20 | USA | NaI, KI, ITZ, FLZ |
| 2009 | Gremião et al.40 | Brazil | ITZ+AMB IL |
| 2010 | Pereira et al.73 | Brazil | ITZ, KTZ |
| 2010 | Madrid et al.57 | Brazil | ITZ |
| 2011 | Gremião et al.41 | Brazil | ITZ+AMB IL |
| 2012 | Reis et al.75 | Brazil | KI |
| 2013 | Rossi et al.81 | Brazil | ITZ |
| 2015 | Gremião et al.42 | Brazil | AMB SC+ITZ, L-AMB IV+ITZ |
| 2015 | Pereira et al.72 | Brazil | L-AMB IV+ITZ |
| 2016 | Reis et al.76 | Brazil | ITZ+KI |
| 2016 | Souza et al.87 | Brazil | Cryosurgery+ITZ |
| 2017 | Han et al.48 | Malaysia | KTZ, ITZ |
| 2018 | Rocha et al.78 | Brazil | ITZ+KI |
| 2018 | Carvalho et al.16 | Brazil | NaI |
| 2018 | Miranda et al.64 | Brazil | ITZ, ITZ+KI |
| 2018 | Souza et al.88 | Brazil | ITZ |
| 2019 | Thomson et al.92 | Australia | ITZ, POS |
KI: potassium iodide; NaI: sodium iodide; SSKI: super saturated potassium iodide solution; ITZ: itraconazole; KTZ: ketaconazole; TRB: terbinafine; FLZ: fluconazole; AMB IL: intralesional amphotericin B; L-AMB IV: intravenous liposomal amphotericin B; POS: posaconazole.
A factor that may hinder reaching a complete cure is the presence of lesions on the bridge of the nose, nasal mucosa lesions and respiratory signs, which are a risk of failure in cats.73,88 The host immune response may also negatively influence the prognosis. Cats with poor general condition and disseminated sporotrichosis show lesions with a high fungal load, which is associated with treatment failure and a longer healing time of the lesion. In these cases, the histopathological findings reveal the predominance of poorly formed granulomas, a reduced number of macrophages, neutrophils and lymphocytes, suggesting an ineffective immune response against S. brasiliensis infection. An optimal lymphocyte CD4/CD8 ratio seems to be determinant in the outcome of the patient, and high levels of CD4 lymphocytes in the peripheral blood are linked to better clinical outcomes, more organized granulomatous inflammation and reduced fungal load.63,88
Interestingly, treatment failure does not seem to be associated with feline immunodeficiency virus (FIV) or feline leukemia virus (FeLV) infections. However, the histological changes observed in skin lesion samples of coinfected cats suggest a less efficient immune response when compared to non-coinfected cats, with the subsequent lower capacity to eliminate the fungus.88 Moreover, cats with sporotrichosis with high levels of IL-10 during FIV and/or FeLV co-infections, and low levels of IL-4 (FeLV-positive) and IL-12 (FIV-positive) seem to develop severe clinical presentations and poor general condition. Nevertheless, severe clinical presentations and treatment failure are frequently described in the absence of feline retrovirus coinfections.63
Considering the role of host factors in host–Sporothrix interaction, immunomodulation has been explored as a potential adjuvant therapy for sporotrichosis. In fact, it seems that those better outcomes when administering KI are related to an influence of iodides on the immune response. The use of KI seems to induce considerable anti-inflammatory effects, notably in neutrophils.52,70,93 In this scenario, it has been shown that KI inhibits Sporothrix species biofilm metabolic activity in both filamentous and yeast forms.13 Other studies also described enhanced antibacterial and antiviral properties in the respiratory mucosa with the use of iodides.30 Although it is tempting to correlate these findings with good results of KI use in the treatment of cats with nasal mucosa involvement, there is no evidence of this association in cats.
New vaccine candidates and immunomodulatory alternatives are currently being investigated in animal models with promising results for prophylactic and therapeutic purposes. A strong protective Th1-mediated response is induced with the use of the enolase-based vaccine and the Montanide PetGel A as an adjuvant.90 Experimental passive immunization of mice with monoclonal antibodies against a Sporothrix 70-kDa glycoprotein has also shown a fungal burden reduction in tissue and an increased interferon-γ production.1,22,67 The treatment with complex carbohydrates as beneficial adjuvants to antifungal therapy, such as β-glucans, that are innate immunity agonists, has also been described.89 These alternatives have been little explored in cats so far, and the current immunomodulators described do not show sufficient evidence to support their use in feline sporotrichosis.
Social and economic factorsTime commitment and the cost of the antifungal drugs are major causes of abandonment. Other economic factors like transportation costs, or costs associated with supplementary medication, negatively influence adherence.7,66,74 In addition, cases of illness in family members are another important reason that leads owners to request euthanasia for their cats84 or abandon them. In Brazil the provision of drugs is free of charge, but it is not enough to guarantee compliance: treatment dropout is frequently described (34–38.5%) and mainly occurs once the clinical signs have dissapeared.17,84 Thus, it is essential to emphasize the importance of both compliance since the treatment is started and the risk of relapse if the medication is discontinued before achieving the clinical cure.
Most treatment protocols are based on daily oral administration of the antifungal drugs, but some cats can be difficult to handle44; giving medication to cats is usually more difficult when compared with dogs due to their discriminating sense of taste.91 Keeping cats indoors or confined within a limited space until reaching the clinical cure is recommended; however, this situation entails a substantial challenge to owners, especially when owning non-neutered cats.6
Therapy-related factorsThe median treatment duration in cats with sporotrichosis is four months, and the treatment should be continued at least four weeks after reaching the clinical cure,44 which might compromise the owner's adherence.53,74 Importantly, pet owners should be aware that an inadequate length of the therapy or an irregular administration may result in prolonged treatment schedule, failure or relapse, which usually occurs 3–18 months after the completion of the therapy.17,84 The occurrence of adverse drug reactions seems to be unrelated with treatment abandonment,17 but may interfere with the therapy schedule and prolong treatment duration. An inadequate drug prescription can also lead to treatment failure. The use of ITZ with H2 receptor antagonists or proton pump blockers should be avoided because alkalinity decreases ITZ absorption.51 The bioavailability of ITZ in oral capsules is also erratic, but it can be maximized through the intake of food or fat at the same time.39
As ITZ may be cost-prohibitive in brand name form, the generic antifungal medication is a satisfactory alternative. In the case of compounded ITZ, this should be avoided whenever possible. Studies in dogs and cats demonstrated that the generic and compounded ITZ are not bioequivalent to the reference drug. Pharmacokinetic data obtained with the generic formulation showed that therapeutic concentrations could be achieved, but compounded ITZ led to low plasma concentrations, being unlikely to be effective.62,77 Furthermore, a study described that the ITZ content in oral capsules acquired from two brand-name products, and three ITZ capsules from compounding pharmacies (for human and veterinary use in all cases) did not match the concentration reported by the manufacturers.96
Antifungal resistanceThe widespread use of antifungal agents is presumed to be a factor that promotes drug resistance.4,19 The emergence of Sporothrix species with in vitro antifungal resistance has been evidenced, and resistance development may be due to the melanin production capacity, genetic diversity and mutations in cytochrome P450.95 The ability of the filamentous saprophytic phase of Sporothrix species to form biofilms has been previously described and is also associated with increased resistance to antifungal agents.12,25
Antifungal-resistant strains of S. brasiliensis recovered from cats have been reported in Brazil.66,82,95 In cats with sporotrichosis there is a trend in relating in vitro resistance to treatment failure (clinical resistance); however, the latter can come of many reasons, not only of drug resistance (Fig. 2).
The in vitro activity of ITZ, KTZ, TRB and AMB was evaluated against 47 isolates of S. brasiliensis from cats followed-up at the Laboratory of Clinical Research on Dermatozoonoses in Domestic Animals at INI/Fiocruz, Rio de Janeiro, Brazil (unpublished data). These isolates were collected before the treatment with ITZ or KTZ. The filamentous phase was subjected to in vitro susceptibility testing using the liquid microdilution method according to the Clinical and Laboratory Standards Institute protocol M38-A2.18 TRB was the most effective antifungal, with in vitro activity showing the lowest MIC values (0.015–0.12μg/mL). AMB showed a MIC range of 0.25 2μg/mL. Among the azoles, KTZ (0.12–2μg/mL) showed better in vitro activity than ITZ (0.50–2μg/mL). These results were similar to those in other studies with S. brasiliensis strains recovered from cats.11,61,82 Curiously, S. schenckii isolates from Malaysia showed low susceptibility towards terbinafine.48 In the same study, when assesing the outcome of the antifungal treatment on 33 cats (17 achieving clinical cure and 16 therapeutic failure), no significant difference was observed concerning the MICs obtained for KTZ and ITZ, suggesting that there was no correlation between the in vitro and in vivo findings in the studied sample. In another study from Southern Brazil that selected 12 feline cases of infection by S. brasiliensis with high MIC values (≥4μg/mL) to ITZ, a correlation between the in vivo therapeutic response and the in vitro antifungal susceptibility assay was observed due to the occurrence of refractory cases with ITZ-resistant S.brasiliensis.66
Additionally, no significant differences were observed between the general condition of one infected cat (presence of lymphadenomegaly, distribution of skin lesions and presence of respiratory signs) and the MIC values obtained from other cats in Rio de Janeiro. This lack of significant association between clinical characteristics and MIC values was already described in a study with human patients from the same region.47 Therefore, the evaluation of the results of the antifungal susceptibility tests must be managed with great caution.
ConclusionsSome of the major advances and significant challenges that remain in the management of feline sporotrichosis have been reviewed. The veterinarian's role is essential to enable owners to increase adherence to therapeutic protocols and to develop easy-to-administer medications for cats (e.g., injectable depot formulations for long-term controlled drug release). Due to the few therapeutic options, studies on drug repositioning, vaccines and immunotherapy candidates should be encouraged. As prompt treatment in cats can rapidly reduce the fungal burden and risk of transmission of Sporothrix from cats, the availability of a first-line treatment for these animals at health facilities in affected areas is essential.
Conflict of interestNone declared.
Laboratório de Pesquisa Clínica em Dermatozoonoses em Animais Domésticos, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil for the technical support.