Surgical treatment of clinically negative neck in maxillary squamous cell carcinoma (SCC) of the upper jaw is controversial. The purpose of this study was to define the incidence of cervical metastasis and to assess if elective neck dissection is justified when the neck is not primarily affected.
MethodsWe retrospectively reviewed 20 patients treated of SCC of the maxillary alveolus and hard palate between 2005 and 2012.
ResultsSix (30%) patients presented with cervical lymph metastasis at initial diagnosis. Two of the14 patients who initially had no signs of metastasis in the neck developed cervical metastasis during follow-up and another patient with cervical metastasis at diagnosis developed contralateral cervical metastasis. All the patients with cervical metastasis (45%) were pT3/T4 SCC. Cervical metastasis developed at a mean of 11.6 months.
ConclusionsDespite this study being limited by its retrospective nature and the sample size, based on our findings and on an extensive review of the literature, we may conclude that cervical metastasis from maxillary alveolus and hard palate SCC appears most frequently in pT3/T4 tumors. Therefore, we find elective neck dissection appropriate for patients with pT3/T4 SCC of the upper jaw.
El tratamiento quirúrgico del cuello clínicamente negativo en el carcinoma de células escamosas (CCE) del maxilar superior es controvertido. El objetivo de este estudio es mostrar la incidencia de metástasis cervical y analizar si la disección cervical electiva está justificada cuando el cuello no está afectado de inicio.
MétodosRevisamos retrospectivamente 20 pacientes tratados por CCE de paladar duro y reborde alveolar superior entre 2005 y 2012.
ResultadosSeis (30%) pacientes presentaron metástasis cervical de inicio. Dos de los 14 pacientes que inicialmente no tuvieron signos de metástasis cervical la desarrollaron durante el seguimiento, y otro paciente con metástasis cervical al inicio desarrolló una metástasis cervical contralateral. Todos los pacientes con metástasis cervical (45%) fueron pT3/T4 CCE. El tiempo medio de aparición de metástasis cervical fue de 11,6 meses.
ConclusionesA pesar de las limitaciones de este estudio (naturaleza retrospectiva, limitado número de pacientes), y tras analizar los resultados obtenidos y revisar la literatura, podemos concluir que la metástasis cervical de CCE de maxilar superior aparece con mayor incidencia en tumores pT3/T4. Por lo tanto, creemos conveniente realizar disección cervical electiva en pacientes con CCE T3/T4 de maxilar superior.
Squamous cell carcinoma (SCC) is the most common malignant type of carcinoma within the oral cavity. Maxillary and mandibular gingivae are filled with lymph systems that cross the midline. In the same way, mucosa of the hard and soft palate is streaked by a dense superficial system of lymph vessels. It is well documented that SCC with a mucosal element has potential risk to metastasize to cervical lymph nodes, especially in cervical levels I and II. The involvement of regional lymph nodes depends on factors such as site, tumor size and several histological features of the primary tumor, as previously reported.1–3
Maxillary SCC is less frequent than SCC from other oral sites such as tongue, floor of mouth or retromolar region. Many studies have evaluated the need for elective neck dissection for these intraoral common sites when there is no clinical or radiological suspicious of lymphadenopathy. Controversies remain regarding the strategy of treatment for patients with maxillary SCC, including indications for unilateral or bilateral elective neck dissection and postoperative adjuvant treatment. Only a few authors4–13 have focused on the management of the neck in SCC of the maxillary gingiva, maxillary alveolus and hard palate.
In general, tumors with cervical lymph node metastasis are associated with increased risk of treatment failures and recurrences. In recent studies,5,10 it has been proven that a high rate of occult cervical metastasis in SCC of the maxilla has been found and elective neck dissection in these patients was recommended in order to reduce recurrences.
The aim of this retrospective study was to determine the rate of cervical lymph node metastasis from the upper maxillary alveolus and hard palate SCC. We also wondered if elective neck dissection should be considered for SCC of the maxillary alveolus and hard palate when the neck is not primarily affected.
Materials and methodsA retrospective study of medical records was carried out using database of oncological patients of the Department of oral and maxillofacial surgery. Twenty patients treated of maxillary SCC involving the alveolus and hard palate between 2005 and 2012 were included in this study. All of these patients received a complete clinical head and neck examination, including fiberscope examination. A biopsy of the surgical specimen was performed before surgery for diagnostic purposes. Magnetic resonance imaging (MRI) and/or computed tomography (CT) scan was performed before surgery, in order to assess tumor radiologic features, tumor extension and also involvement of neighboring structures, if present.
Inclusion criteriaTwenty patients presenting SCC of the maxilla met the following inclusion criteria:
- 1.
Tumors diagnosed as SCC at the examination of pathological features of the surgical specimens obtained by biopsy.
- 2.
Tumors located at maxillary gingiva, maxillary alveolus and hard palate.
- 3.
Tumors that were not originated in paranasal sinuses or the nasal cavity shown by the examination from radiologic tests. Tumors involving these sites and other malignancies of the maxilla were excluded from this study.
Surgery consisted on resection of the primary tumor with at least a 1-cm margin around the lesion. All patients with possible lymph neck node metastasis from the physical and radiologic examination were considered clinically positive neck (cN+), and were subsequently submitted for a modified type III radical neck dissection of the involved side. If no positive cervical lymph nodes were pre-operatively demonstrated, cervical dissection was not performed. Patients with tumors that cross the midline were operated, performing cervical neck dissection when neck was clinically or radiologically positive.
Local-regional post-operative RT was also administered if at least one of the following criteria was present: close (1–5mm) or involved (<1mm) surgical margins, vascular invasion, perineural infiltration, bone or cartilage involvement and/or pathologic lymph neck node involvement (pN+) after histologic examination of the surgical specimen.
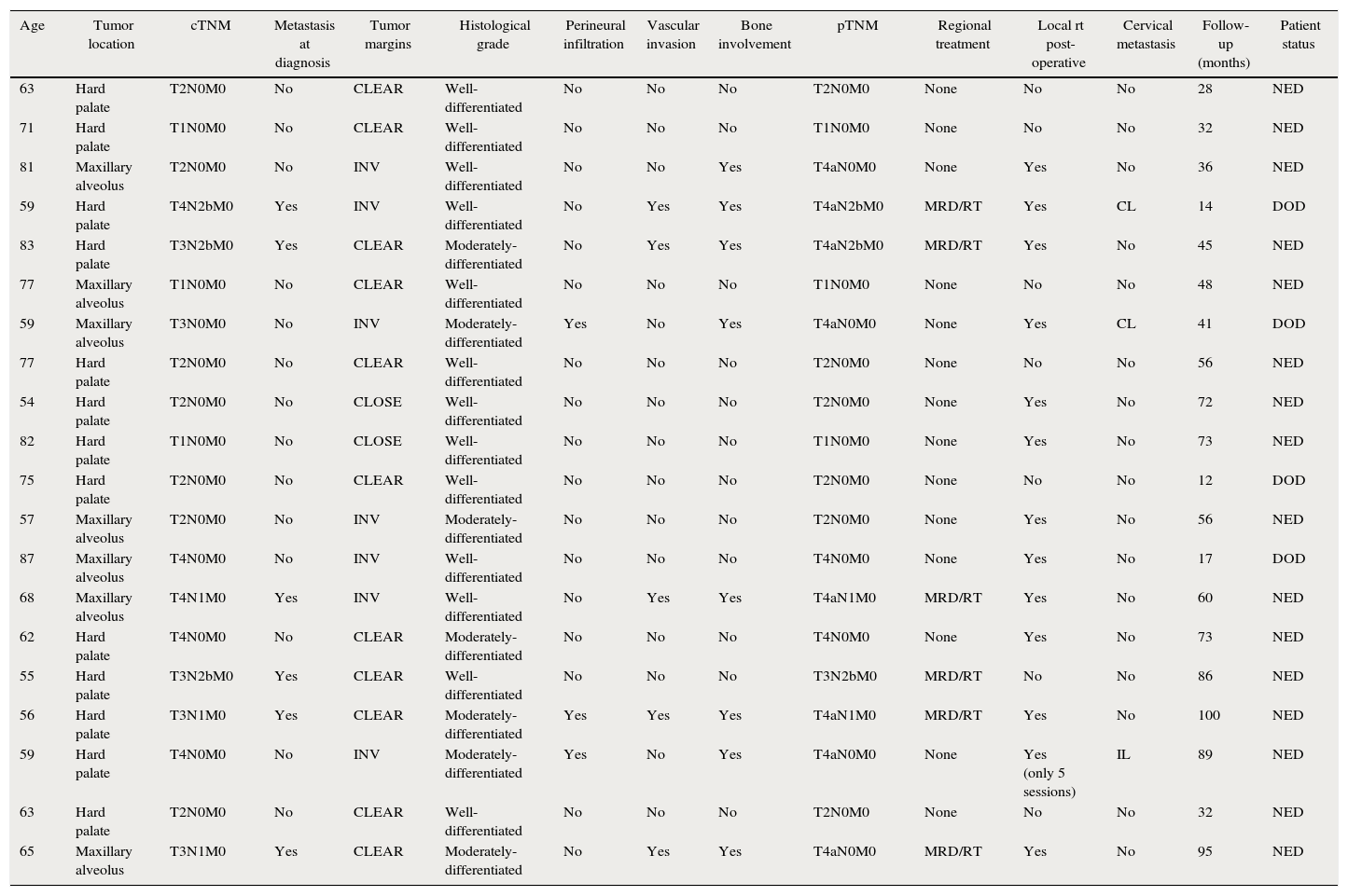
Registered treatment features (Table 1) were: (1) lymph neck node extension at diagnosis; (2) type of surgical treatment; and (3) administration of post-operative radiotherapy.
Patient's data (maxillary alveolus and hard palate squamous cell carcinoma).
| Age | Tumor location | cTNM | Metastasis at diagnosis | Tumor margins | Histological grade | Perineural infiltration | Vascular invasion | Bone involvement | pTNM | Regional treatment | Local rt post-operative | Cervical metastasis | Follow-up (months) | Patient status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | Hard palate | T2N0M0 | No | CLEAR | Well-differentiated | No | No | No | T2N0M0 | None | No | No | 28 | NED |
| 71 | Hard palate | T1N0M0 | No | CLEAR | Well-differentiated | No | No | No | T1N0M0 | None | No | No | 32 | NED |
| 81 | Maxillary alveolus | T2N0M0 | No | INV | Well-differentiated | No | No | Yes | T4aN0M0 | None | Yes | No | 36 | NED |
| 59 | Hard palate | T4N2bM0 | Yes | INV | Well-differentiated | No | Yes | Yes | T4aN2bM0 | MRD/RT | Yes | CL | 14 | DOD |
| 83 | Hard palate | T3N2bM0 | Yes | CLEAR | Moderately-differentiated | No | Yes | Yes | T4aN2bM0 | MRD/RT | Yes | No | 45 | NED |
| 77 | Maxillary alveolus | T1N0M0 | No | CLEAR | Well-differentiated | No | No | No | T1N0M0 | None | No | No | 48 | NED |
| 59 | Maxillary alveolus | T3N0M0 | No | INV | Moderately-differentiated | Yes | No | Yes | T4aN0M0 | None | Yes | CL | 41 | DOD |
| 77 | Hard palate | T2N0M0 | No | CLEAR | Well-differentiated | No | No | No | T2N0M0 | None | No | No | 56 | NED |
| 54 | Hard palate | T2N0M0 | No | CLOSE | Well-differentiated | No | No | No | T2N0M0 | None | Yes | No | 72 | NED |
| 82 | Hard palate | T1N0M0 | No | CLOSE | Well-differentiated | No | No | No | T1N0M0 | None | Yes | No | 73 | NED |
| 75 | Hard palate | T2N0M0 | No | CLEAR | Well-differentiated | No | No | No | T2N0M0 | None | No | No | 12 | DOD |
| 57 | Maxillary alveolus | T2N0M0 | No | INV | Moderately-differentiated | No | No | No | T2N0M0 | None | Yes | No | 56 | NED |
| 87 | Maxillary alveolus | T4N0M0 | No | INV | Well-differentiated | No | No | No | T4N0M0 | None | Yes | No | 17 | DOD |
| 68 | Maxillary alveolus | T4N1M0 | Yes | INV | Well-differentiated | No | Yes | Yes | T4aN1M0 | MRD/RT | Yes | No | 60 | NED |
| 62 | Hard palate | T4N0M0 | No | CLEAR | Moderately-differentiated | No | No | No | T4N0M0 | None | Yes | No | 73 | NED |
| 55 | Hard palate | T3N2bM0 | Yes | CLEAR | Well-differentiated | No | No | No | T3N2bM0 | MRD/RT | No | No | 86 | NED |
| 56 | Hard palate | T3N1M0 | Yes | CLEAR | Moderately-differentiated | Yes | Yes | Yes | T4aN1M0 | MRD/RT | Yes | No | 100 | NED |
| 59 | Hard palate | T4N0M0 | No | INV | Moderately-differentiated | Yes | No | Yes | T4aN0M0 | None | Yes (only 5 sessions) | IL | 89 | NED |
| 63 | Hard palate | T2N0M0 | No | CLEAR | Well-differentiated | No | No | No | T2N0M0 | None | No | No | 32 | NED |
| 65 | Maxillary alveolus | T3N1M0 | Yes | CLEAR | Moderately-differentiated | No | Yes | Yes | T4aN0M0 | MRD/RT | Yes | No | 95 | NED |
Abbreviations: INV, involvement; MRD, modified radical neck dissection; RT, radiotherapy. NED, no evidence of disease; DOD, died of disease; CL, contralateral; IL, ipsilateral.
All the surgical specimens were analyzed and revised specifically for this study by the Department of Pathology of our Institution. A complete supply of histologic information was included in our records (Table 1), such as: (1) pathological TNM staging (pTNM); (2) tumor grading; (3) tumor margins: free (>5mm), close (1–5mm); involved (<1mm) bone involvement: (4) vascular invasion; and (5) perineural infiltration.
Follow-upAll patients underwent a CT-scan at 6 months and at one year after surgery as a control for regional recurrence or cervical metastasis. As long as regional recurrence was suspected, a cervicofacial CT-scan with or without positron-emission-tomography (PET)-CT scan and a cytological study using fine-needle aspiration (FNA) of the cervical mass were performed.
Registered follow-up features were: (1) presence of regional recurrence; and (2) clinical outcome at the end of the follow-up period.
This study was approved by the Hospital Ethical Committee and by the Institutional Human Studies (IRB) Committee.
Data analysisData analysis was performed by using SPSS® software for Windows (version 21.0; IBM®, Chicago, IL). The level of statistical significance was set at 0.5. The descriptive statistical analysis was based on the mean and standard deviation for continuous variables, whereas the frequency and percentage were used for categorical variables.
ResultsThe study group included 20 patients, 12 men and 8 women, with SCC of the maxilla and with an average age of 67.65 years at initial diagnosis. Data regarding tumor location, tumor staging, pathological features, performed treatment and appearance of regional recurrence are depicted in Table 1. Average post-operatory follow-up was 53.25 months. Thirteen patients (65%) had tumors primarily located in the hard palate, whereas 7 patients (35%) had them in the maxillary alveolus. Tumor grade was well-differentiated in 12 (60%) of the patients, while it was moderately-differentiated in 8 (40%). Perineural infiltration was present in 3 (15%) patients, while vascular invasion appeared in 5 (25%). Bone involvement was identified in 8 (40%) patients. Regarding tumor size, pTNM classification revealed advanced disease in 11 (55%) patients, thence tumors were classified as pT3 and pT4.
Fourteen patients (70%) did not undergo cervical management, 6 (30%) underwent a modified type III radical neck dissection plus post-operative RT. Post-operative local RT was administered in 13 patients (65%).
At diagnosis, 6 of the 20 (30%) patients with maxillary SCC presented cervical nodal disease, as evidenced by clinical examination and radiological findings consistent with malignancy. Five of these six patients had bone invasion. These six patients underwent cervical dissection and the pathological examination showed lymph neck node metastasis in all of them. Interestingly, they were also classified as pT3 and pT4 from the histological study of the surgical specimen attending to tumor size considerations and bone invasion. Among these patients, no regional recurrence in the ipsilateral neck appeared during the follow-up period. Meanwhile, 3 of the 20 (15%) patients developed cervical metastasis in the follow-up period (these 3 patients had bone invasion): (1) Two patients at 10 and 11 months, in the contralateral neck; (2) one patient at 14 months in the ipsilateral neck. Cervical metastasis developed at 11.6 months as average time. Local recurrence appeared in 2 of 20 (10%) patients. In this series, 9 of the 20 (45%) patients with SCC involving the palate or the maxillary alveolus developed cervical metastasis during disease, including those present at initial diagnosis (30%) and those appearing during follow-up (15%).
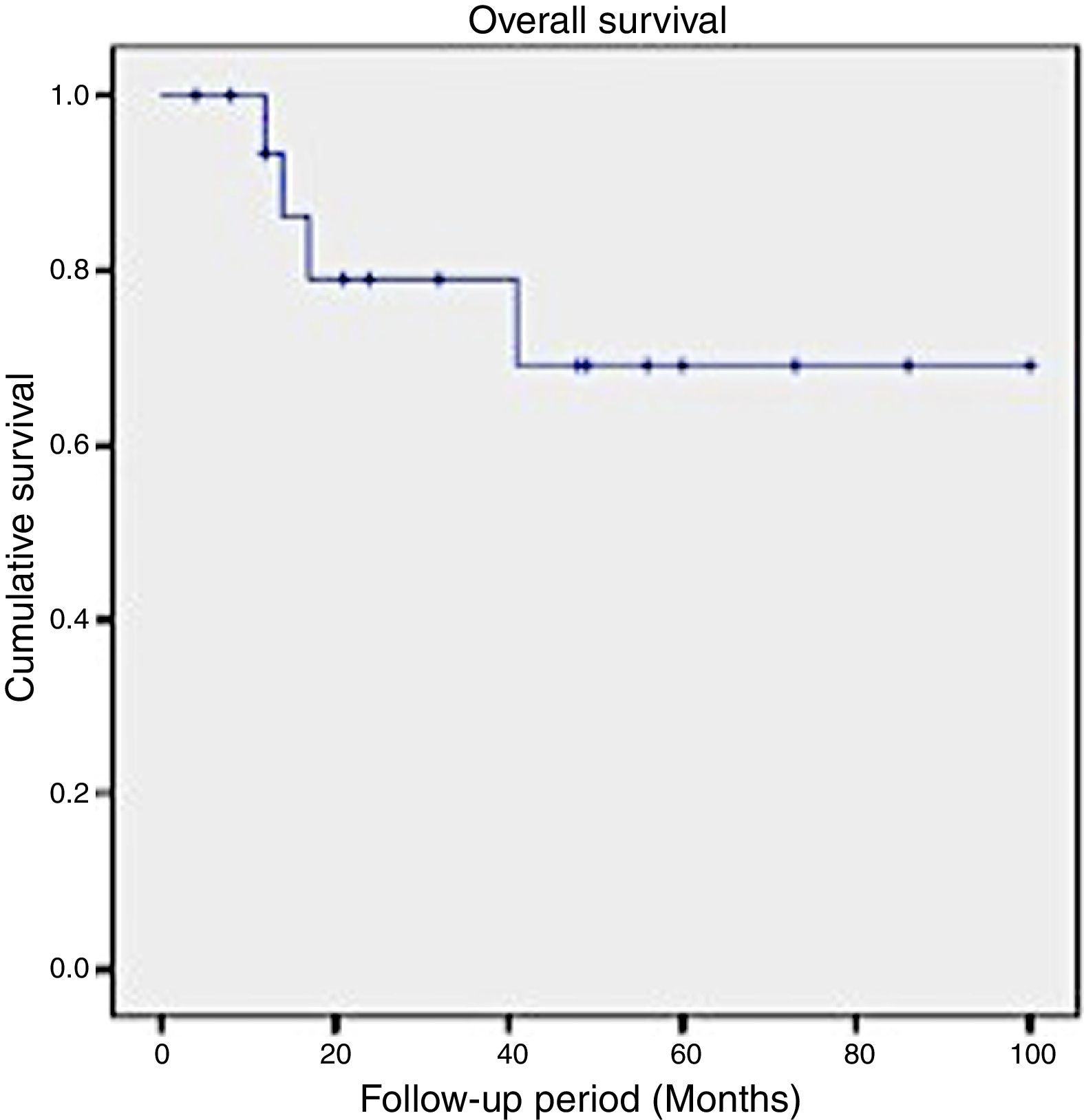
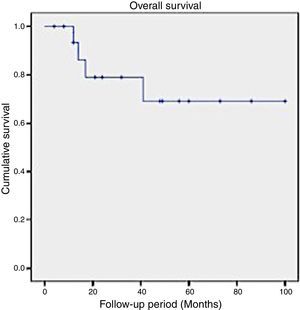
In this series, the 2-year survival rate for patients suffering SCC of the maxilla was 79%, while the 5-year survival rate decreased to 69% (Fig. 1).
DiscussionIn the last century, few studies have been focused on cervical metastasis from SCC of the maxilla. Nevertheless, cervical metastasis from SCC of the tongue or the floor of mouth has been well studied, both sites presenting a high incidence.14,15 Janeway, in 1918, first reported cervical metastasis from maxillary SCC on a rate of 33.3% (7/21), while most of patients were treated with radiotherapy.16
In our series, 9 of the 20 (45%) patients with SCC involving the palate or the maxillary alveolus developed cervical metastasis during disease, including those present at initial diagnosis (30%) and those appearing during follow-up (15%). These outcomes are similar to others published in recent studies. Montes and Schmidt,4 in a series of 14 patients with SCC of the maxilla, reported a 42.9% rate of regional nodal disease. Brown et al.5 in a series of 43 patients, reported a rate of 37.2% of cervical metastasis. Simental et al.6 in a series of 26 patients with SCC of the maxillary alveolus and hard palate found cervical metastasis in 34.6%, similar to that observed by Kruse and Grätz7 (33.6%) in a series of 30 patients. Mourouzis et al.8 reported a 23.5% incidence of cervical metastasis at presentation with maxillary SCC in a series of 17 patients. These reported incidences of cervical metastasis are comparable to those observed for SCC of the tongue or the floor of mouth. Ogura et al.9 reported a 28.5% incidence of cervical disease at presentation. In this study, they found a significant relationship between the presence of bone invasion and the presence of cervical metastasis. In our study, we observed the presence of bone invasion in 8 patients. Seven of these 8 (87.5%) patients developed cervical metastasis, including those found at presentation (5 patients) and those found during follow-up (2 patients).
Interestingly, in our study two of the three cervical metastasis occurred in the contralateral neck during follow-up. A possible explanation for that higher observed contralateral neck metastasis in SCC of the maxilla compared with other locations within the oral cavity could be due to the fact that the mucosa of the hard palate has a dense superficial lymphatic vessel system. A few crossing lymph vessels are located in the midline of the hard palate, and a significant transition of the midline can be observed in the deeper and the caudal part of the soft palate. Due to this, contralateral regional recurrence from SCC of the hard palate and maxillary alveolus could be probably present in lesions crossing the midline,17 being this a yet non-evidenced contrasted hypothesis.
In our series, neck metastasis developed at 11.6 months as average time. No regional recurrence appeared during the follow-up period. Simental et al.6 and Montes and Schmidt4 reported regional recurrences at 10.4 and 9.75 months, respectively, whereas Morris et al.10 pointed it out at 6 months. Presumably, most of regional recurrences may appear during the first year. Therefore, close monitoring should be kept over these patients.
It is very significant that all cervical metastasis from SCC of the maxilla in this series (45%) corresponded with a tumor size larger than 4cm (T3 and T4 tumors). This rate of cervical metastasis from big-sized tumors may suggest performing elective neck dissection only in patients with advanced disease. This finding has also been observed by an American multicenter study by Montes et al.11 about maxillary SCC, which reported a high percentage of cervical metastasis in T3 and T4 tumors. Meng et al.18 in their series of 78 patients with SCC of the maxilla, reported that rates for positive nodal metastasis from T1 and T2 tumors were lower than 15%, whereas those for T3 and T4 tumors were higher than 40%. Analyzing pathological features, moderately differentiated SCC was present in 71.4% of all the patients who presented or developed cervical metastasis.
Weiss et al.19 advised performing elective treatment of the neck in patients with SCC of the oral cavity when risk of clinically occult metastasis exceeded 15–20%. Recently, several studies have been published reporting higher-than-expected incidence of regional recurrence in patients without neck metastasis at diagnosis, ranging from 14% to 29%,4–11 which resulted in poor survival in most of the cases. Morris et al.10 reported a 21% of isolated regional recurrence rate and they suggested that elective neck dissection would seem prudent in most locally advanced cases. The rate of cervical metastasis in our study was 45% (9 of 20). Based on these findings and in our results, we emphasize the importance of considering elective neck dissection in the treatment of locally advanced SCC of the maxilla.
Brown et al.5 suggested that management of the N0 neck for maxillary SCC should include the same approach to the neck than to other oral sites. They carried out a comparison between SCC in the maxillary alveolus and hard palate to other oral sites in terms of neck management and outcomes. They reported a higher regional recurrence rate (26%) in tumors of the maxillary alveolus and hard palate compared with tumors from other oral sites (7%). This could be due to the fact that elective neck dissection was performed in 55% of patients with tumors in other sites of the oral cavity while, in contrast, in only 28% of patients with tumors in the upper maxilla.
This study has some limitations since it is a retrospective study with a short number of patients. Despite that, it is pointed out the high incidence of cervical metastasis from SCC of upper maxilla, especially in cases of advance stages.
All these studies add evidence of a need in changing the management of the N0 neck in maxillary SCC. Not only elective neck dissection is an option in cN0 patients, but also other treatments such as sentinel node biopsy.
ConclusionsDespite this study is limited by its retrospective nature and the sample size, and based on our findings and on an extensive review of the literature, we may conclude that cervical metastasis from maxillary alveolus and hard palate SCC appears most frequently in pT3/T4 tumors and in presence of bone invasion. Therefore, we recommend elective neck dissection for patients with pT3/T4 SCC of the maxillary alveolus and the hard palate.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
DisclaimerThe authors do not have any financial interests, either directly or indirectly, in the products or information listed in the paper.
Conflict of interestThe authors declare no conflict of interest.
FundingNone.