Coronavirus disease-2019 (COVID-19) is a global public health emergency with numerous clinical facets, including acute kidney injury and acute cerebrovascular disease. Further knowledge of its various pathogenic mechanisms is essential, including coagulation disorders. Monoclonal gammopathy is characterized by the overproduction of a monoclonal immunoglobulin caused by clonal proliferation. Using a postmortem study of ultrasound-guided percutaneous core biopsies, the aim of this report is to present our observations on the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection pathology associated with monoclonal gammopathy. The clinical presentation was acute renal failure. Pathological findings revealed kappa light chain cast nephropathy. SARS-CoV-2 immunohistochemistry was positive in some renal tubular cells. Another notable finding was the presence of a high density of alveolar megakaryocytes, which probably explained the final outcome (acute cerebrovascular disease). Immunohistochemical study for SARS-CoV-2 does not verify the pathogenic effect of the virus and thus its contribution to the acute kidney injury.
La enfermedad por coronavirus de 2019 (COVID-19) es una emergencia sanitaria pública global con numerosas facetas clínicas que incluyen enfermedad renal aguda y enfermedad cerebrovascular aguda. Es necesario un conocimiento adicional de su mecanismo patogénico. Los trastornos de coagulación están claramente incluidos en dichos mecanismos. La gammapatía monoclonal se caracteriza por la sobreproducción de inmunoglobulina monoclonal causada por proliferación clonal. Utilizando un estudio postmortem de biopsias percutáneas ecoguiadas, el objetivo de este informe es presentar nuestras observaciones sobre la patología del síndrome respiratorio agudo severo por infección de coronavirus 2 (SARS-CoV-2) con gammapatía monoclonal. La presentación clínica fue insuficiencia renal aguda. Los hallazgos patológicos revelaron nefropatía por cilindros de cadenas ligeras kappa. La inmunohistoquímica de SARS-CoV-2 fue positiva en ciertas células tubulares renales. La presencia de megacariocitos alveolares (alta densidad) fue un hallazgo notable, que explica probablemente el resultado final del paciente (enfermedad cerebrovascular aguda). El estudio inmunohistoquímico frente a SARS-CoV-2 no verifica el efecto patogénico del virus y, por tanto, su contribución a la nefropatía aguda.
Coronavirus disease-2019 (COVID-19) is a global public health emergency caused by the recently discovered coronavirus, SARS-CoV-2. It has been found to have numerous clinical facets.1 Although initially COVID-19 was described as a purely respiratory disease, it is now known that infected individuals can rapidly progress to a multiple organ dysfunction syndrome. Clinical manifestations include acute kidney injury (AKI)2 and acute cerebrovascular disease (ACVD).3 Additional knowledge of its pathogenic mechanisms is necessary, including coagulation disorders.1
Monoclonal gammopathy (MG) is characterized by the overproduction of a monoclonal immunoglobulin (MIg). Etiologies include hematologic malignancy, such as multiple myeloma (MM) and other non-malignant clonal proliferations. Clonal cells can secrete intact MIg or its fragments. The development of renal disease in this setting depends on the nephrotoxic potential of the secreted M protein.4
Autopsies may improve our understanding of numerous disease processes and ultrasound-guided minimally invasive autopsy (MIA-US) is an alternative to the standard postmortem procedure. This technique allows us to obtain tissue samples in cases in which standard autopsies cannot be performed due to a lack of autopsy rooms complying with biosecurity recommendations.5 Recent postmortem studies1 have offered explanations for some of the clinical alterations found in this disease, including coagulation disorders.
This study presents the clinical and histopathological characteristics revealed by ultrasound-guided percutaneous biopsies performed on a patient with MG, infected with SARS-CoV-2.
Case reportA 70-year-old, overweight woman, with a past medical history of hypertension, presented in the emergency department with malaise, generalized weakness, dysuria, and oliguria. Seven days prior to admission, she had been diagnosed with SARS-CoV-2 infection (she had tested positive for SARS-CoV-2 RNA by RT-PCR assay [reverse transcription polymerase chain reaction]) although she had no cough, dyspnea or fever. Upon admission, she tested positive for SARS-CoV-2 RNA by RT-PCR assay, was afebrile and hypotensive with a heart rate of 94 and oxyhemoglobin saturation on room air of 96%.
A physical examination revealed that she was dehydrated and drowsy. Further examination revealed hemoglobin of 10.9g/dL (11.8–15.8) and normal platelet count. Her serum creatinine was 7mg/dL (0.5–1), urea was 130mg/dL (11–49), potassium was 4.2mmol/L (3.4–5.5), total calcium was 10.3mg/dL (8.6–10.2) and she had an elevated D-dimer of 10500ng/mL (0–500).
A CT scan of the chest and abdomen revealed no findings consistent with COVID-19 and no evidence of urinary obstruction. Atheromatosis was observed in the aortoiliac territory.
Intravenous isotonic fluid therapy and prophylactic anticoagulation with enoxaparin were initiated and AKI diagnostic evaluation was performed. After a few days, the patient remained anuric. In the absence of volume depletion, treatment with loop diuretics was initiated with no improvement and renal replacement therapy was performed.
Four days following admission, the laboratory test results were obtained: urinary protein excretion of 1774mg/24h and urinary albumin excretion of 98mg/24h. All antibodies ANCA, anti-GMB, ANA, anti-dsDNA, were negative. Serum complements C3 and C4 were normal; tests for hepatitis B surface antigen and antibody to hepatitis C were all negative. Serum immunoglobulin (Ig) G 412mg/dL (725–1900 md/dL), (Ig) A 25mg/dL (50–340),(Ig) M 9mg/dL (45–280). Bence Jones protein kappa was observed in blood and urine, the serum free light chain kappa was 4830mg/dL (3.30–19), the serum kappa/lambda ratio was 287.5 (0.260–1.650). The SARS-CoV-2 RT-PCR assay remained positive.
Given the suspicion of MM, hematology assessment was requested. The patient remained in anuric renal failure and was on intermittent hemodialysis. Six days later, she suddenly presented dysarthria and lost consciousness, expiring a few minutes later. A brain stroke was suspected. A clinical autopsy was requested.
The patient underwent a MIA-US and several needle core biopsies, from 10 to 15mm in length, were obtained from the lungs, kidneys, liver, heart, skin, adipose tissue and skeletal muscle. The following histopathological findings were reported:
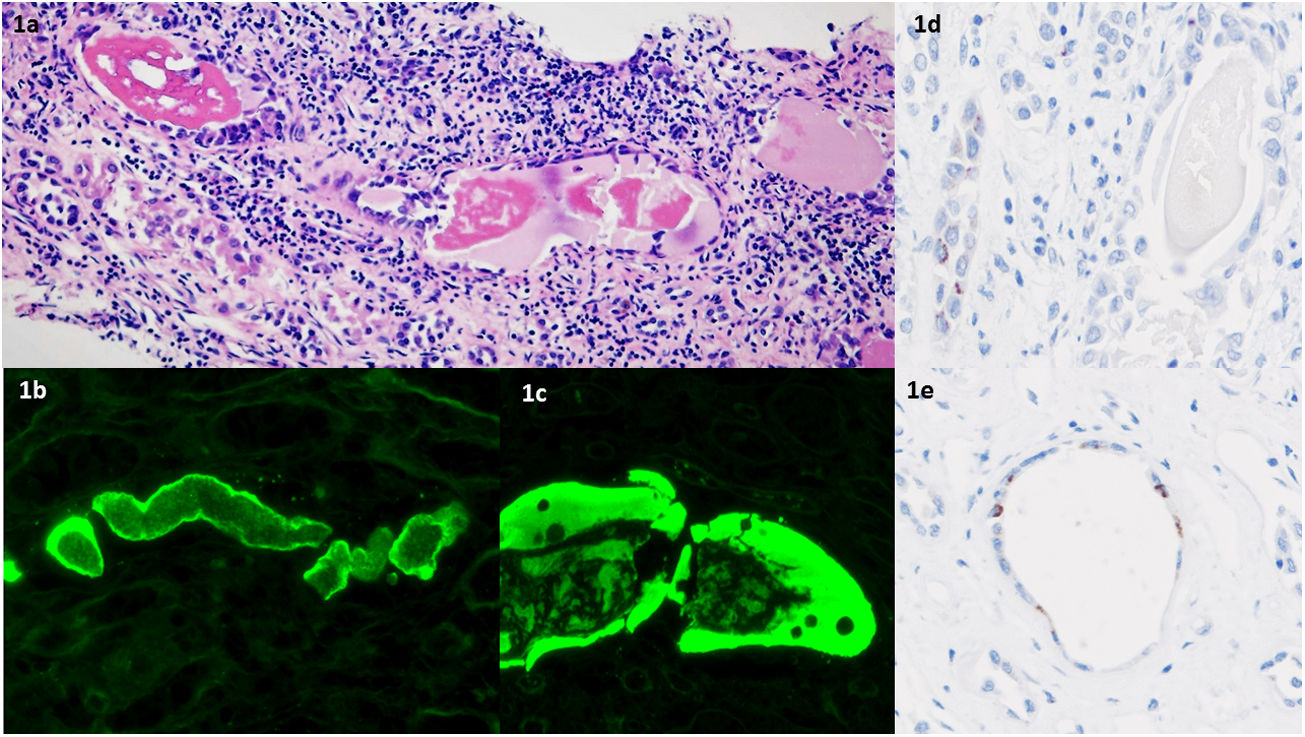
Kidney tissues: distal and collecting tubules showed a flattened and/or reactive epithelium; they were obstructed with a fractured hypereosinophilic, dense cast; a giant cell reaction was observed around some of the tubules as well as interstitial inflammation with macrophages and mononuclear cells (Fig. 1a). Proximal tubules did not showed casts. Glomeruli were not involved. Vessels did not reveal specific findings. Hemosiderin granules and pigmented casts were not observed in the tubular epithelium. Congo red stain was negative.
(a) Hematoxylin–eosin stain: hypereosinophilic casts in the distal nephron segments; interstitial inflammation with macrophages and mononuclear cells (H–E ×200). (b and c) Restricted light-chain immunofluorescence: Kappa-light chain immunofluorescence microscopy stains tubular casts. Lambda-light chain staining was negative (not shown). (d and e) Renal tubular epithelium positive for SARS-CoV-2 NP protein by immunohistochemistry (×400).
Immunofluorescence microscopy with anti-human kappa, lambda, IgG, IgM, IgA, C3, C1q, and fibrinogen antiserum revealed variable staining of intratubular immunoglobulin kappa-light chain casts (Fig. 1b and c). Lambda light chain, IgG, IgM, IgA, C3, C1q, and fibrinogen were negative. The diagnosis was kappa-light chain cast nephropathy,
Immunohistochemical (IHC) stain against SARS-CoV-2 NP protein in the kidney revealed cytoplasmic granular immunoreactivity in some of the distal tubular epithelial cells (Fig. 1d and e). IHC stain was negative in glomeruli and vessels. IHC stain against SARS-CoV-2 spike protein was similar to NP, although weaker.
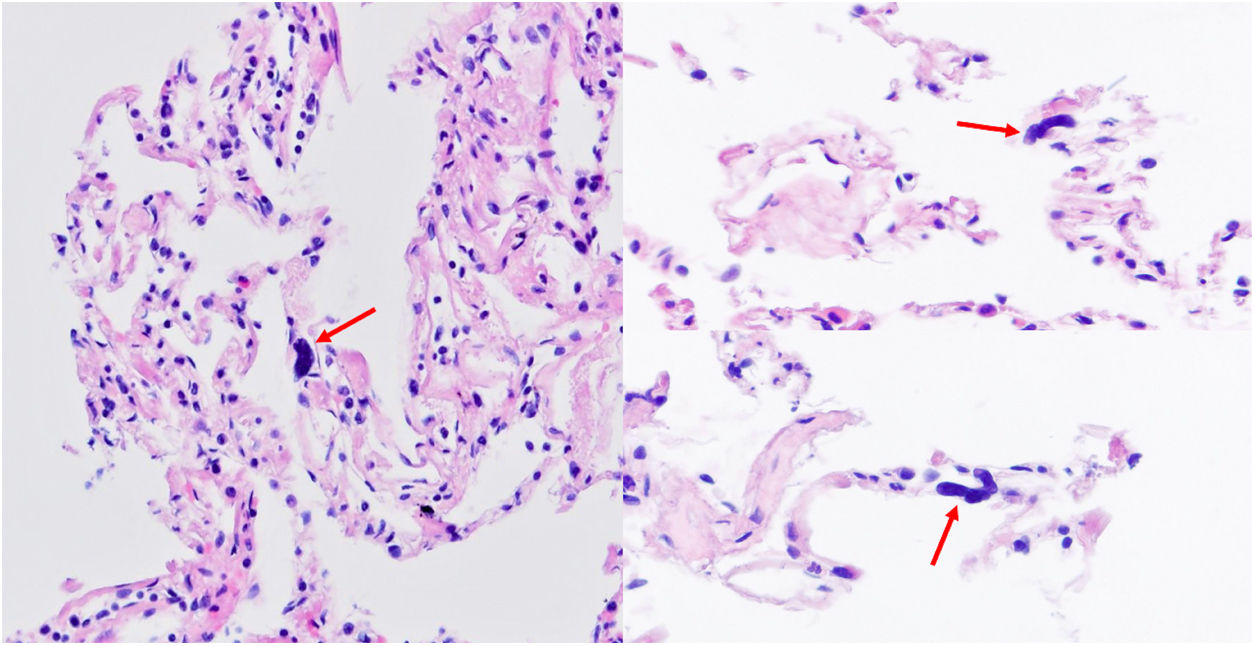
Lung tissues: there was no evidence of tissue damage. The main finding in lung tissue was the presence of various large cell nuclei (naked nuclei) morphologically consistent with megakaryocytes in alveolar walls (Fig. 2). SARS-CoV-2 IHC (spike protein and NP protein) was negative.
Liver tissue revealed mixed steatosis, Grade 1, and mild siderosis. SARS-CoV-2 IHC was not assessable (lipofuscin and hemosiderin granules limit assessment). No relevant findings were observed in the remaining tissues. SARS-CoV-2 IHC was negative in these tissues.
DiscussionWe present the histopathological findings associated with SARS-CoV-2 infection and its clinical presentation as AKI in a patient with recently diagnosed MG.
To date, little information is available on pathological findings and outcomes of MG patients with COVID-19, it only having been mentioned in one research letter.6
Postmortem examination is necessary to understand the physiopathology of illnesses and to contribute to future therapeutic decision-making. Given that our facilities lack an autopsy room that fulfills biosecurity recommendations, we performed ultrasound-guided percutaneous core biopsies. This is a minimally invasive technique offering rapid and precise responses to the study of the pathogenesis of new infectious agents.5
The patient's histological findings may be divided into two main categories:
- 1.
Findings attributed to the primary disease: light-chain cast nephropathy (LCCN).
- 2.
Pulmonary findings in deceased COVID-19 patients: several alveolar megakaryocytes.
Cast nephropathy is the predominant pattern of renal injury in MM. It has been rarely reported in patients suffering from other MGs. Rapidly progressive renal failure is the most frequent clinical presentation.4 Common precipitating factors include dehydration and infection,7 as occurred in our patient. AKI (with or without proteinuria) has been described in a variable percentage of COVID-19 patients. The mechanisms of renal injury during COVID-19 are difficult to study due to the interference of certain coexisting factors, such as polypharmacy, hypoxia, and cytokine storm.2 Our patient revealed no evidence of pulmonary or systemic disease related to COVID-19, so LCCN alone could explain the AKI. Nevertheless, we cannot determine if the virus contributed to the AKI in addition to the LCCN; its detection via immunohistochemical (IHC) technique is a potential means of demonstrating direct renal infection by SARS-CoV-2.8 However, solely the presence of viral particles does not demonstrate a direct cytopathic effect or a replicative potential.2
Finally, various megakaryocytes were observed in the randomly obtained small lung tissue extract (a high density), although fibrin thrombus and other lesions were not found. Although this finding is in agreement with previous reports,1 in our case, the cells were found in lung tissue having no pathology and in absence of a severe clinical case of COVID-19.
Various studies have demonstrated that the lungs are involved in the biogenesis of platelets9 and that these precursor cells (megakaryocytes) increase in number during infections. The bare nuclei suggest that their cytoplasm was consumed during platelet production.1 Roncati et al.1 were the first to use a postmortem study to describe the increase of megakaryocytes in the lungs and bone marrow of critical COVID-19 patients. Thier work offers a morphological study, revealing the existence of abnormal immunothrombosis in severe cases of COVID-19, with interleukin-6 (IL-6) being the key molecule in the coagulation disorders and the influx of cytokines in these cases.
Compared to healthy individuals, SARS-CoV-2 patients have increased serum concentrations of many proinflammatory mediators, including IL-1β, IL-2, IL-6, IL-7, IL-8, interferon (IFN)-γ-induced protein 10 (IP-10), granulocyte colony-stimulating factor (G-CFS), macrophage inflammatory protein (MIP)-1α, platelet-derived growth factor (PDGF), TNF-α, vascular endothelial growth factor (VEGF) among others. This cytokine storm is even more evident in individuals admitted to the intensive care unit, as demonstrated by greater increases in serum concentration of several of these pro-inflammatory mediators, which suggests a correlation with disease severity.10
As for the likely relation of MG to MM, there are various coagulation abnormalities in this malignancy, leading to a hypercoagulable state. This includes the secretion of growth factors and cytokines such as IL-6.11 This cytokine stimulates megakaryocytopoiesis and platelet production in bone marrow and the lungs1 and may sustain normal platelet counts despite bone marrow infiltration by plasma cells.12
Regarding our patient's final outcome, a study by Mao et al.3 highlighted the existence of ACVD as a notable COVID-19 complication. Furthermore, Oxley et al.13also found large-vessel stroke as a presenting feature of SARS-CoV-2 infection in young patients, some of whom presented no symptoms of COVID-19 and had no clinical histories predisposing them to strokes. These findings are similar to those in our case in which, although a severe case of COVID 19 was not present, a clinical pattern of ACVA was found.
Regarding the existing neuropathological studies, only a few of these studies6,14 confirm these clinical cases. Finally, a recent study15 has demonstrated for the first time that COVID-19 significantly alters platelet gene expression, triggering a robust platelet hyperreactivity.
Our patient died from a clinical case compatible with ACVD. The finding of a high density of megakaryocytes in the lung biopsy serves as morphological evidence of abnormal immunothrombosis and the patient's final outcome. However, cerebral tissue was not available for histological study, due to limitations of the MIA-US, and thus it was not possible to establish definitively the cause of death.
We acknowledge the limitations of a report of only a single case together with the present limited use of immunohistochemistry for SARS-CoV-2. Therefore, more extensive studies are needed to assess accurately the role of immunohistochemistry in COVID-19 diagnosis.
Conclusions- 1.
An increased number of megakaryocytes in the lungs of SARS CoV-2 patients is not always found within the context of severe lung damage. This increase may be observed in lungs that are free of lesions. These cells offer morphological evidence of abnormal immunothrombosis, explaining the patient's final clinical outcome, despite prophylactic anticoagulation; both the SARS CoV-2 infection and the neoplastic disease may be involved in its pathogeny.
- 2.
The evidence of SARS CoV-2 by IHC in renal tubular epithelium does not demonstrate a direct cytopathic effect or a replicative potential, therefore it is not possible to prove its pathogenic effect and its contribution to the AKI.
This research has not received specific aid from agencies from public or commercial sectors or non-profit entities.
Conflict of interestsThe authors declare that no conflicts of interest exist regarding the publication of this paper.
We wish to thank all of the healthcare workers from La Paz University Hospital for their extraordinary effort during this pandemic.