The number of fungal infections has increased in recent years in Rio Grande do Sul (RS), Brazil. Epidemiological studies are important for proper control of infections.
AimsTo evaluate the etiology of fungal infections in patients in RS, from 2003 to 2015.
MethodsThis is a retrospective and longitudinal study carried out at Mycology Department of Central Laboratory of RS; 13,707 samples were evaluated. The variables sex, age, site of infection, and etiologic agent were analyzed. Susceptibility of Candida to fluconazole was tested in isolates from samples collected in 2015 from 51 outpatients.
ResultsOf the 13,707 samples, 840 cases (6.12%) of fungal infections were found and included in the analyses; female gender accounted for the 55.9% of the cases. The main fungus was Candida albicans (450 cases, 53.38%; p<0.001). Onychomycosis was the most frequent infection in superficial mycoses. Systemic mycoses accounted for 54.05% of the cases, from which 68.8% occurred in males, mainly HIV-positive (33.11%), and the main etiologic agent in these cases was Cryptococcus neoformans (73.13%). Among 51 samples tested for susceptibility to fluconazole, 78.43% of Candida isolates were susceptible; 5.88% were susceptible in a dose-dependent manner, and 15.69% were resistant.
ConclusionsC. albicans is a common cause of fungal infections in RS, accounting for half of the cases; resistance to antifungals was found in non-hospitalized patients. In addition, women seem to be more susceptible to fungal infections than men, however men show more systemic mycoses than women. The nails are the most common site of infection.
El número de casos de infecciones fúngicas ha aumentado en los últimos años en Rio Grande do Sul (RS), Brasil. Los estudios epidemiológicos son importantes para el control de estas infecciones.
ObjetivosEvaluar la etiología de las infecciones fúngicas en pacientes de RS desde 2003 hasta 2015.
MétodosEste es un estudio retrospectivo y longitudinal realizado en el Departamento de Micología del Laboratorio Central de RS; se evaluaron 13.707 casos de infecciones. Se analizaron las variables sexo, edad, lugar de infección y agente etiológico. La sensibilidad de Candida al fluconazol se analizó en 51 aislamientos de muestras recogidas en el año 2015.
ResultadosDe las 13.707 muestras, 840 casos (6,12%) de infecciones fúngicas se incluyeron en el análisis; el 55,9% correspondieron a mujeres. El hongo predominante fue Candida albicans (450 casos, 53,38%; p<0,001). La onicomicosis fue la infección más frecuente de las micosis superficiales. Las micosis sistémicas representaron el 54,05% de los casos; de estos, el 68,8% tuvieron lugar en hombres, principalmente VIH-positivos (33,11%), y el principal agente etiológico en estos casos fue Cryptococcus neoformans (73,13%). Entre las 51 muestras analizadas para determinar la sensibilidad al fluconazol, el 78,43% de los aislamientos de Candida fueron sensibles; el 5,88% fueron sensibles de forma dosis-dependiente y el 15,69% fueron resistentes.
ConclusionesC. albicans es una causa común de infecciones fúngicas en RS, pues representa la mitad de los casos; su resistencia a los antifúngicos se encontró entre pacientes ambulatorios. Además, parece que las mujeres son más susceptibles a las infecciones fúngicas que los hombres, mientras que los hombres presentan más micosis sistémicas que las mujeres; las uñas son el lugar más frecuente de infección.
Fungi can cause diseases ranging from cutaneous skin infections to lethal acute or chronic infections of deep tissues. Around 600 fungal species are human pathogens, including ascomycetes of the genus Candida.5,28Candida albicans has the potential of causing life-threatening systemic infections and is the major pathogen associated with invasive mycoses; other Candida species, in turn, have been reported as the cause of infections in patients with underlying diseases.3,31,35
The epidemiology of such infections varies among different geographic regions and in medical centers within the same region.4,13 Local studies are important in order to obtain epidemiological data and to understand local factors that may contribute to acquire an infection. In addition, information about the susceptibility of local strains to antifungical medications is necessary for the management and treatment of patients with a candidal infection.1 Despite the fact that the number of fungal infections has increased considerably in the past decades, mainly among immunosuppressed patients, data about the epidemiology and the prognostic factors associated with candidemia in patients admitted to ICUs in Latin America are scarce.8,23,30 In Rio Grande do Sul (RS), the Southernmost State in Brazil, fungal infections are a significant cause of morbidity and mortality, but there are no clinical or epidemiological data regarding this situation.
The long-term use of fluconazole (FCZ) in the treatment of invasive candidiasis can cause the emergence of non-C. albicans Candida species; moreover, those strains with low susceptibility have appeared in the hospital environment. Accordingly, some strains of Candida glabrata and Candida krusei already display decreased susceptibility or even resistance to FCZ.7,17
Hence, with the emergence of new pathogenic fungal strains and the increase in fungal resistance to the current antimycotic medications it is important to address the aspects of fungal infections, including analysis based on susceptibility tests in clinical isolates.18,37 Determining the sensibility profile of these agents enables to give a proper treatment, the control of the resistant strains, and provides information for the study of the evolution of resistance by the etiologic agents.
The ARTEMIS DISK, one of the most important surveillance programs on fungal resistance to FCZ and voriconazole in 134 institutions from 40 countries, including Brazil, makes use of the disk diffusion method to estimate resistance.32 Among several available methods to evaluate the sensibility, the disk diffusion method is considered an accurate method of assessing the susceptibility of Candida species to FCZ because it is affordable and easily reproducible to apply in clinical laboratories.14,32
In this retrospective and longitudinal study we estimated the prevalence of fungal infection in RS from 2003 to 2015. The rate and etiology of the infections were evaluated, and the fungal susceptibility to FCZ of the strains isolated in the year 2015 was analyzed.
Materials and methodsPatients and samplesClinical samples from patients with fungal infections attended in hospitals and ambulatories in RS from December 2003 to December 2015 were analyzed. The inclusion criterion was a positive culture in the samples for one or more of the following pathogens: Candida species, Cryptococcus neoformans, Fusarium species, Trichosporon species, Aspergillus species, Scytalidium species, Curvularia species, Chaloropsis species, Scopulariopsis species, Acremonium species, Onychocola species, Penicillium species, Scedosporium species, Hendersonula species and any species included in the Mucorales order.
We also verified the fungal etiology and the clinical profile of the patients, including age, gender, lesion topography, state region of notification, presence of superficial mycoses (cutaneous tegument, nails) or systemic mycoses (internal organs), and HIV serological status. To test the susceptibility to FCZ, 51 strains of Candida species isolated in 2015 from outpatients were selected. The identification of the Candida isolates and the FCZ disk diffusion susceptibility test were performed at the Mycology Department of LACEN/SES-RS.
This study was approved by the Ethics Committees of Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA, Ethics Statement n. 23523513.4.0000.5345) and LACEN/SES-RS (Ethics Statement n. 23523513.4.3001.5320).
Candida species identificationIsolates of Candida species were identified based on their morphophysiological characteristics. Cultures in CHROMagar-Candida medium (Conda Laboratories, Spain) were performed to confirm the viability and pureness, and to identify the isolates by the production of chromogen pigments (green: C. albicans, blue: Candida tropicalis, and pink: C. krusei).26 The identification of C. albicans was based on the presence of the germinative tube in human serum and on the production of chlamydospores in corn meal agar (BD, EUA) with tween 80 (Synth, Brazil).12,39 The biochemical identification was made through the API ID 32C commercial system (bioMérieux, France) according to the manufacturer's recommendation. The isolates were maintained in sterile distilled water at room temperature up to the moment of performing the susceptibility tests.
Antifungal susceptibility testThe agar disk diffusion test was performed with paper disks containing 25μg of FCZ (CECON, Brazil) and Petri dishes (90mm diameter) containing Mueller-Hinton agar (Oxoid, Hampshire, England) supplemented with glucose 2%, as described in the M44-A2 document (CLSI, 2009).20 In order to verify the precision, accuracy and performance of the test, C. albicans ATCC 14053 and Candida parapsilosis ATCC 22019 were used as control strains. Fifty one isolates of Candida species were subcultured on plates with Sabouraud dextrose agar (Difco, England) and incubated at 35–37°C for 24h. Five colonies of each isolate were selected and suspended in 5ml of sterile saline (0.85%). The turbidity of the was adjusted to the 0.5 McFarland scale (106cell/ml). Suspensions were inoculated using a sterile swab over the surface of agar Mueller-Hinton. The disks with FCZ were aseptically placed on the inoculated agar and the plate was incubated aerobically at 35–37°C for 24h. The diameters of the inhibition area were measured to determine the susceptibility, and the minimal inhibitory concentration (MIC) was calculated. The interpretative criteria of the FCZ disk diffusion test were those suggested by CLSI: susceptible (S): ≥19mm; susceptible dose-dependent (SDD): 15–18mm; resistant (R): ≤14mm.
Statistical analysisStatistical analyses were carried out with the SPSS software version 17.0 (Chicago, IL, USA). Categorical variables were compared using chi-square test and continuous variables by ANOVA test. Values of p<0.05 were considered as statistically significant.
ResultsEtiology of the infections and characteristics of the patientsA total of 13,707 epidemiological records of fungal infections in RS from December 2003 to December 2015 were obtained, out of which 840 cases (6.12%) were positive for at least one of the agents considered in the inclusion criteria, and were analyzed in the study. The period with the highest number of cases (38%) was from 2007 to 2010. Most of the cases (72.4%, p<0.001) were from the metropolitan region of Porto Alegre, state capital of RS.
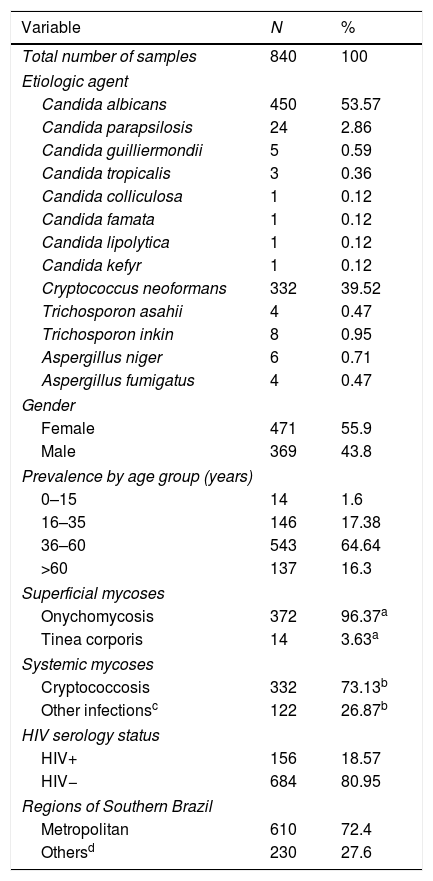
Table 1 shows the distribution of cases according to the etiological agent, patients’ sex, prevalence by age group, type of mycosis (superficial or systemic), HIV serologic status, and geographical regions. Of the total number of positive cases evaluated (n=840), 471 (55.9%) were women and 369 (44.1%) corresponded to men (p<0.001). The average age was 43 years (0–90 years age range), and the age range most affected by fungal infections was 36–60 years, with 543 cases (64.6%).
General characteristics of the study.
| Variable | N | % |
|---|---|---|
| Total number of samples | 840 | 100 |
| Etiologic agent | ||
| Candida albicans | 450 | 53.57 |
| Candida parapsilosis | 24 | 2.86 |
| Candida guilliermondii | 5 | 0.59 |
| Candida tropicalis | 3 | 0.36 |
| Candida colliculosa | 1 | 0.12 |
| Candida famata | 1 | 0.12 |
| Candida lipolytica | 1 | 0.12 |
| Candida kefyr | 1 | 0.12 |
| Cryptococcus neoformans | 332 | 39.52 |
| Trichosporon asahii | 4 | 0.47 |
| Trichosporon inkin | 8 | 0.95 |
| Aspergillus niger | 6 | 0.71 |
| Aspergillus fumigatus | 4 | 0.47 |
| Gender | ||
| Female | 471 | 55.9 |
| Male | 369 | 43.8 |
| Prevalence by age group (years) | ||
| 0–15 | 14 | 1.6 |
| 16–35 | 146 | 17.38 |
| 36–60 | 543 | 64.64 |
| >60 | 137 | 16.3 |
| Superficial mycoses | ||
| Onychomycosis | 372 | 96.37a |
| Tinea corporis | 14 | 3.63a |
| Systemic mycoses | ||
| Cryptococcosis | 332 | 73.13b |
| Other infectionsc | 122 | 26.87b |
| HIV serology status | ||
| HIV+ | 156 | 18.57 |
| HIV− | 684 | 80.95 |
| Regions of Southern Brazil | ||
| Metropolitan | 610 | 72.4 |
| Othersd | 230 | 27.6 |
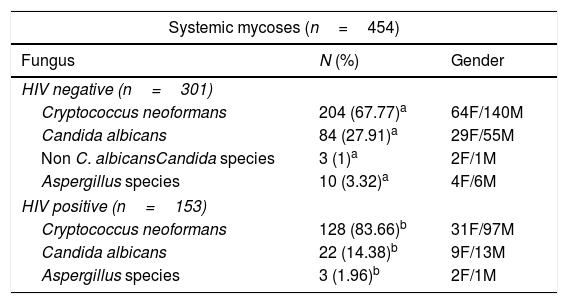
The species C. albicans was the main isolated fungus (53.57%, p<0.001), followed by C. neoformans (39.52%) and C. parapsilosis (2.86%) (Table 1). It is worth noting that 71.1% of the samples positive for C. albicans were from female patients, and this species was found mainly in superficial infections; in contrast, 71.38% of the cases positive for C. neoformans were in male patients with systemic infections (Table 2). Furthermore, 322 cerebrospinal fluid (CSF) samples were analyzed from patients with meningitis, among which 317 (98.4%) were positive for C. neoformans (not shown); this species was also found in 83.7% of the HIV-seropositive group.
Systemic and superficial mycoses in Rio Grande do Sul, Brazil, 2003–2015.
| Systemic mycoses (n=454) | ||
|---|---|---|
| Fungus | N (%) | Gender |
| HIV negative (n=301) | ||
| Cryptococcus neoformans | 204 (67.77)a | 64F/140M |
| Candida albicans | 84 (27.91)a | 29F/55M |
| Non C. albicansCandida species | 3 (1)a | 2F/1M |
| Aspergillus species | 10 (3.32)a | 4F/6M |
| HIV positive (n=153) | ||
| Cryptococcus neoformans | 128 (83.66)b | 31F/97M |
| Candida albicans | 22 (14.38)b | 9F/13M |
| Aspergillus species | 3 (1.96)b | 2F/1M |
| Superficial mycoses (n=386) | ||
|---|---|---|
| Fungus | N (%) | Gender |
| HIV negative (n=383) | ||
| Candida albicans | 341 (89.03)a | 294F/47M |
| Non C. albicansCandida species | 33 (8.62)a | 27F/6M |
| Trichosporon species | 8 (2.09)a | 5F/3M |
| Aspergillus species | 1 (0.26)a | 1F |
| HIV positive (n=3) | ||
| Candida albicans | 3 (100)b | 3F |
F: female; M: male.
Systemic mycoses (SIM) accounted for 454 cases (54.05%), and the major etiologic agent in SIM was C. neoformans, with 332 cases, followed by Candida and Aspergillus species (109 and 13 species, respectively). Men presented the highest frequency of SIM (68.94%, p<0.001). We found that 33.7% of SIM cases were seropositive for HIV (111 male and 42 female), with C. neoformans accounting for 83.66% of the infections in this group (Table 2).
Superficial mycosesSuperficial mycoses (SM) accounted for 386 cases (45.95%) and most of them were onychomycoses (96.37%, p<0.001); it is worth noting that 330 cases (85.49%) occurred in women (Table 1). We found that Candida was the most frequent agent (97.67%), followed by Trichosporon species (2.07%) and Aspergillus species (0.26%) (Table 2). Only three patients, all women infected with C. albicans, were HIV-positive.
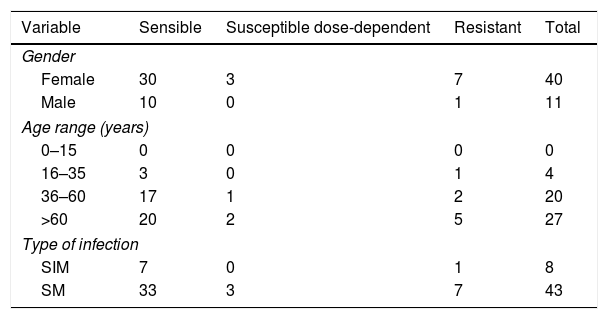
Susceptibility profileAmong the 51 isolates of Candida studied, 40 (78.43%) were susceptible to FCZ, three (5.88%) were susceptible in a dose-dependent manner, and eight (15.69%) showed resistance (Table 3). Resistance to FCZ was predominantly found in isolates from patients older than 60 years (average 69 years old).
Susceptibility profile of Candida to fluconazole by the disk diffusion method.
| Species | Susceptible | Susceptible dose-dependent | Resistant | Total |
|---|---|---|---|---|
| Candida albicans | 18 | 3 | 5 | 26 |
| Non C. albicansCandida speciesa | 22 | 0 | 3 | 25 |
| Total (%) | 40 (78.43) | 3 (5.88) | 8 (15.69) | 51 |
The diameters of the inhibition zones produced in the agar disk diffusion method for all the isolates tested varied from 10 to 25mm. The results of the susceptibility profile from 51 isolates of Candida species to the antifungal agents tested by the disk diffusion method are shown in Table 4.
Resistance profile to the antifungal fluconazole.
| Variable | Sensible | Susceptible dose-dependent | Resistant | Total |
|---|---|---|---|---|
| Gender | ||||
| Female | 30 | 3 | 7 | 40 |
| Male | 10 | 0 | 1 | 11 |
| Age range (years) | ||||
| 0–15 | 0 | 0 | 0 | 0 |
| 16–35 | 3 | 0 | 1 | 4 |
| 36–60 | 17 | 1 | 2 | 20 |
| >60 | 20 | 2 | 5 | 27 |
| Type of infection | ||||
| SIM | 7 | 0 | 1 | 8 |
| SM | 33 | 3 | 7 | 43 |
SIM: systemic mycosis; SM: superficial mycosis.
In the last two decades, fungi have been identified at high frequency as agents responsible for serious infections. The emergence of organisms with less susceptibility to the available antifungal medications emphasizes the clinical importance of establishing routine laboratory diagnostics for fungal infections.25,28 To date, the present work is the largest longitudinal study conducted on this subject in Southern Brazil.
During the period of this study (2003–2015), most of the fungal infections analyzed were in females, and the age range varied widely, with average of 43 years. It should be emphasized that adults between 36–60 years old were highly affected, corresponding to 64% of the cases. Observational prospective studies in Latin America with systemic and superficial infections indicate that 22.3% and 35.8% of the episodes, respectively, also occurred in adult patients.33,36 Our data corroborate these studies, showing that this age group is more prone to these types of infections. Factors that might contribute for that are lifestyle, being part of working class, and being exposed to public environments.
C. albicans was the most prevalent agent found in our samples, followed by C. neoformans and other Candida species, with greater representation of C. parapsilosis. These data agree with other Brazilian and South American studies concerning nosocomial fungal infections, which show C. albicans as the most prevalent species isolated.9,10,21,40
Most of the notifications (72.4%) were in the metropolitan region of Porto Alegre, probably because the city has many health centers, being this region a reference for the treatment of diseases. Understanding the epidemiological characteristics of a specific community has both local and global importance considering the constant flow of people within a country and around the world.24 Recently, Magalhaes et al.19 evaluated 108 isolates of yeast from various clinical samples collected in three hospitals in São Luis do Maranhão, Northeastern Brazil. Most species were of the genus Candida, mainly C. albicans, followed by other species of the genus. According to Lagrou,16C. albicans remains the major cause of invasive infections; nevertheless, the incidence of C. parapsilosis has grown significantly, surpassing C. albicans in some studies, depending on the period and the geographical area evaluated.
In the present study, SIM were more frequent when compared to SM (53.4% and 46.3%, respectively), perhaps because LACEN/SES-RS, as a reference laboratory, receives many samples from hospitals and ambulatories that are specialized in the treatment of patients with HIV, as well as oncologic and transplant patients; these patients are more susceptible to systemic fungal infections. In fact, we found that concomitant HIV and fungal infection rates were higher in SIM than in SM (98.03% and 1.97%, respectively, p<0.01), so HIV infection could be considered a risk factor for SIM. C.neoformans was the most representative agent in males, the most prevalent among SIM (73.13%), and the most frequent species in seropositive patients (83.66%). Similar findings have shown that infections by C. neoformans in hospitalized patients have increased in recent years, and more than 80% of the cryptococcosis cases worldwide are associated with HIV infection.38
Men showed the highest frequency of SIM, which can be related to the fact that most HIV-positive patients were male. Female, on the other hand, presented predominance of SM, especially onychomycosis; having the nails done in a beauty salon is a frequent habit of many women in Brazil, and the use of manicure tools that are shared among clients or that are not well sterilized might be a cause of a high rate of fungal infections among women. In addition, women are more concerned than men in searching for health care in case of an onychomycosis. A study by Ribeiro et al.34 at a hospital in São Paulo found that 66% of the patients treated for onychomycosis were women and 34% were men. Resembling our findings, a recent study conducted in Porto Alegre, RS, evaluated the prevalence of SM and found that the genus Candida was the most prevalent, with women being the most affected patients in the fingernails.15 These authors argue that the epidemiology of onychomycosis is of multifactorial influence and its prevalence is directly related to age, geography, climate of the region, lifestyle and association to other diseases.
In relation to the susceptibility tests to FCZ, 15.69% of the isolates of Candida were resistant. It is important to consider that all isolates were from outpatient services. A similar Brazilian study22 with 93 isolates of Candida species from hospitals demonstrated a similar distribution of the susceptibility rates, in which 62.5% of the isolates presented a susceptible profile, 6.5% were susceptible in a dose-dependent way (6.5%), and 31% were resistant to FCZ (31%). In our study, even with a limited number of strains, a high resistance rate of Candida species was found, somewhat higher than those of other authors.2,11 Indeed, in 2013, the CDC announced increasing incidence of Candida infections due to azole-resistant strains and considered this event a serious public health threat that must be addressed through improved use of antifungal agents.6 Most of the isolates with resistance to FCZ (62.5%) were found among patients older than 60 years. An epidemiological study carried out in Spain27 showed similar results with a rate of resistance to FCZ ten times higher in patients over 65 years (46.7%). Pfaller et al. (SENTRY-Asia-Pacific, European, Latin American and North American),29 in contrast, observed a higher percentage of FCZ-resistant isolates in the 29–59 age group.
One of the limitations of this study was that only strains from 2015 were available for susceptibility tests. Fungal cultures were not carried out due to a lack of laboratorial infrastructure before 2015. Besides, data concerning the status of HIV infection for HIV-positive patients was limited. Another limitation of the study was that we were able to perform the antifungal susceptibility testing only to some of the isolates recovered in 2015 due to limited availability of resources; the analysis was performed using the broth microdilution method according to CLSI document M44-A2.
Fungal infections are important in susceptible patients such as immunosuppressed individuals. Thus, it is important to consider underlying health conditions in different group of patients when studying fungal infections. However, during routine assistance, many data are neglected; this was the situation in this study since specific clinical aspects regarding underlying diseases were not available, therefore not being included in the analyses.
In conclusion, in this study we observed that females seem to be more affected by fungal infections, especially concerning onychomycosis. The Candida genus presents a high incidence rate, with a predominance of C. albicans. SIM was predominant in males, mainly in CSF, and HIV infection was shown to be a risk factor. According to FCZ susceptibility tests, an alarming resistance rate of Candida species was found in non-hospitalized patients in this sampling of Southern Brazil. Susceptibility tests to antifungal agents are necessary in the clinical practice; hence, improvements and investments in health care services, such as the training of technicians in areas where these tests are carried out, are important measures in the control of fungal infections.
This is the first work conducted in Southern Brazil with a large number of samples of fungal infections at the Reference Laboratory for Mycoses. The results present original epidemiological data for RS and show the necessity to implement fungal diagnosis in public health laboratories. We consider important to proceed with further analyses of fungal infections with a larger number of strains, including comparisons between different fungal agents and different susceptibility tests, adding in this way epidemiological data that are useful for the clinical management of patients in public health services.
Conflict of interestThe authors declare that no competing interests exist.
This work was supported by Laboratório Central da Secretaria de Saúde do Estado do Rio Grande do Sul (LACEN/SES-RS, Brazil), Programa de Apoio ao Desenvolvimento Científico e Tecnológico PADCT/FEPPS-RS, Centro de Estudos Avançados em Dermatologia do Rio Grande do Sul, and Graduate Program in Pathology of UFCSPA. We thank Maria Ines Gonzalez Solari for reviewing the Spanish.