Spinal metastases (SM) account for 5–30% of patients with cancer, causing pain, deformity and/or neurological deficit. Postoperative complications are a concerning subject and wound-related complications (WRC) may delay adjuvant treatment. The objective of this study was to analyze the incidence of WRC in patients with SM that underwent surgical treatment as well as possible risk factors related to the occurrence of complications.
Materials and methodsPatients with SM operated between 2011 and 2021 were analyzed. Demographics characteristics, primary tumor, general and neurological status, Tokuhashi score, type of surgical treatment, surgical length, preoperative serum albumin and hemoglobin, pre and postoperative adjuvant treatment were analyzed. The incidence and risk factors of WRC – surgical site infection, hematoma, and/or dehiscence – at 90 days was evaluated. Patients were classified in two groups according to the absence/presence of WRC.
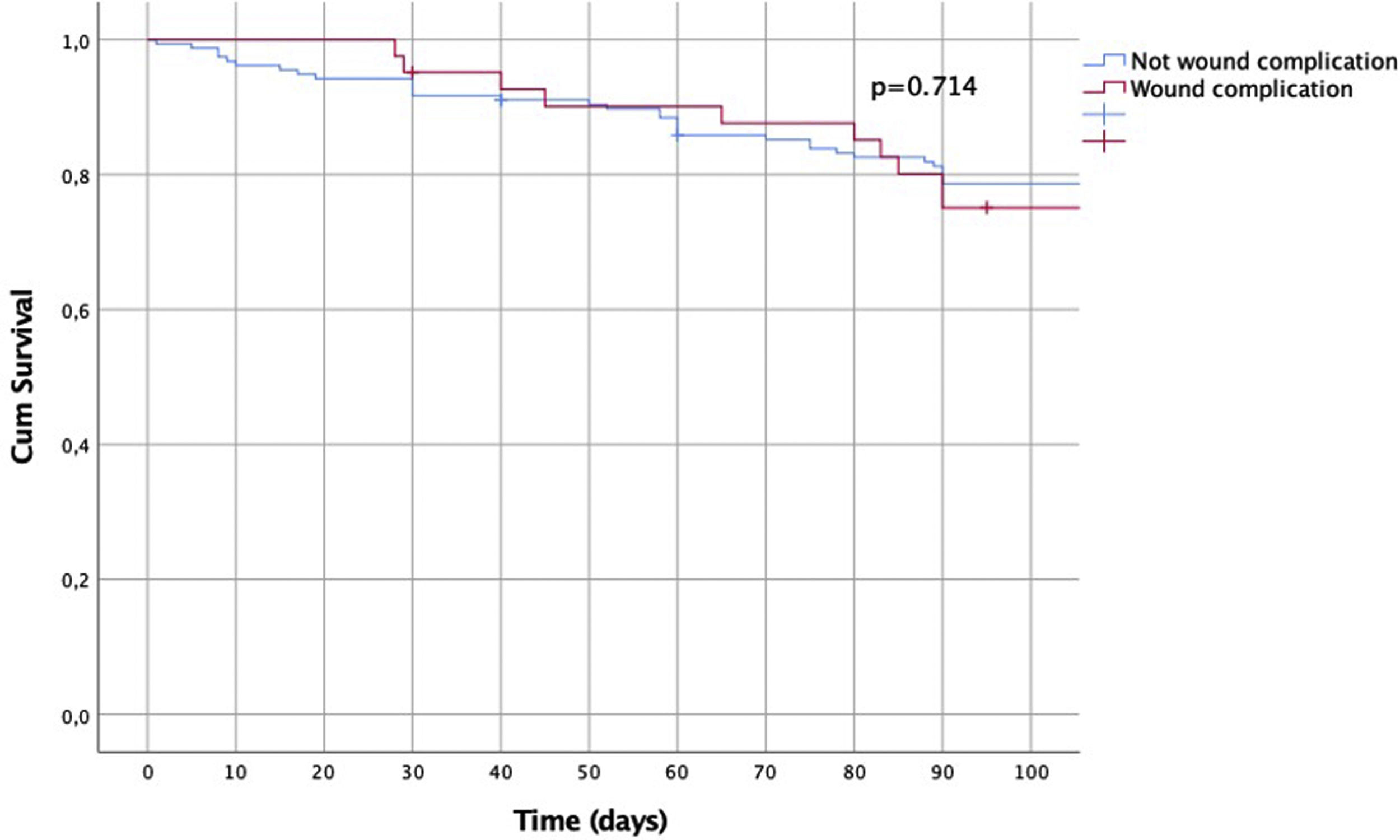
Results198 patients (121 males and 77 females) with an average age of 65 years (range 54–73 years) were analyzed. WRC were observed in 44 patients (22%). On multivariable analysis, significant predictors for developing WRC were low Tokuhashi score (OR=7.89, 95% CI=1.37–45.35, p=0.021), prostate cancer as primary tumor (6.73, 1.14–39.65, p=0.035), and preoperative serum albumin level ≤3.5g/dL (2.31, 1.02–5.22, p=0.044). There was no difference between groups on 90 days survival rate (p=0.714).
ConclusionsIn our series, the incidence of WRC was 22%, main risk factors for complications were low Tokuhashi score, lower preoperative serum albumin, and prostate cancer. Finally, short-term survival rate was not affected by the occurrence of WRC.
Las metástasis espinales (MV) pueden ocurrir en el 5-30% de los pacientes con cáncer, provocando dolor, deformidad y/o déficit neurológico. Las complicaciones postoperatorias son un motivo de preocupación y las complicaciones relacionadas con la herida (CRH) pueden retrasar el inicio del tratamiento adyuvante. El objetivo de este estudio fue analizar la incidencia de CRH en pacientes con MV sometidos a tratamiento quirúrgico y evaluar los posibles factores de riesgo relacionados con estas complicaciones.
Materiales y métodosSe analizaron pacientes operados por MV entre 2011 y 2021. Se analizaron características demográficas, tumor primario, estado general y neurológico, score de Tokuhashi, tipo de tratamiento quirúrgico, duración de la cirugía, albúmina sérica y hemoglobina preoperatorias, tratamiento adyuvante pre- y postoperatorio. Se evaluó la incidencia y los factores de riesgo de la CRH (infección del sitio quirúrgico, hematoma y/o dehiscencia) a los 90 días. Los pacientes se clasificaron en dos grupos según la ausencia/presencia de CRH.
ResultadosSe analizaron 198 pacientes (121 hombres y 77 mujeres) con una edad promedio de 65 años (rango 54-73 años). Se observaron CRH en 44 pacientes (22%). En el análisis multivariado, los predictores significativos para el desarrollo de CRH fueron un score de Tokuhashi bajo (OR=7,89; IC del 95%=1,37-45,35; p=0,021), cáncer de próstata como tumor primario (OR=6,73; IC del 95%=1,14-39,65; p=0,035) y albúmina sérica preoperatoria≤3,5g/dL (OR=2.31; IC del 95%=1,02-5,22; p=0,044). No hubo diferencias entre los grupos en la supervivencia a los 90 días (p=0,714).
ConclusionesEn nuestra serie, la incidencia de CRH fue del 22%, los principales factores de riesgo para su aparición fueron el score de Tokuhashi bajo, la albúmina sérica preoperatoria más baja y el cáncer de próstata. Finalmente, la tasa de supervivencia a corto plazo no se vio afectada por la ocurrencia de CRH.
Spinal metastases are present in 5–30% of patients with cancer at the time of diagnosis, being a common cause of back pain, deformity, and weakness in this selected group of patients.1 Nowadays, an increased survival rate has been observed in different oncologic subgroups, probably related to early diagnosis as well as medical and surgical treatment improvements. An increase in the prevalence of oncologic conditions and associated comorbidities requiring surgical treatment is expected along with growing life expectancy and population aging.2,3 Despite surgical innovations, undesirable adverse events continue to occur in highly vulnerable patients.4,5
Surgical treatment of patients with SM can be associated with higher risk of local complications such as surgical site infection and wound dehiscence,6,7 those events are related to the high prevalence of associated comorbidities, poor nutritional status, and use of adjuvant therapies.8 While intense efforts have been made to analyze postoperative mortality rates, little emphasis has been put on immediate postoperative morbidity rates.9,10
Wound-related complications are associated with longer hospital length of stay, higher re-operation rates and delay in starting the adjuvant therapy. The aim of this study is to report the incidence of wound-related postoperative complications in patients with metastatic spinal disease and analyze related risk factors as well as their impact on the short-term survival rate.
Material and methodsStudy design and data collectionAfter the approval of the Institutional Review Board (Protocol number 5989, IRB00010193), a retrospective reviewed study of patients with metastatic vertebral disease who underwent surgical treatment in a single institution between January 2011 and February 2021 was conducted. Patients with lymphoproliferative lesions (n=22) and primary tumors (n=13) were excluded. Data analysis included: baseline patient's demographics parameters, type of primary tumor, neurological status measured by Frankel classification (classified as neurological compromise Frankel A–D and no neurological compromise Frankel E), Tokuhashi score (classified in three groups: Tokuhashi group 1: score 0–8, Tokuhashi group 2: score 9–11 and Tokuhashi group 3: score 12–15), type of surgical treatment: patients were classified as decompression alone versus instrumentation with/without decompression, length of surgery (minutes), pre and postoperative adjuvant treatment – radiotherapy and/or chemotherapy. Additionally, preoperative analytics, such as serum albumin levels (g/dL), hematocrit (%) and hemoglobin (g/dL) were studied.
Wound-related complicationsWound related complications were defined as a deviation of the postoperative wound evolution that required surgical intervention, including wound hematoma requiring drainage, dehiscence and deep surgical site infection needing closure and/or surgical debridement within the first 90 postoperative days. Non-surgically treated superficial wound infections were excluded. Patients were classified in two cohorts according to the absence – not wound related-complications cohort (NWRC) – or the presence of wound-related complications cohort (WRC). The postoperative mortality rate within the first 90 days was compared between cohorts.
Statistical analysisFor each continuous variable, the Shapiro–Wilk test was performed to analyze the normal distribution of quantitative variables. Among the variables of interest, age and hematocrit were found to have a normal distribution and therefore, were dichotomized using the media as the cutoff value — e.g., with 62 years for age (≤62 or >62), and with 35% for hematocrit (≤35 or >35); preoperative hemoglobin, preoperative serum albumin level, and length of surgery were found to have a non-normal distribution and therefore, were dichotomized using the median as the cutoff value — e.g., with 12g/dL for hemoglobin (≤12 or >12), with 3.5g/dL for albumin (≤3.5 or >3.5), and with 180min for length of surgery (≤180 or >180). For inter-group comparisons of categorical variables, Pearson χ2 analysis and Fisher's Exact test were employed, as appropriate based upon cell numbers. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated for each variable, in terms of its impact upon the presence of WRC (yes/no). Multivariable analysis was performed by binary logistic regression analysis. For each model, independent variables were introduced into the model by forward entry and retained in the final model when p<0.1. The analysis of survival was performed using Kaplan–Meier method and comparison between groups using the log-rank test. The statistical software program SPSS version 25 for Mac (IBM, Armonk, NY) was employed. Any two-tailed p value <0.05 was considered statistically significant.
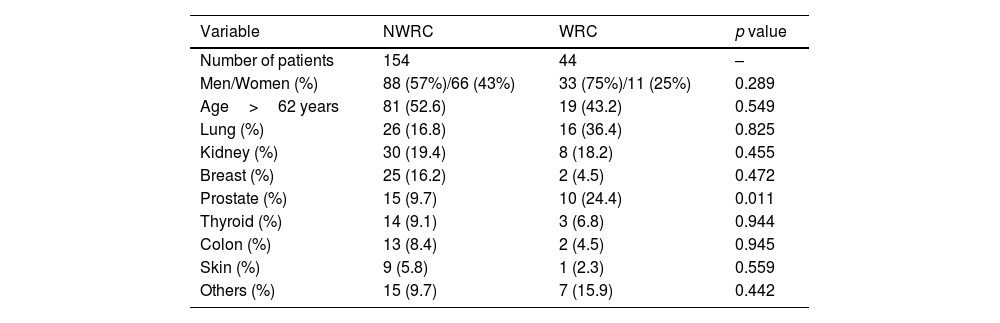
Results198 patients (121 males and 77 females) were included with an average age of 62.1±13.01 years. 44 patients (22.2%) were enrolled in the WRC cohort (Table 1). The most common primary tumors were Lung (20%), kidney (18.6%) and Breast (13%).
Demographic characteristics of the patient population according to the groups.
| Variable | NWRC | WRC | p value |
|---|---|---|---|
| Number of patients | 154 | 44 | – |
| Men/Women (%) | 88 (57%)/66 (43%) | 33 (75%)/11 (25%) | 0.289 |
| Age>62 years | 81 (52.6) | 19 (43.2) | 0.549 |
| Lung (%) | 26 (16.8) | 16 (36.4) | 0.825 |
| Kidney (%) | 30 (19.4) | 8 (18.2) | 0.455 |
| Breast (%) | 25 (16.2) | 2 (4.5) | 0.472 |
| Prostate (%) | 15 (9.7) | 10 (24.4) | 0.011 |
| Thyroid (%) | 14 (9.1) | 3 (6.8) | 0.944 |
| Colon (%) | 13 (8.4) | 2 (4.5) | 0.945 |
| Skin (%) | 9 (5.8) | 1 (2.3) | 0.559 |
| Others (%) | 15 (9.7) | 7 (15.9) | 0.442 |
Abbreviations: NWRC, not wound-related complications; WRC, wound-related complications.
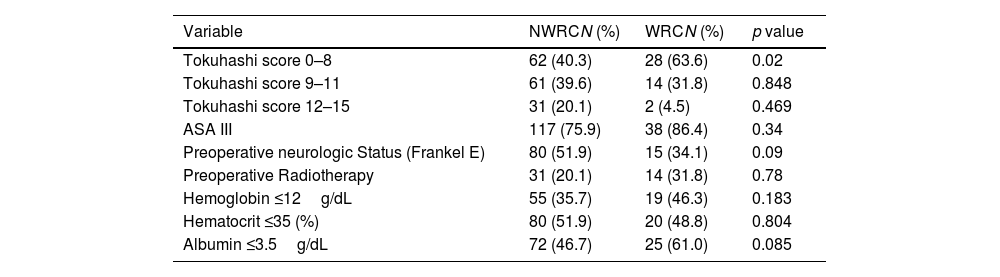
From 44 patients, 27 suffered deep infection (61.3.%), followed by 9 wound dehiscence (20.4%) and 8 wound hematomas (18.2%). Patients in the wound-related complication cohort had significantly lower Tokuhashi score of 7.34±2.5 (p<0.01), higher prevalence of prostate cancer and lower preoperative albumin (Table 2). Regarding postoperative characteristics, the prevalence of postoperative Frankel E neurological status was significantly lower in the WRC group (Table 3).
Clinical Characteristics and preoperative variables.
| Variable | NWRCN (%) | WRCN (%) | p value |
|---|---|---|---|
| Tokuhashi score 0–8 | 62 (40.3) | 28 (63.6) | 0.02 |
| Tokuhashi score 9–11 | 61 (39.6) | 14 (31.8) | 0.848 |
| Tokuhashi score 12–15 | 31 (20.1) | 2 (4.5) | 0.469 |
| ASA III | 117 (75.9) | 38 (86.4) | 0.34 |
| Preoperative neurologic Status (Frankel E) | 80 (51.9) | 15 (34.1) | 0.09 |
| Preoperative Radiotherapy | 31 (20.1) | 14 (31.8) | 0.78 |
| Hemoglobin ≤12g/dL | 55 (35.7) | 19 (46.3) | 0.183 |
| Hematocrit ≤35 (%) | 80 (51.9) | 20 (48.8) | 0.804 |
| Albumin ≤3.5g/dL | 72 (46.7) | 25 (61.0) | 0.085 |
Operative and postoperative variables.
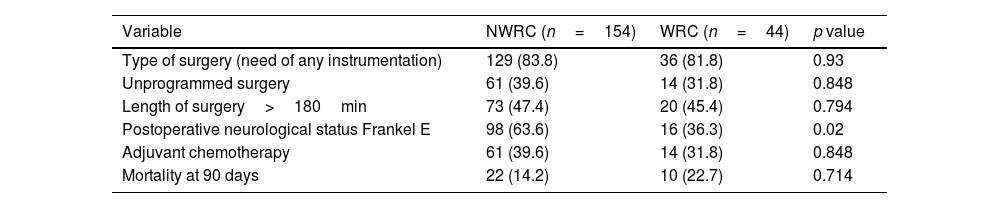
| Variable | NWRC (n=154) | WRC (n=44) | p value |
|---|---|---|---|
| Type of surgery (need of any instrumentation) | 129 (83.8) | 36 (81.8) | 0.93 |
| Unprogrammed surgery | 61 (39.6) | 14 (31.8) | 0.848 |
| Length of surgery>180min | 73 (47.4) | 20 (45.4) | 0.794 |
| Postoperative neurological status Frankel E | 98 (63.6) | 16 (36.3) | 0.02 |
| Adjuvant chemotherapy | 61 (39.6) | 14 (31.8) | 0.848 |
| Mortality at 90 days | 22 (14.2) | 10 (22.7) | 0.714 |
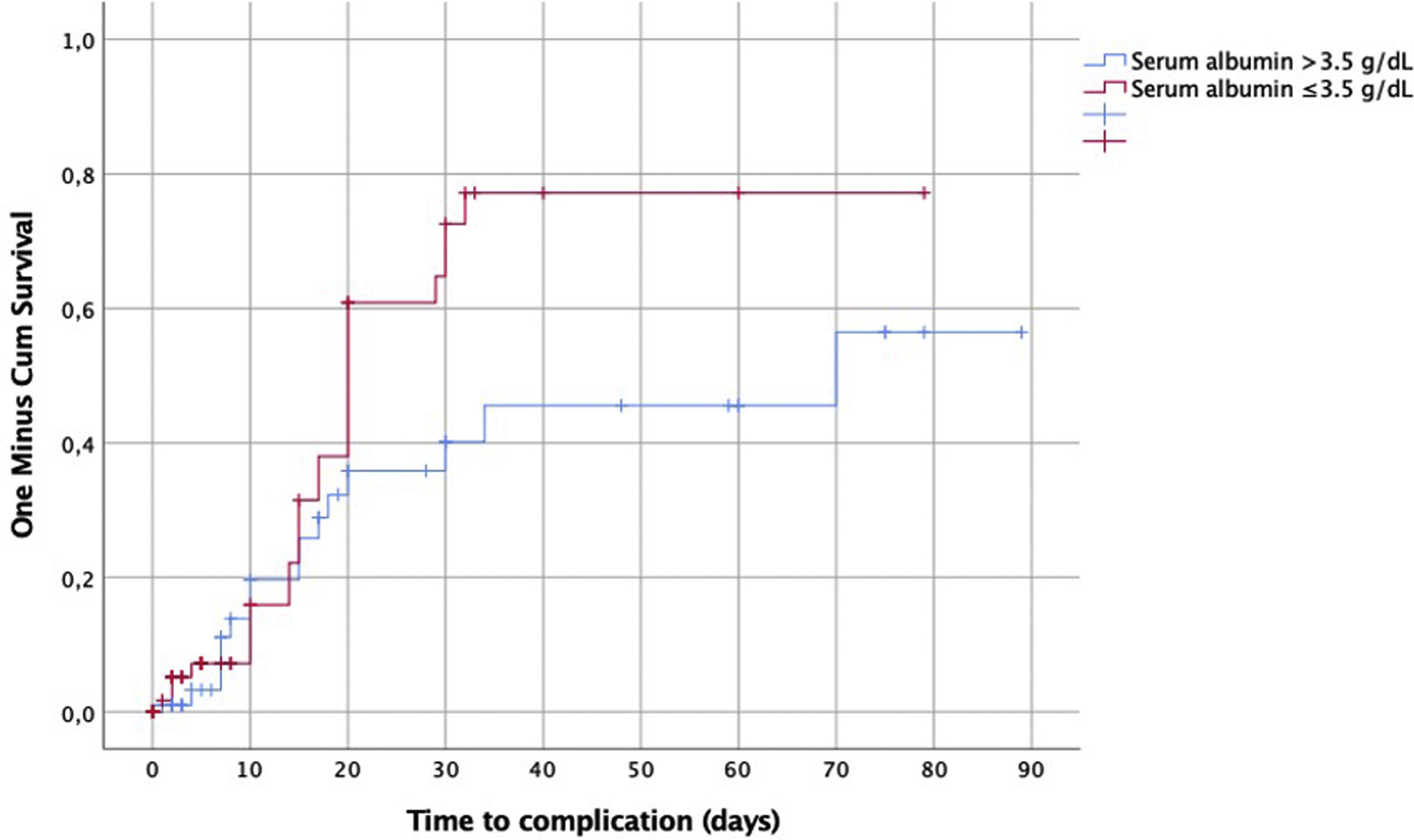
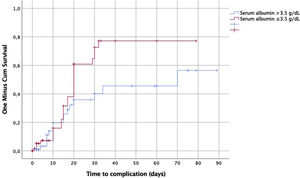
The comparison between serum albumin level ≤3.5 versus >3.5g/dL to the time of the WRC showed a significant rise of occurrence at 20th postoperative day in the group ≤3.5g/dL (p=0.06) (Fig. 1).
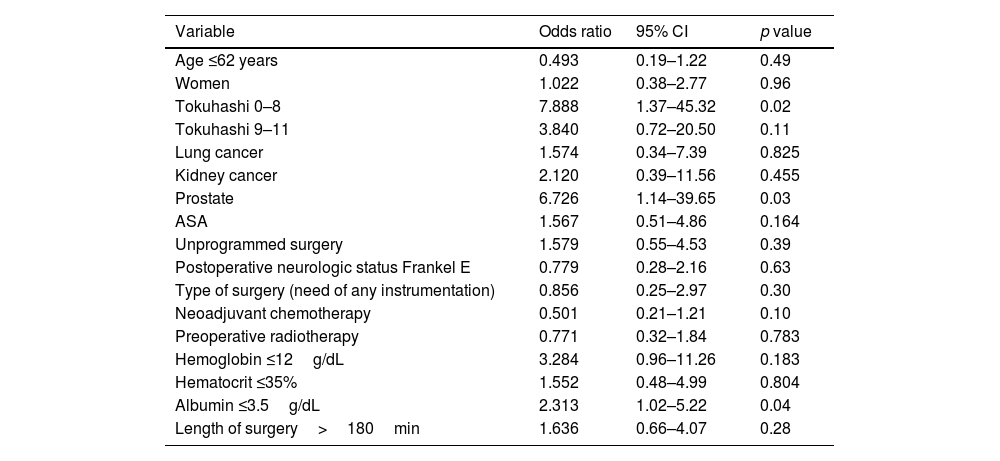
Multivariate analysisRegarding risk factors related to the occurrence of wound related complications, multivariate analysis showed that lower Tokuhashi Score (<8) OR: 7.88 (95% CI 1.3–45.3) (p 0.0021), primary prostate cancer OR 6.72 (95% CI 1.14–39.65) (p=0.03) and hypoalbuminemia (<3.5mg/dL) OR: 2.31 (95% CI 1.02–5.22) (p 0.044) were statistically significant, Table 4.
Multivariate analysis for developing wound-related complications.
| Variable | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Age ≤62 years | 0.493 | 0.19–1.22 | 0.49 |
| Women | 1.022 | 0.38–2.77 | 0.96 |
| Tokuhashi 0–8 | 7.888 | 1.37–45.32 | 0.02 |
| Tokuhashi 9–11 | 3.840 | 0.72–20.50 | 0.11 |
| Lung cancer | 1.574 | 0.34–7.39 | 0.825 |
| Kidney cancer | 2.120 | 0.39–11.56 | 0.455 |
| Prostate | 6.726 | 1.14–39.65 | 0.03 |
| ASA | 1.567 | 0.51–4.86 | 0.164 |
| Unprogrammed surgery | 1.579 | 0.55–4.53 | 0.39 |
| Postoperative neurologic status Frankel E | 0.779 | 0.28–2.16 | 0.63 |
| Type of surgery (need of any instrumentation) | 0.856 | 0.25–2.97 | 0.30 |
| Neoadjuvant chemotherapy | 0.501 | 0.21–1.21 | 0.10 |
| Preoperative radiotherapy | 0.771 | 0.32–1.84 | 0.783 |
| Hemoglobin ≤12g/dL | 3.284 | 0.96–11.26 | 0.183 |
| Hematocrit ≤35% | 1.552 | 0.48–4.99 | 0.804 |
| Albumin ≤3.5g/dL | 2.313 | 1.02–5.22 | 0.04 |
| Length of surgery>180min | 1.636 | 0.66–4.07 | 0.28 |
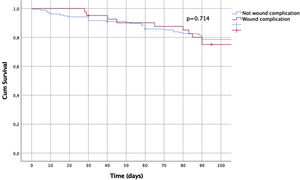
Survival rates at 90 days of follow-up between did not show a statistically significant difference between the groups (p=0.714) (Fig. 2).
DiscussionPostoperative wound-related complications in patients with spinal metastasis are associated with higher morbidity, hospital length of stay and may delay postoperative oncologic treatment affecting quality of life and mortality rate.11,12 In our study, we found that 22% of patients with spinal metastasis who underwent spinal surgery suffered a wound-related complication that required re-operation (dehiscence, hematoma or surgical site infection). Moreover, patients with lower survival rate (based on Tokuhashi score), lower preoperative albumin level and prostate cancer were more likely to develop wound-related complications.
Hypoalbuminemia is a known independent risk factor for developing spinal wound complications, increased hospital length of stay and readmission,13–15 this is probably associated with poor nutritional status, comorbidities and inflammation that affect normal wound healing mechanisms.16 He et al.17 showed significant association between lower albumin levels and wound dehiscence in 554 patients that underwent single-level posterior lumbar fusion surgery. In patients with spinal metastasis, the role of albumin levels appears to be also related to higher mortality.18 In our study, we observed that lower preoperative albumin levels were associated with higher incidence of wound-related complications. We observed a substantial increment of wound-related complications in patients with lower albumin by the end of the third week, this finding has been highlighted in other studies.3,19–21
The role of Tokuhashi score in predicting survival has been extensively studied in the literature2,22–24 with an overall acceptable survival prediction. This study analyzes the role of Tokuhashi score in predicting wound related complication, showing that a significantly higher complication rate was related to those patients with a score of 0–8 (survival rate of less than 6 months). Contrasting the available evidence,25,26 this study found that prostate cancer, as primary tumor, was associated with higher WRC regardless the history of preoperative radiotherapy.
Even though the presence of these type of complications can eventually lead to a sepsis and death,27,28 the occurrence of wound-related complication and subsequent reoperation was not associated with higher mortality at 90 days in our series. This data demonstrate that short-term mortality rate is not affected by these complications and the need of further procedures.
Our study has some limitations. First, this is a retrospective case series analysis level IV evidence study. Nevertheless, we consider this series interesting due to the number of patients included and the short follow-up related to the primary outcome that could influence the lower loss of patients during the 90-days period making our results more reliable. Another limitation is related to the amount of radiotherapy and/or type of chemotherapy in our series which were considered as a binary variable (presence/absence) without specification regarding time, dose and type of treatments, these variables are known factors that can affect wound healing.26 Regardless this matter, this study showed no significant influence on the occurrence of WRC, perhaps related to a type II error (false negative error), this probably could be addressed with a large series or multicenteric analysis.
ConclusionsThe incidence of wound-related complications after surgical treatment of spinal metastasis was 22%. The main risk factors to develop wound-related complications in our study were low Tokuhashi score, prostate cancer as primary tumor and low preoperative serum albumin level. The occurrence of WRC was not associated with a lower survival rate. These factors should be considered at the time of surgical decision-making.
Level of evidenceLevel of evidence IV.
Conflict of interestThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.